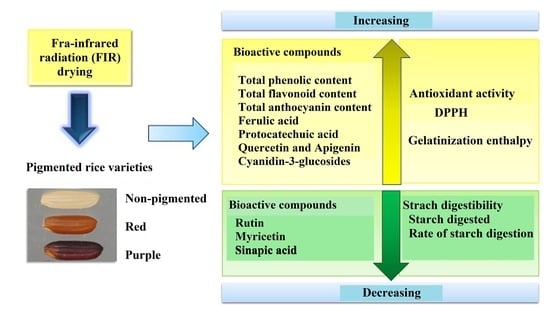
Effects of Far-Infrared Radiation Drying on Starch Digestibility and the Content of Bioactive Compounds in Differently Pigmented Rice Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Determination of Total Phenolic Content
2.4. Determination of Total Flavonoid Content
2.5. Determination of Total Anthocyanin Content
2.6. Determination of Antioxidant Activity Using DPPH Radical-Scavenging Activity
2.7. Amylose Content
2.8. Identification of Phenolic Compounds
2.9. Identification of Flavonoids
2.10. Identification of Anthocyanins
2.11. Differential Scanning Calorimetry (DSC)
2.12. Starch Digestion
2.13. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content (TPC)
3.2. Total Flavonoid Content (TFC)
3.3. The Total Anthocyanin Content (TAC)
3.4. Antioxidant Activity
3.5. Phenolic Compounds
3.6. Composition of Flavonoids
3.7. Composition of Anthocyanin
3.8. Amylose Content
3.9. Differential Scanning Calorimetry (DSC)
3.10. In Vitro Digestibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, Y.; Jeong, H.S.; Lee, J. Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chem. 2007, 103, 130–138. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. The distribution of phenolic acids in rice. Food Chem. 2004, 87, 401–406. [Google Scholar] [CrossRef]
- Wolever, T.M.; Jenkins, D.J.; Vuksan, V.; Josse, R.G.; Wong, G.S.; Jenkins, A.L. Glycemic index of foods in individual subjects. Diabetes Care 1990, 13, 126–132. [Google Scholar] [CrossRef]
- Ratseewo, J.; Meeso, N.; Siriamornpun, S. Changes in amino acids and bioactive compounds of pigmented rice as affected by far-infrared radiation and hot air drying. Food Chem. 2020, 306, 125644. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Mitchell, S.; Sahye-Pudaruth, S.; Mejia, S.B.; Vidgen, E. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Nagao, N.; Itani, T.; Irifune, K. Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem. 2012, 135, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Ziegler, V.; Bubolz, V.K.; Da Silva, J.; Cardozo, M.M.C.; Elias, M.C.; De Oliveira, M. Effects of the roasting process over the content of secondary metabolites from peanut grains (Arachishypogaea L.) with different colorations of testa. J. Food Qual. 2016, 39, 685–694. [Google Scholar] [CrossRef]
- Daiponmak, W.; Senakun, C.; Siriamornpun, S. Antiglycation capacity and antioxidant activities of different pigmented Thai rice. Int. J. Food Sci. Technol. 2014, 49, 1805–1810. [Google Scholar] [CrossRef]
- Deepa, G.; Singh, V.; Naidu, K.A. A comparative study on starch digestibility, glycemic index and resistant starch of pigmented (‘Njavara’and ‘Jyothi’) and a non-pigmented (‘IR 64’) rice varieties. J. Food Sci. Technol. 2010, 47, 644–649. [Google Scholar] [CrossRef]
- Ponjanta, J.; Chomsri, N.O.; Meechoui, S. Correlation of pasting behaviors with total phenolic compounds and starch digestibility of indigenous pigmented rice grown in upper Northern Thailand. Funct. Foods Health Dis. 2016, 6, 133–143. [Google Scholar] [CrossRef]
- Ratseewo, J.; Warren, F.J.; Siriamornpun, S. The influence of starch structure and anthocyanin content on the digestibility of Thai pigmented rice. Food Chem. 2019, 298, 124949. [Google Scholar] [CrossRef] [PubMed]
- Ademiluyi, A.O.; Oboh, G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef]
- Shobana, S.; Sreerama, Y.N.; Malleshi, N.G. Composition and enzyme inhibitory properties of finger millet (Eleusinecoracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Ramdath, D.D.; Padhi, E.; Hawke, A.; Sivaramalingam, T.; Tsao, R. The glycemic index of pigmented potatoes is related to their polyphenol content. Food Funct. 2014, 5, 909–915. [Google Scholar] [CrossRef]
- Wanyo, P.; Meeso, N.; Siriamornpun, S. Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 2014, 157, 457–463. [Google Scholar] [CrossRef]
- Raksakantong, P.; Siriamornpun, S.; Meeso, N. Effect of drying methods on volatile compounds, fatty acids and antioxidant property of Thai kaffir lime (Citrus hystrix DC). Int. J. Food Sci. Technol. 2012, 47, 603–612. [Google Scholar] [CrossRef]
- Lee, S.C.; Jeong, S.M.; Kim, S.Y.; Park, H.R.; Nam, K.C.; Ahn, D.U. Effect of far-infrared radiation and heat treatment on the antioxidant activity of water extracts from peanut hulls. Food Chem. 2006, 94, 489–493. [Google Scholar] [CrossRef]
- Tangkhawanit, E.; Meeso, N.; Siriamornpun, S. Changes in bioactive components, biological activities and starch digestibility of soymilk residues as affected by far-infrared radiation combined with hot-air and hot-air drying. Dry. Technol. 2022, 1–14. [Google Scholar] [CrossRef]
- Ratseewo, J.; Tangkhawanit, E.; Meeso, N.; Kaewseejan, N.; Siriamornpun, S. Changes in antioxidant properties and volatile compounds of kaffir lime leaf as affected by cooking processes. Int. Food Res. J. 2016, 23, 188. [Google Scholar]
- Knutson, C.A.; Grove, M.J. Rapid method for estimation of amylose in maize starches. Cereal Chem. 1994, 71, 469–471. [Google Scholar]
- Warren, F.J.; Zhang, B.; Waltzer, G.; Gidley, M.J.; Dhital, S. The interplay of α-amylase and amyloglucosidase activities on the digestion of starch in in vitro enzymic systems. Carbohydr. Polym. 2015, 117, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; García-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Butterworth, P.J.; Warren, F.J.; Grassby, T.; Patel, H.; Ellis, P.R. Analysis of starch amylolysis using plots for first-order kinetics. Carbohydr. Polym. 2012, 87, 2189–2197. [Google Scholar] [CrossRef]
- Edwards, C.H.; Warren, F.J.; Campbell, G.M.; Gaisford, S.; Royall, P.G.; Butterworth, P.J.; Ellis, P.R. A study of starch gelatinization behaviour in hydrothermally processed plant food tissues and implications for in vitro digestibility. Food Funct. 2015, 6, 3634–3641. [Google Scholar] [CrossRef]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Adak, N.; Heybeli, N.; Ertekin, C. Infrared drying of strawberry. Food Chem. 2017, 219, 109–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Pan, H.; Fan, L. Optimisation of the microwave-assisted extraction process for six phenolic compounds in Agaricus blazei murrill. Int. J. Food Sci. Technol. 2012, 47, 24–31. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, S.M.; Park, W.P.; Nam, K.C.; Ahn, D.U.; Lee, S.C. Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts. Food Chem. 2006, 97, 472–479. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Iannoccari, T.; Summa, C.; Morelli, R.; Rapisarda, P. Effect of thermal treatments on antioxidant and antiradical activity of blood orange juice. Food Chem. 2004, 85, 41–47. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Chaiyasut, C.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sivamaruthi, B.S. Physicochemical and antioxidative properties of black, brown and red rice varieties of northern Thailand. Food Sci. Technol. 2015, 35, 331–338. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Ratseewo, J.; Kaewseejan, N.; Meeso, N. Effect of osmotic treatments and drying methods on bioactive compounds in papaya and tomato. RSC Adv. 2015, 5, 18579–18587. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates–nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Ghimeray, A.K.; Sharma, P.; Phoutaxay, P.; Salitxay, T.; Woo, S.H.; Park, S.U.; Park, C.H. Far infrared irradiation alters total polyphenol, total flavonoid, antioxidant property and quercetin production in tartary buckwheat sprout powder. J. Cereal Sci. 2014, 59, 167–172. [Google Scholar] [CrossRef]
- Vetrova, E.V.; Maksimenko, E.V.; Khizrieva, S.S.; Bugaeva, A.F.; Borisenko, N.I.; Minkin, V.I. A simple way for the preparation of natural antioxidant quercetin from rutin by subcritical water. J. Nat. Sci. Biol. Med. 2017, 8, 213. [Google Scholar] [CrossRef]
- Adu-Kwarteng, E.; Ellis, W.O.; Oduro, I.; Manful, J.T. Rice grain quality: A comparison of local varieties with new varieties under study in Ghana. Food Control 2003, 14, 507–514. [Google Scholar] [CrossRef]
- Saikia, S.; Dutta, H.; Saikia, D.; Mahanta, C.L. Quality characterisation and estimation of phytochemicals content and antioxidant capacity of aromatic pigmented and non-pigmented rice varieties. Food Res. Int. 2012, 46, 334–340. [Google Scholar] [CrossRef]
- IRRI. 2018. Available online: http://www.knowledgebank.irri.org/millingprocess/index.php/ricequality-mainmenu-281/quality-characteristics-of-milled-rice-mainmenu-283 (accessed on 5 October 2022).
- Chatthongpisut, R.; Schwartz, S.J.; Yongsawatdigul, J. Antioxidant activities and antiproliferative activity of Thai purple rice cooked by various methods on human colon cancer cells. Food Chem. 2015, 188, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cheng, J.; Lin, Q.; Wang, Q.; Wang, J.; Yu, G. Effects of endogenous proteins and lipids on structural, thermal, rheological, and pasting properties and digestibility of adlay seed (Coix lacryma-jobi L.) starch. Food Hydrocoll 2021, 111, 106254. [Google Scholar] [CrossRef]
- Tarahi, M.; Shahidi, F.; Hedayati, S. Physicochemical, Pasting, and Thermal Properties of Native Corn Starch–Mung Bean Protein Isolate Composites. Gels 2022, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Acquistucci, R. Health-Promoting Compounds in Pigmented Thai and Wild Rice. Foods 2017, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Charoenlertkul, P.; Yibchok-anun, S. α-Glucosidase inhibitory activity of cyanidin-3-galactoside and synergistic effect with acarbose. J. Enzym. Inhib. Med. Chem. 2009, 24, 65–69. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Jung, E.H.; Ran Kim, S.; Hwang, I.K.; Youl Ha, T. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef]

| Rice Varieties | Treatments | Phenolic Acids (µg/100 g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GA | PCCA | VA | ChA | CFA | SyA | p-CA | FA | SNA | Total | ||
| KDML105 | Unheated | 2.42 ± 0.07 f | 14.48 ± 0.56 d | 2.24 ± 0.08 g | 2.66 ± 0.12 c | 1.45 ± 0.04 c | 1.53 ± 0.09 f | 3.32 ± 0.26 h | 16.50 ± 0.63 e | 1.14 ± 0.06 j | 45.75 ± 2.42 e |
| (White) | FIR | 2.98 ± 0.11 e | 21.12 ± 0.11 a | 2.49 ± 0.09 g | 2.99 ± 0.19 ab | 1.46 ± 0.01 c | 1.66 ± 0.04 ef | 3.55 ± 0.09 h | 22.12 ± 0.87 c | 1.21 ± 0.08 i | 59.58 ± 2.52 c |
| Sung Yod | Unheated | 2.35 ± 0.07 f | 13.58 ± 0.19 e | 3.49 ± 0.07 f | 1.41 ± 0.09 e | 1.26 ± 0.07 d | 1.51 ± 0.05 f | 3.72 ± 0.17 g | 16.85 ± 0.21 e | 1.49 ± 0.04 h | 45.66 ± 3.45 |
| (Red) | FIR | 2.97 ± 0.13 e | 20.32 ± 0.13 b | 5.81 ± 0.21 c | 1.45 ± 0.12 de | 1.29 ± 0.03 d | 1.74 ± 0.06 de | 3.98 ± 0.07 f | 22.33 ± 1.43 c | 1.62 ± 0.05 g | 61.51 ± 4.58 c |
| Mun Poo | Unheated | 2.29 ± 0.25 f | 12.64 ± 0.67 f | 3.74 ± 0.12 e | 2.52 ± 0.05 c | 1.46 ± 0.05 bc | 1.55 ± 0.07 e | 5.51 ± 0.22 e | 16.83 ± 0.15 e | 1.50 ± 0.09 h | 48.04 ± 3.71 e |
| (Red) | FIR | 2.97 ± 0.14 e | 18.38 ± 0.12 d | 4.24 ± 0.22 d | 2.61 ± 0.11 c | 1.44 ± 0.02 c | 1.75 ± 0.05 d | 6.12 ± 0.12 d | 23.57 ± 1.22 c | 1.65 ± 0.04 g | 62.35 ± 4.79 c |
| Mali Dang | Unheated | 3.31 ± 0.11 d | 13.21 ± 0.26 ef | 3.76 ± 0.09 e | 1.50 ± 0.07 de | 1.54 ± 0.05 b | 1.63 ± 0.05 e | 5.43 ± 0.23 e | 17.71 ± 0.26 d | 2.32 ± 0.05 f | 50.42 ± 4.91 d |
| (Red) | FIR | 4.98 ± 0.11 c | 18.87 ± 0.13 c | 4.31 ± 0.18 d | 1.68 ± 0.11 d | 1.58 ± 0.05 b | 1.89 ± 0.01 c | 5.77 ± 0.11 e | 22.65 ± 1.27 c | 2.61 ± 0.18 e | 64.34 ± 4.31 c |
| Hom Nil | Unheated | 6.29 ± 0.15 b | 14.82 ± 0.72 d | 6.71 ± 0.06 b | 2.75 ± 0.08 bc | 1.71 ± 0.06 a | 1.74 ± 0.08 d | 6.58 ± 0.23 c | 33.11 ± 0.42 b | 5.01 ± 0.17 d | 78.72 ± 4.28 b |
| (Purple) | FIR | 6.96 ± 0.21 a | 20.98 ± 0.15 a | 7.01 ± 0.31 a | 3.21 ± 0.15 a | 1.72 ± 0.09 a | 1.99 ± 0.01 b | 6.81 ± 0.21 c | 40.22 ± 1.35 a | 5.32 ± 0.11 c | 94.22 ± 4.71 a |
| Riceberry | Unheated | 6.24 ± 0.09 b | 14.76 ± 0.34 d | 6.70 ± 0.08 b | 2.76 ± 0.04 bc | 1.76 ± 0.04 a | 1.97 ± 0.05 a | 7.21 ± 0.13 b | 32.32 ± 0.37 b | 5.14 ± 0.26 b | 78.76 ± 3.98 b |
| (Purple) | FIR | 6.87 ± 0.16 a | 21.07 ± 0.15 a | 7.12 ± 0.24 a | 3.11 ± 0.16 a | 1.76 ± 0.07 a | 2.03 ± 0.06 a | 7.66 ± 0.21 a | 40.28 ± 2.39 a | 5.43 ± 0.11 a | 95.33 ± 4.64 a |
| Rice Varieties | Treatments | Flavonoids (µg/100 g) | Anthocyanin (µg/100 g) | |||||
|---|---|---|---|---|---|---|---|---|
| Rutin | Myricetin | Quercetin | Apigenin | Cyanidin-3-glucoside | Pelargonidin | Malvidin | ||
| KDML105 | Unheated | 2.73 ± 0.04 g | 9.43 ± 0.11 e | 5.43 ± 0.21 e | 2.42 ± 0.06 f | ND | ND | ND |
| (White) | FIR | 2.02 ± 0.07 h | 7.87 ± 0.15 f | 7.52 ± 0.22 d | 2.76 ± 0.04 d | ND | ND | ND |
| Sung Yod | Unheated | 3.72 ± 0.14 c | 15.32 ± 0.45 c | 9.12 ± 1.08 c | 2.44 ± 0.01 f | 9.56 ± 1.43 g | 0.43 ± 0.04 f | 1.36 ± 0.07 g |
| (Red) | FIR | 2.96 ± 0.11 f | 10.32 ± 1.17 e | 15.37 ± 1.27 b | 3.44 ± 0.07 c | 20.44 ± 0.75 e | 3.43 ± 0.09 d | 1.47 ± 0.02 e |
| Mun Poo | Unheated | 3.32 ± 0.09 de | 15.32 ± 1.26 b | 10.48 ± 1.68 c | 2.54 ± 0.03 e | 9.55 ± 1.48 g | 0.24 ± 0.02 g | 1.43 ± 0.08 f |
| (Red) | FIR | 2.76 ± 0.08 g | 9.91 ± 1.18 e | 15.21 ± 1.82 b | 3.64 ± 0.11 c | 18.31 ± 0.33 f | 3.21 ± 0.01 e | 1.58 ± 0.05 d |
| Mali Dang | Unheated | 4.38 ± 0.21 b | 17.55 ± 1.76 b | 14.38 ± 1.96 b | 2.49 ± 0.09 ef | 10.45 ± 1.32 g | 0.45 ± 0.02 f | 1.52 ± 0.04 d |
| (Red) | FIR | 3.21 ± 0.02 e | 12.43 ± 1.64 de | 23.43 ± 1.39 a | 3.54 ± 0.08 c | 25.76 ± 0.04 d | 4.11 ± 0.07 c | 1.73 ± 0.09 c |
| Hom Nil | Unheated | 5.51 ± 0.02 a | 22.32 ± 2.11 a | 15.34 ± 1.36 b | 4.54 ± 0.10 b | 41.56 ± 3.87 c | 2.36 ± 0.13 b | 3.62 ± 0.12 b |
| (Purple) | FIR | 3.41 ± 0.04 d | 15.94 ± 1.15 b | 23.96 ± 1.32 a | 5.42 ± 0.11 a | 127.70 ± 0.13 a | 6.32 ± 0.27 a | 4.87 ± 0.14 a |
| Riceberry | Unheated | 5.43 ± 0.13 a | 20.31 ± 2.09 a | 14.02 ± 2.33 b | 4.43 ± 0.05 b | 37.29 ± 2.94 c | 2.46 ± 0.09 b | 3.78 ± 0.29 b |
| (Purple) | FIR | 3.32 ± 0.17 de | 14.66 ± 1.07 b | 23.94 ± 2.72 a | 5.46 ± 0.14 a | 61.71 ± 0.14 b | 5.87 ± 0.39 a | 4.85 ± 0.11 a |
| Samples | Treatments | % Amylose Content | To (°C) | Tp ( °C) | Tc ( °C) | ΔgelH (J g−1 Starch) |
|---|---|---|---|---|---|---|
| KDML105 | Unheated | 12.36 ± 0.12 d | 61.19 ± 0.12 e | 67.88 ± 0.11 e | 74.11 ± 0.10 d | 9.63 ± 0.36 g |
| (White) | FIR | 12.45 ± 0.11 d | 61.12 ± 0.05 e | 67.64 ± 0.07 e | 74.10 ± 0.07 d | 10.33 ± 0.21 ef |
| Sung Yod | Unheated | 14.42 ± 0.13 bc | 74.18 ± 0.08 a | 79.18 ± 0.09 a | 82.90 ± 0.23 a | 12.55 ± 0.42 b |
| (Red) | FIR | 14.55 ± 0.12 b | 73.73 ± 0.05 b | 78.81 ± 0.05 b | 82.72 ± 0.11 a | 13.54 ± 0.02 a |
| Mun Poo | Unheated | 15.77 ± 0.18 a | 62.45 ± 0.15 c | 69.09 ± 0.04 d | 74.96 ± 0.16 c | 9.55 ± 0.39 g |
| (Red) | FIR | 15.75 ± 0.12 a | 62.39 ± 0.19 cd | 69.10 ± 0.54 cd | 74.90 ± 0.12 c | 10.51 ± 0.32 e |
| Mali Dang | Unheated | 15.65 ± 0.14 a | 62.21 ± 0.11 cd | 68.77 ± 0.11 d | 74.95 ± 0.29 c | 11.53 ± 0.43 d |
| (Red) | FIR | 15.70 ± 0.14 a | 62.20 ± 0.09 d | 68.87 ± 0.53 d | 74.86 ± 0.24 c | 12.44 ± 0.33 b |
| Hom Nil | Unheated | 14.20 ± 0.18 c | 60.59 ± 0.11 f | 69.54 ± 0.12 c | 75.74 ± 0.12 b | 9.78 ± 0.76 fg |
| (Purple) | FIR | 14.31 ± 0.15 c | 60.43 ± 0.11 f | 69.32 ± 0.54 cd | 75.70 ± 0.21 b | 11.02 ± 0.31 d |
| Riceberry | Unheated | 14.08 ± 0.12 c | 58.04 ± 0.08 g | 65.75 ± 0.08 f | 73.82 ± 0.25 d | 10.81 ± 0.39 de |
| (Purple) | FIR | 14.18 ± 0.12 c | 58.08 ± 0.11 g | 65.64 ± 0.05 f | 73.38 ± 0.06 e | 11.74 ± 0.28 c |
| Sample | Treatments | %Starch Digestedat 60 min. | Single or First Phase | Second Phase | Total C∞ (%) | ||
|---|---|---|---|---|---|---|---|
| C1∞ (%) | k1 (min−1) | C2∞ (%) | k2 (min−1) | ||||
| KDML105 | Unheated | 16.21 ± 0.19 a | 17.21 ± 0.59 a | 0.071 ± 0.001 f | N/A | N/A | 17.44 ± 0.59 a |
| (White) | FIR | 12.17 ± 0.19 c | 12.81 ± 0.59 b | 0.059 ± 0.001 g | N/A | N/A | 12.81 ± 0.10 e |
| Sung Yod | Unheated | 9.57 ± 0.02 d | 3.46 ± 0.11 f | 0.198 ± 0.001 d | 8.99 ± 0.12 b | 0.041 ± 0.002 c | 12.45 ± 0.75 e |
| (Red) | FIR | 7.43 ± 0.02 f | 2.32 ± 0.11 g | 0.185 ± 0.002 e | 5.64 ± 0.11 c | 0.030 ± 0.001 d | 7.96 ± 0.18 g |
| Mun Poo | Unheated | 13.31 ± 0.40 b | 13.54 ± 2.23 b | 0.057 ± 0.003 h | N/A | N/A | 13.54 ± 0.11 d |
| (Red) | FIR | 8.55 ± 0.32 e | 8.68 ± 1.11 c | 0.044 ± 0.002 i | N/A | N/A | 8.68 ± 0.06 f |
| Mali Dang | Unheated | 12.13 ± 1.35 bc | 6.00 ± 0.71 d | 0.364 ± 0.003 a | 9.76 ± 0.15 a | 0.065 ± 0.001 a | 15.76 ± 0.23 b |
| (Red) | FIR | 7. 54 ± 0.04 f | 3.32 ± 0.21 f | 0.221 ± 0.003 c | 4.57 ± 0.11 d | 0.052 ± 0.001 b | 7.89 ± 0.11 g |
| Hom Nil | Unheated | 11.69 ± 1.11 c | 3.89 ± 0.49 f | 0.362 ± 0.002 a | 9.57 ± 0.14 a | 0.058 ± 0.002 b | 13.46 ± 0.13 d |
| (Purple) | FIR | 7.33 ± 0.30 f | 3.28 ± 0.29 f | 0.211 ± 0.001 c | 4.61 ± 0.31 d | 0.039 ± 0.001 c | 7.79 ± 0.17 g |
| Riceberry | Unheated | 12.50 ± 1.32 bc | 4.96 ± 0.07 e | 0.313 ± 0.003 b | 9.71 ± 0.17 a | 0.059 ± 0.001 b | 14.67 ± 0.14 c |
| (Purple) | FIR | 7.65 ± 0.81 f | 3.34 ± 0.07 f | 0.219 ± 0.004 c | 4.59 ± 0.07 d | 0.041 ± 0.004 c | 7.93 ± 0.13 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratseewo, J.; Warren, F.J.; Meeso, N.; Siriamornpun, S. Effects of Far-Infrared Radiation Drying on Starch Digestibility and the Content of Bioactive Compounds in Differently Pigmented Rice Varieties. Foods 2022, 11, 4079. https://doi.org/10.3390/foods11244079
Ratseewo J, Warren FJ, Meeso N, Siriamornpun S. Effects of Far-Infrared Radiation Drying on Starch Digestibility and the Content of Bioactive Compounds in Differently Pigmented Rice Varieties. Foods. 2022; 11(24):4079. https://doi.org/10.3390/foods11244079
Chicago/Turabian StyleRatseewo, Jiranan, Frederick Jame Warren, Naret Meeso, and Sirithon Siriamornpun. 2022. "Effects of Far-Infrared Radiation Drying on Starch Digestibility and the Content of Bioactive Compounds in Differently Pigmented Rice Varieties" Foods 11, no. 24: 4079. https://doi.org/10.3390/foods11244079
APA StyleRatseewo, J., Warren, F. J., Meeso, N., & Siriamornpun, S. (2022). Effects of Far-Infrared Radiation Drying on Starch Digestibility and the Content of Bioactive Compounds in Differently Pigmented Rice Varieties. Foods, 11(24), 4079. https://doi.org/10.3390/foods11244079