A Homogalacturonan from Peel of Winter Jujube (Zizyphus jujuba Mill. cv. Dongzao): Characterization and Protective Effects against CCl4-Induced Liver Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Fractionation of Galacturonan Pectin from Peel of Winter Jujube
2.3. Monosaccharide Composition Analysis
2.4. Methylation and GC-MS Analysis
2.5. NMR Study
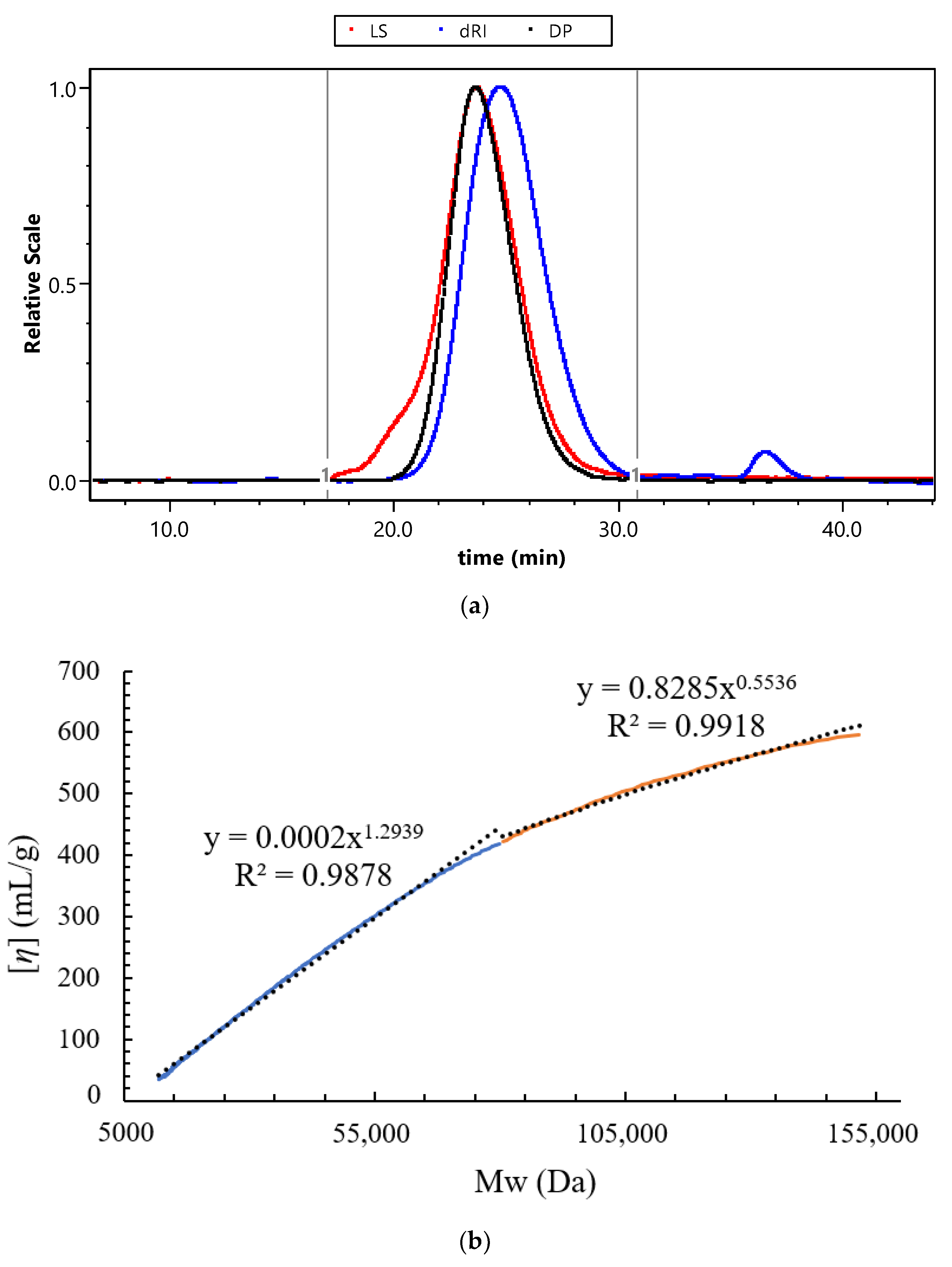
2.6. Determination of Molecular Parameters
2.7. Animal Grouping and Experimental Design
2.8. Biochemical Examinations and Histopathological Images
2.9. Statistical Analysis
3. Results and Discussions
3.1. Fractionation and Monosaccharide Composition
3.2. Structural Characterizations
3.3. Molecular Parameters Analysis of WJP-F80
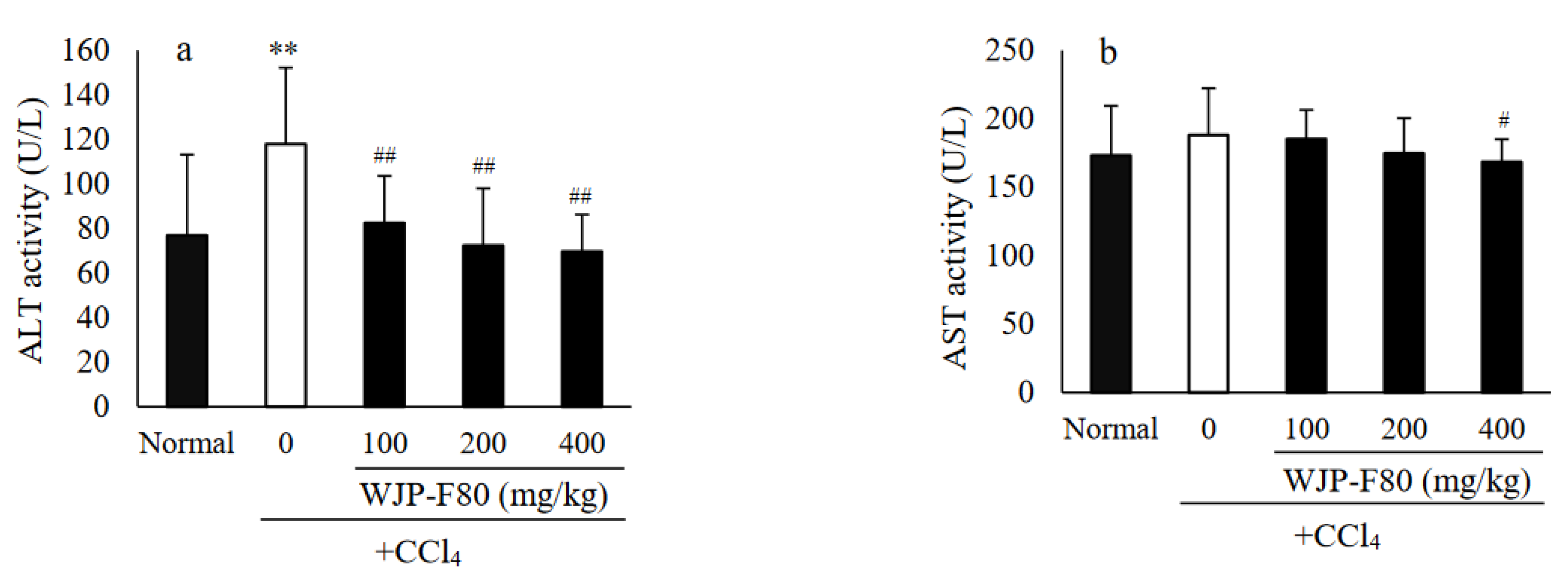
3.4. Effects of WJP-F80 on Serum ALT and AST Levels in Mice
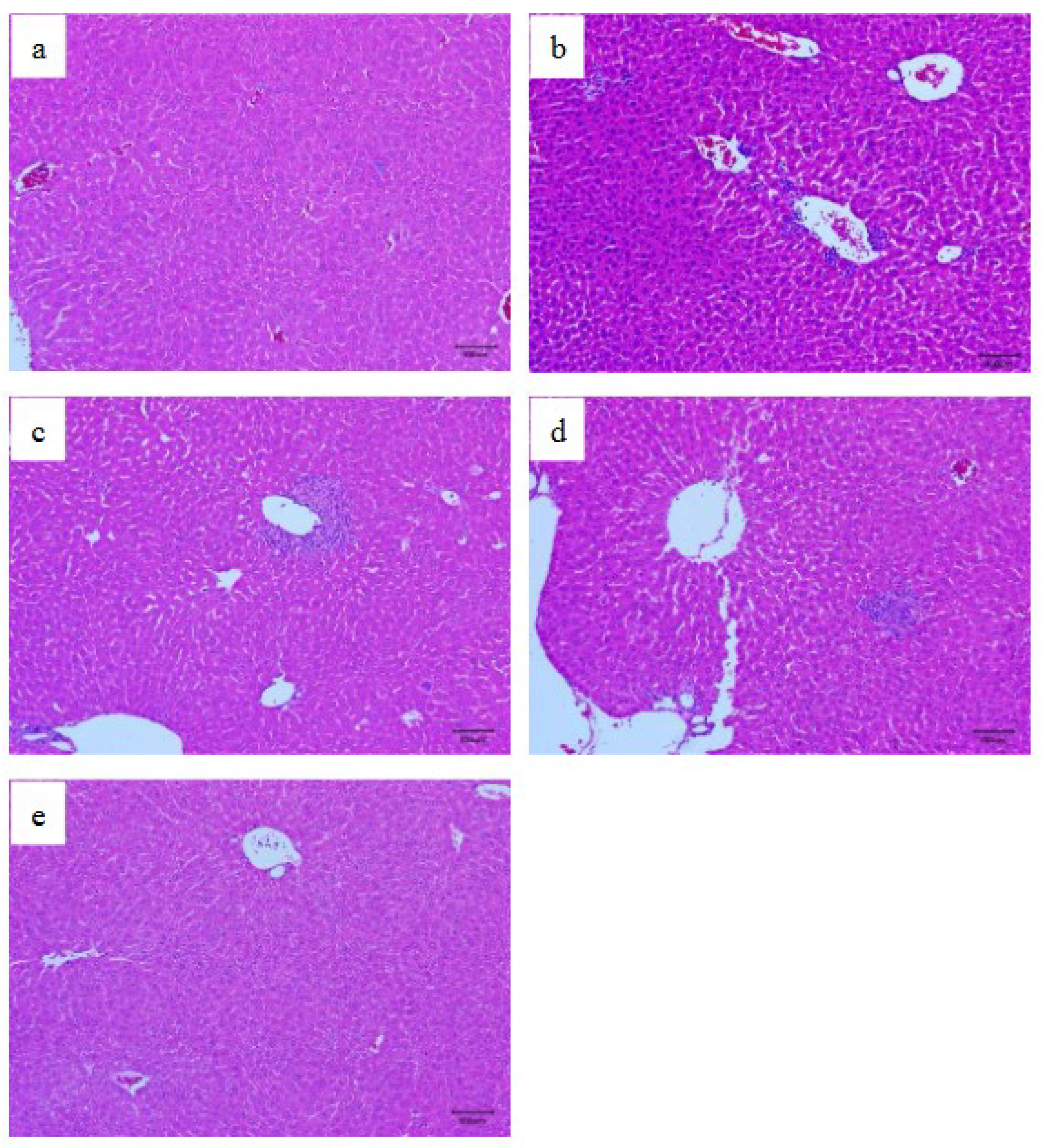
3.5. Histopathological Examination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tsim, K.W.K. A review of edible jujube, the Ziziphus jujuba fruit: A heath food supplement for anemia prevalence. Front. Pharmacol. 2020, 11, 593655. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Maiwulanjiang, M.; Lam, K.Y.C.; Zhang, W.L.; Zhan, J.Y.X.; Lam, C.T.W.; Xu, S.L.; Zhu, K.Y.; Yao, P.; Lau, D.T.W.; et al. A standardized extract of the fruit of Ziziphus jujuba (Jujube) induces neuronal differentiation of cultured PC12 cells: A signaling mediated by protein kinase A. J. Agric. Food Chem. 2014, 62, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Bao, T.; Mo, J.; Ni, J.; Chen, W. Research advances in bioactive components and health benefits of jujube (Ziziphus jujuba Mill.) fruit. J. Zhejiang Univ. Sci. B 2021, 22, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.T.W.; Gong, A.G.W.; Lam, K.Y.C.; Zhang, L.M.; Chen, J.P.; Dong, T.T.X.; Lin, H.Q.; Tsim, K.W.K. Jujube-containing herbal decoctions induce neuronal differentiation and the expression of anti-oxidant enzymes in cultured PC12 cells. J. Ethnopharmacol. 2016, 188, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Han, Y.; Kennedy, J.F.; Jiang, H.; Cao, H.; Zhang, Y.; Wang, T. A review on polysaccharides from jujube and their pharmacological activities. Carbohydr. Polym. Technol. Appl. 2022, 3, 100220. [Google Scholar] [CrossRef]
- Ji, X.; Cheng, Y.; Tian, J.; Zhang, S.; Jing, Y.; Shi, M. Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 2021, 8, 54. [Google Scholar] [CrossRef]
- Liu, C.; Wang, F.; Zhang, R. An acidic polysaccharide with anti-inflammatory effects from Blackened jujube: Conformation and rheological properties. Foods 2022, 11, 2488. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.; Wu, X.; Dai, H.; Gao, X.; Liu, M.; Tu, P.F. Structures and immunological activities of two pectic polysaccharides from the fruits of Ziziphus jujuba Mill. cv. jinsixiaozao Hort. Food Res. Int. 2006, 39, 917–923. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, F.; Zhang, R.; Liu, F.; Peng, Q.; Wang, M. An acidic polysaccharide from Ziziphus Jujuba cv. Muzao: Purification and structural characterization. Food Chem. 2019, 274, 494–499. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Jiang, J.G. Anti-fatigue effects of active ingredients from Traditional Chinese Medicine: A Review. Curr. Med. Chem. 2019, 26, 1833–1848. [Google Scholar] [CrossRef]
- Li, J.; Shan, L.; Liu, Y.; Fan, L.; Ai, L. Screening of a functional polysaccharide from Zizyphus Jujuba cv. Jinsixiaozao and its property. Int. J. Biol. Macromol. 2011, 49, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Chen, Y.; Sun-Waterhouse, D.; Zhang, Y.; Li, F. Immunomodulatory acidic polysaccharides from Zizyphus jujuba cv. Huizao: Insights into their chemical characteristics and modes of action. Food Chem. 2018, 258, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zhan, R.; Xia, L.; Shao, J.; Wang, C.; Chen, D. Polysaccharide isolated from Chinese jujube fruit (Zizyphus jujuba cv. Junzao) exerts anti-inflammatory effects through MAPK signaling. J. Funct. Foods 2018, 40, 461–470. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Y.; Jiao, Y.; Yu, L.; Yang, S.; Yang, X. Antioxidative and hepatoprotective effects of the polysaccharides from Zizyphus jujube cv. Shaanbeitanzao. Carbohydr. Polym. 2012, 88, 1453–1459. [Google Scholar] [CrossRef]
- Liu, G.; Liu, X.; Zhang, Y.; Zhang, F.; Wei, T.; Yang, M.; Wang, K.; Wang, Y.; Liu, N.; Cheng, H.; et al. Hepatoprotective effects of polysaccharides extracted from Zizyphus jujube cv. Huanghetanzao. Int. J. Biol. Macromol. 2015, 76, 169–175. [Google Scholar] [CrossRef]
- Chang, S.C.; Hsu, B.Y.; Chen, B.H. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int. J. Biol. Macromol. 2010, 47, 445–453. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, J.; Liu, G.; Liu, Y.; Wang, K.; Yang, M.; Cheng, H.; Zhao, Z. Structural characterization and in vitro antitumor activity of polysaccharides from Zizyphus jujuba cv. Muzao. RSC Adv. 2015, 5, 7860–7867. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, L.; Liu, S.; Guo, X.; Qu, Y.; Gao, M.; Cui, X.; Yang, Y. A novel acidic polysaccharide from the residue of Panax notoginseng and its hepatoprotective effect on alcoholic liver damage in mice. Int. J. Biol. Macromol. 2020, 149, 1084–1097. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Ding, J.; Lai, P.F.; Xia, Y.; Ai, L. Structural features and emulsifying stability of a highly branched arabinogalactan from immature peach (Prunus persica) exudates. Food Hydrocoll. 2020, 104, 105721. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.J.; Nie, S.P.; Chen, Y.; Wang, Y.X.; Xie, M.Y. Structural characterisation of a novel bioactive polysaccharide from Ganoderma atrum. Carbohydr. Polym. 2012, 88, 1047–1054. [Google Scholar] [CrossRef]
- Cui, S.W. (Ed.) Structural analysis of polysaccharides. In Food Carbohydrates: Chemistry, Physical Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2005; pp. 108–161. [Google Scholar]
- Zhang, H.; Zhao, T.; Wang, J.; Xia, Y.; Song, Z.; Ai, L. An amendment to the fine structure of galactoxyloglucan from Tamarind (Tamarindus indica L.) seed. Int. J. Biol. Macromol. 2020, 149, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nie, S.P.; Guo, Q.B.; Wang, Q.; Cui, S.W.; Xie, M.Y. Conformational properties of a bioactive polysaccharide from Ganoderma atrum by light scattering and molecular modeling. Food Hydrocoll. 2018, 84, 16–25. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yan, Y.; Hou, C.; Shi, M.; Liu, Y. Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus Jujuba cv. Muzao. Int. J. Biol. Macromol. 2020, 147, 844–852. [Google Scholar] [CrossRef]
- John, A.; Yang, J.; Liu, J.; Jiang, Y.; Yang, B. The structure changes of water-soluble polysaccharides in papaya during ripening. Int. J. Biol. Macromol. 2018, 115, 152–156. [Google Scholar] [CrossRef]
- Petersen, B.O.; Meier, S.; Duus, J.Ø.; Clausen, M.H. Structural characterization of homogalacturonan by NMR spectroscopy—Assignment of reference compounds. Carbohydr. Res. 2008, 343, 2830–2833. [Google Scholar] [CrossRef]
- Golovchenko, V.V.; Khlopin, V.A.; Patova, O.A.; Feltsinger, L.S.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S. Pectin from leaves of birch (Betula pendula Roth.): Results of NMR experiments and hypothesis of the RG-I structure. Carbohydr. Polym. 2022, 284, 119186. [Google Scholar] [CrossRef]
- Shakhmatov, E.G.; Toukach, P.V.; Makarova, E.N. Structural studies of the pectic polysaccharide from fruits of Punica granatum. Carbohydr. Polym. 2020, 235, 115978. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, S.W. Understanding the physical properties of food polysaccharides. In Food Carbohydrates: Chemistry, Physical Properties, and Applications; Cui, S.W., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 162–214. [Google Scholar]
- Morris, G.A.; Ralet, M.C.; Bonnin, E.; Thibault, J.F.; Harding, S.E. Physical characterisation of the rhamnogalacturonan and homogalacturonan fractions of sugar beet (Beta vulgaris) pectin. Carbohydr. Polym. 2010, 82, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.B.; Wang, Q.; Cui, S.W.; Kang, J.; Hu, X.; Xing, X.; Yada, R.Y. Conformational properties of high molecular weight heteropolysaccharide isolated from seeds of Artemisia sphaerocephala Krasch. Food Hydrocoll. 2013, 32, 155–161. [Google Scholar] [CrossRef]
- Pieczywek, P.M.; Cieśla, J.; Płaziński, W.; Zdunek, A. Aggregation and weak gel formation by pectic polysaccharide homogalacturonan. Carbohydr. Polym. 2021, 256, 117566. [Google Scholar] [CrossRef] [PubMed]
- McCay, P.B.; Lai, E.K.; Poyer, J.L.; DuBose, C.M.; Janzen, E.G. Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism. Observation of lipid radicals in vivo and in vitro. J. Biol. Chem. 1984, 259, 2135–2143. [Google Scholar] [CrossRef]
- Jayakumar, T.; Ramesh, E.; Geraldine, P. Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem. Toxicol. 2006, 44, 1989–1996. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wang, F.; Wu, C. Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J. Agric. Food Chem. 2010, 58, 6525–6531. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Fan, L.; Ai, L.; Shan, L. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao. Carbohydr. Polym. 2011, 84, 390–394. [Google Scholar] [CrossRef]

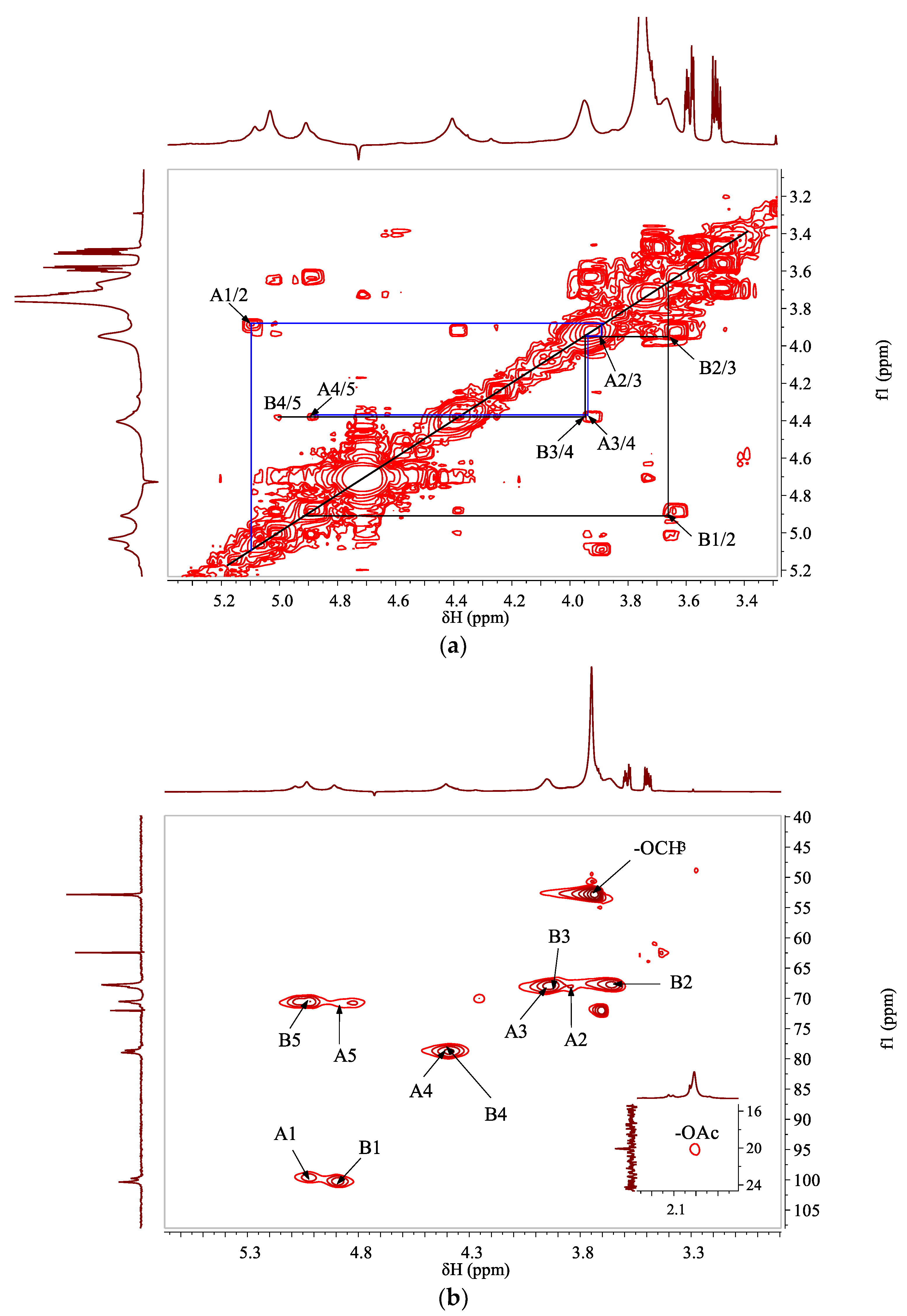

| Code | Sugar Residue | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | C6 | -OCH3 | -OAc |
|---|---|---|---|---|---|---|---|---|---|
| A | →4)-α-GalpA-(1→ | 5.08/99.77 | 3.85/68.18 | 3.94/68.18 | 4.38/78.60 | 4.89/70.89 | 173.46 | ||
| B | →4)-α-GalpA6Me-(1→ | 4.91/100.37 | 3.67/67.76 | 3.96/68.18 | 4.40/78.97 | 5.03/70.46 | 170.66 | 3.75/52.85 | 2.01/20.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Lan, W.; Ji, L.; Ai, L.; Wu, Y.; Zhang, H. A Homogalacturonan from Peel of Winter Jujube (Zizyphus jujuba Mill. cv. Dongzao): Characterization and Protective Effects against CCl4-Induced Liver Injury. Foods 2022, 11, 4087. https://doi.org/10.3390/foods11244087
Sun S, Lan W, Ji L, Ai L, Wu Y, Zhang H. A Homogalacturonan from Peel of Winter Jujube (Zizyphus jujuba Mill. cv. Dongzao): Characterization and Protective Effects against CCl4-Induced Liver Injury. Foods. 2022; 11(24):4087. https://doi.org/10.3390/foods11244087
Chicago/Turabian StyleSun, Shuguang, Wenzhong Lan, Li Ji, Lianzhong Ai, Yan Wu, and Hui Zhang. 2022. "A Homogalacturonan from Peel of Winter Jujube (Zizyphus jujuba Mill. cv. Dongzao): Characterization and Protective Effects against CCl4-Induced Liver Injury" Foods 11, no. 24: 4087. https://doi.org/10.3390/foods11244087






