Effects of Atmospheric Cold Plasma Treatment on the Storage Quality and Chlorophyll Metabolism of Postharvest Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tomato Preparation
2.2. Plasma Device and Treatment
2.3. Firmness
2.4. Respiration Intensity
2.5. Weight Loss
2.6. Color
2.7. Total Soluble Solids (TSS)
2.8. Titratable Acids (TA)
2.9. Chlorophylls and Carotenoids
2.10. Chlorophyllase, Pheophorbide a Mono-Oxygenase
2.11. Expression of the Key Enzyme Genes of Chlorophyll Metabolism
2.12. Statistical Analysis of the Results
3. Results and Discussion
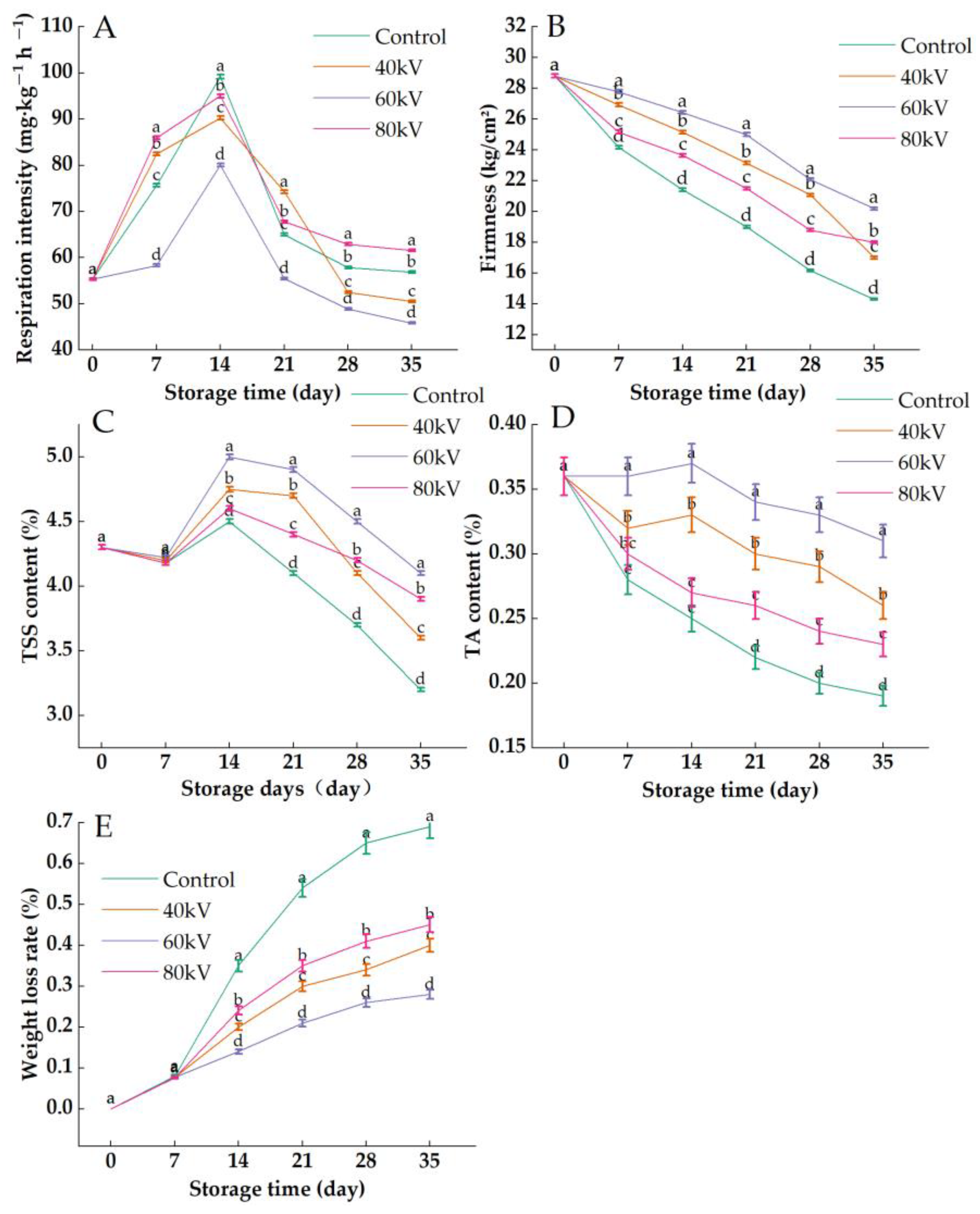
3.1. Effects of Different Intensities of the ACP Treatment on the Respiration Intensity, Firmness, TSS Content, TA Content, and Weight Loss Rate of Tomatoes
3.2. Effects of ACP Treatment of Different Intensities on the Tomato Color Difference
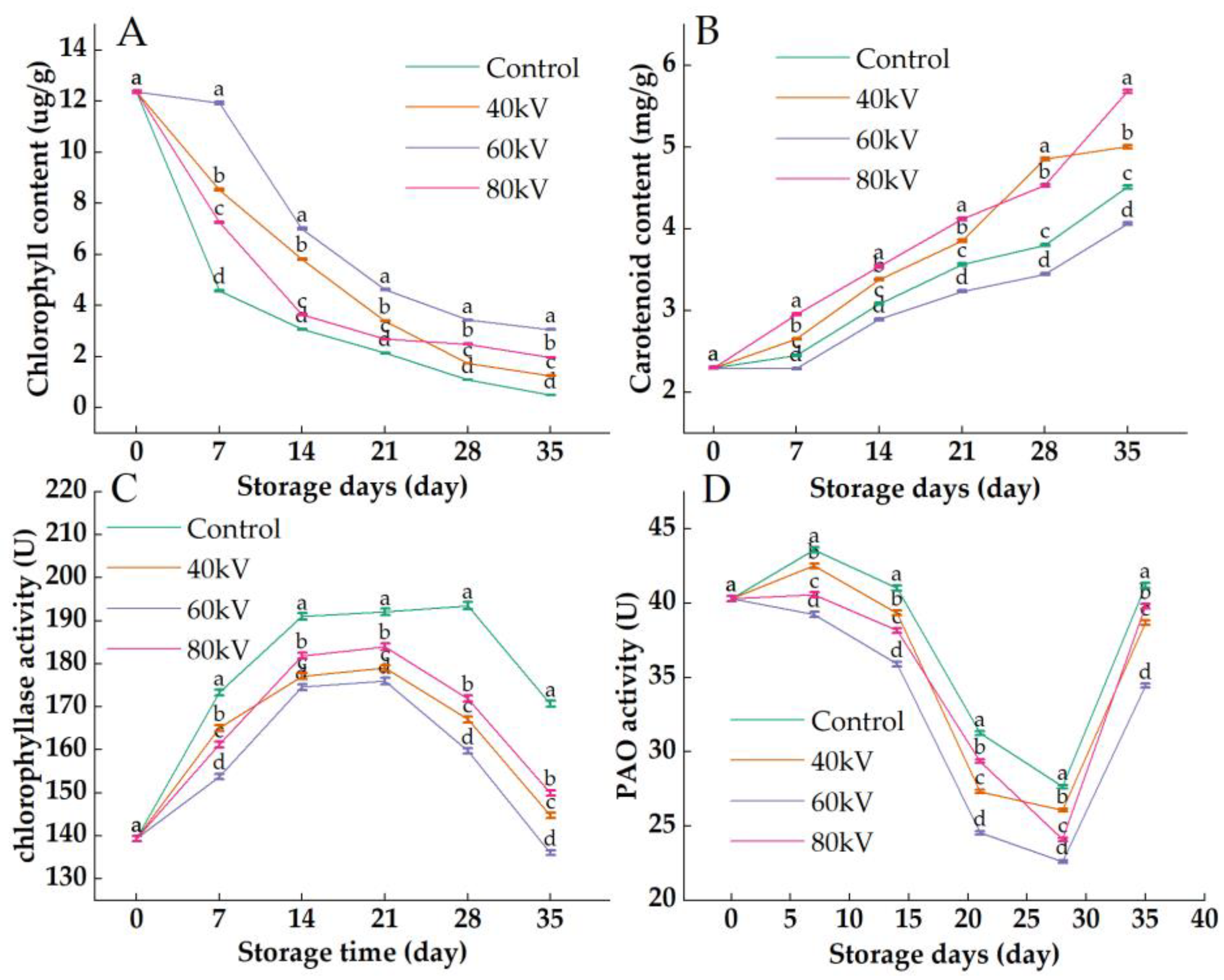
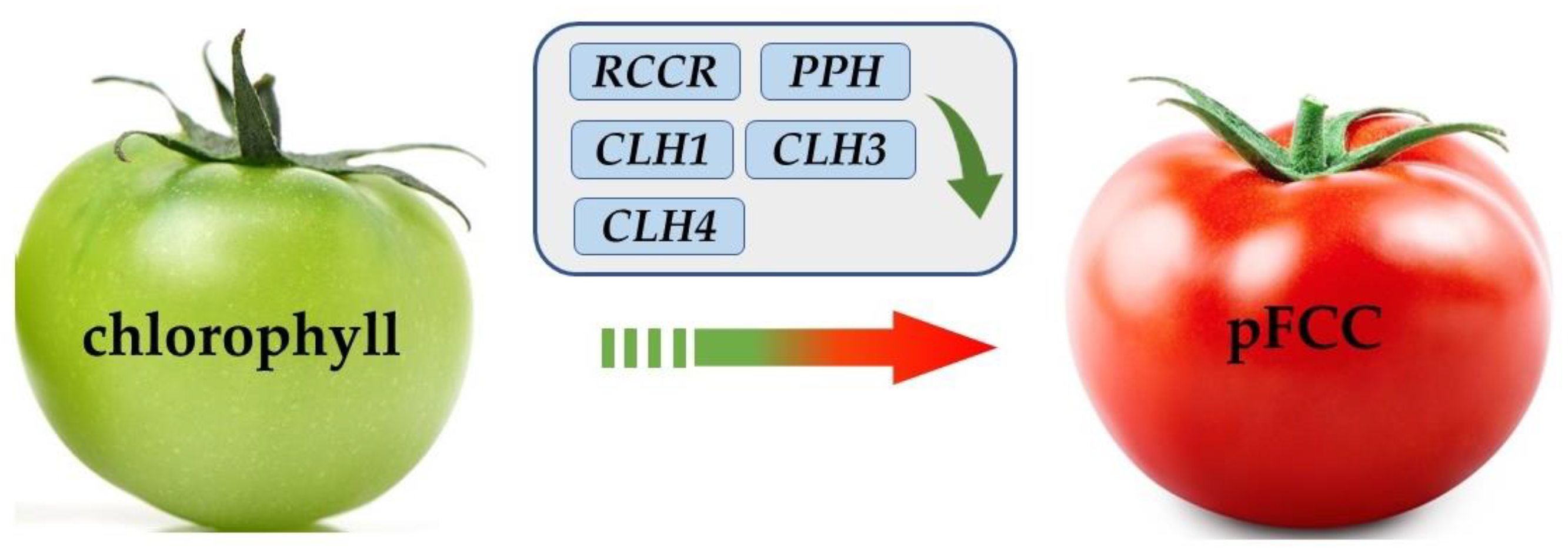
3.3. Effects of Different Intensities of ACP Treatment on the Chlorophyll Content, Carotenoid Content, Chlorophyllase Activity, and Pheophorbide a Mono-Oxygenase Activity of Tomato
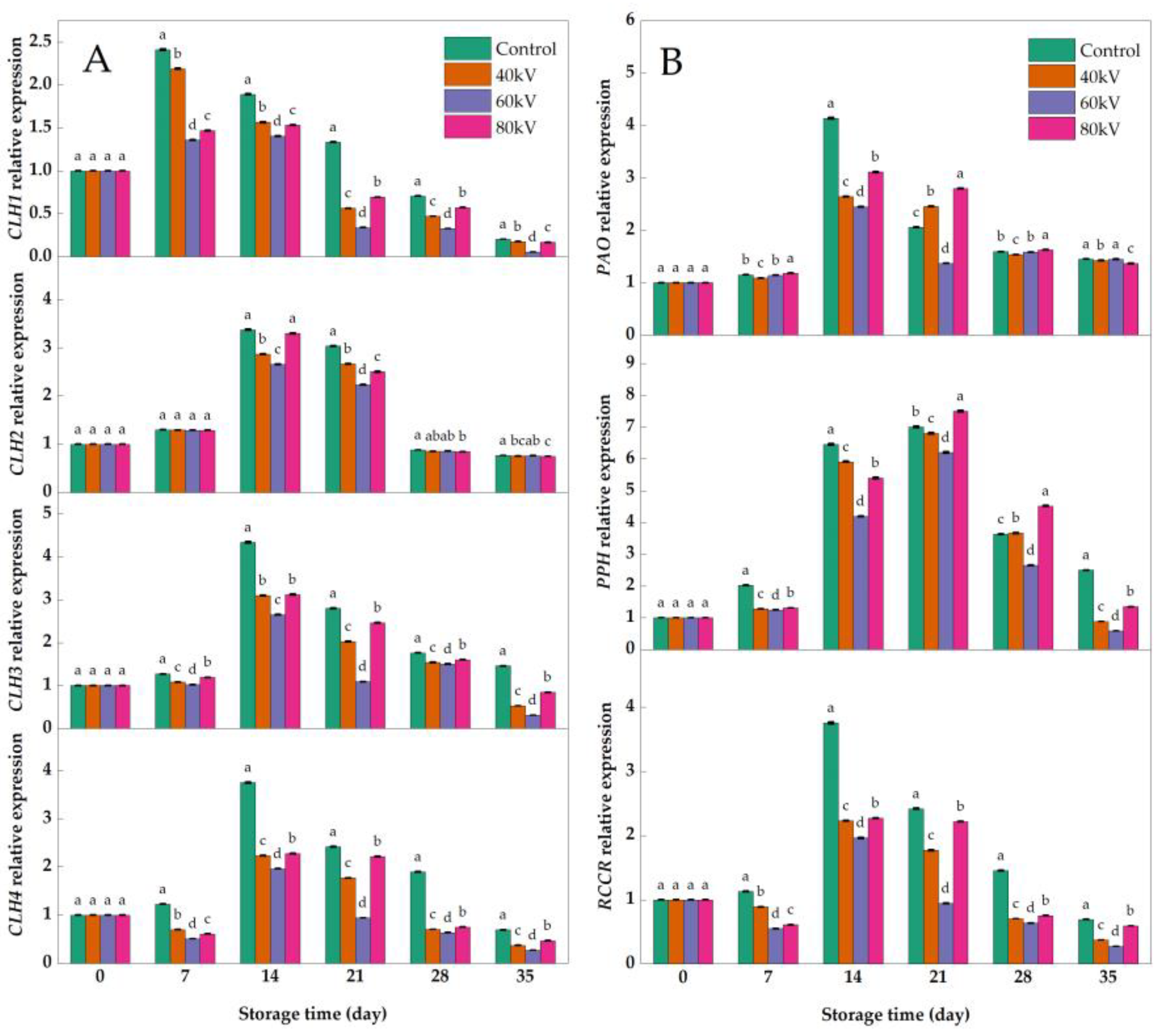
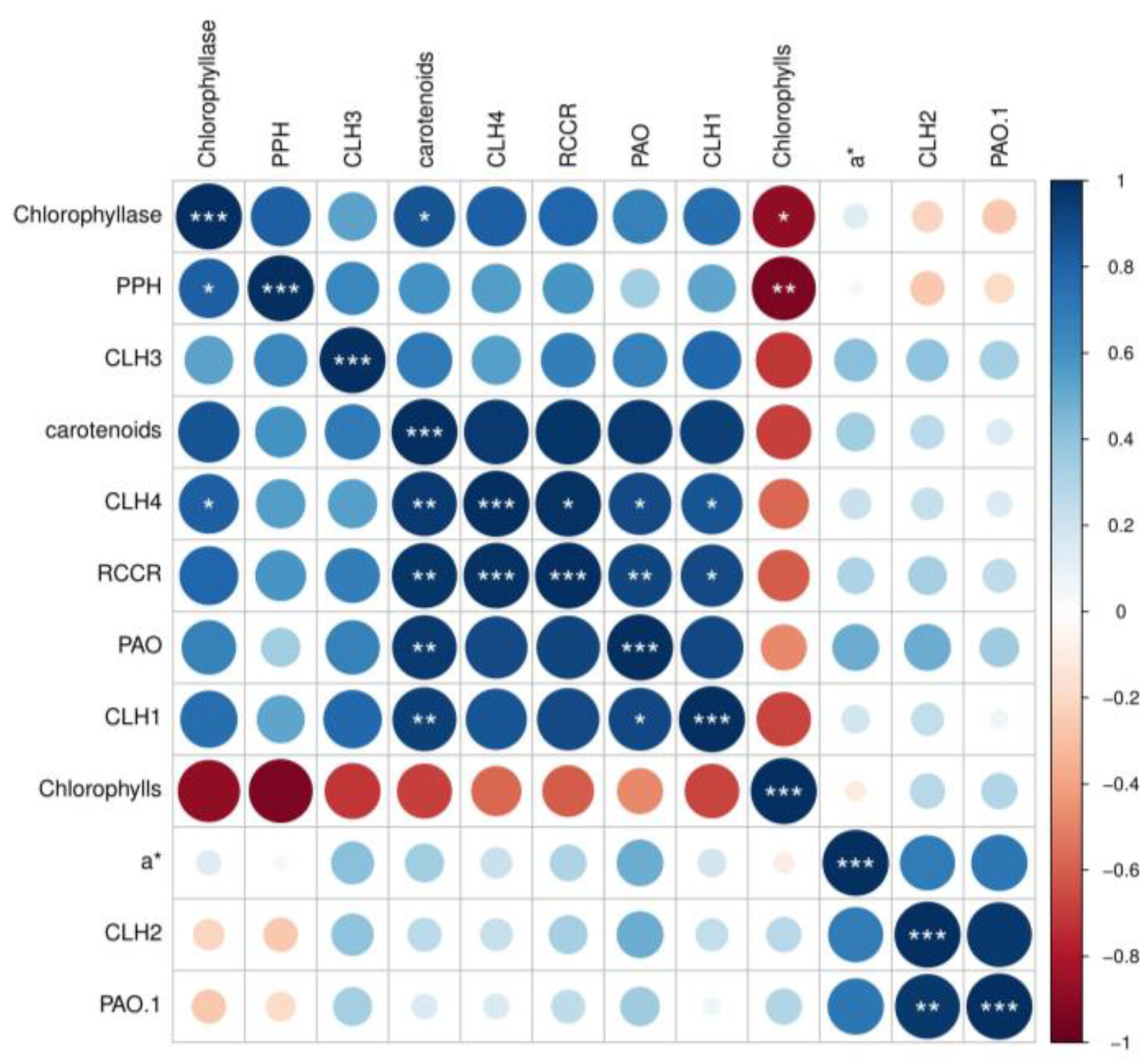
3.4. Effects of Different Intensities of ACP Treatment on the Expression of Chlorophyll-Metabolism-Related Genes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esua, O.J.; Chin, N.L.; Yusof, Y.A.; Sukor, R. Effects of simultaneous UV-C radiation and ultrasonic energy postharvest treatment on bioactive compounds and antioxidant activity of tomatoes during storage. Food Chem. 2019, 270, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Skibsted, L.H. Importance of carotenoid structure in radical-scavenging reactions. J. Agric. Food Chem. 1997, 45, 2970–2977. [Google Scholar] [CrossRef]
- Konagaya, K.; Al Riza, D.F.; Nie, S.; Yoneda, M.; Hirata, T.; Takahashi, N.; Kuramoto, M.; Ogawa, Y.; Suzuki, T.; Kondo, N. Monitoring mature tomato (red stage) quality during storage using ultraviolet-induced visible fluorescence image. Postharvest Biol. Technol. 2020, 160, 111031. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Ye, M.; Zhu, L.; Zhang, L.; Zhu, S. Improvement in storage quality of postharvest tomato fruits by nitroxyl liposomes treatment. Food Chem. 2021, 359, 129933. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Non-equilibriurn plasma-based sterilization: Overview, state-of-the-art, and challenges. In Proceedings of the 31st IEEE International Conference on Plasma Science, 2004. ICOPS 2004. IEEE Conference Record—Abstracts, Baltimore, MD, USA, 1 July 2004; IEEE: Piscataway, NJ, USA; p. 107. [Google Scholar]
- Min, S.C.; Roh, S.H.; Niemira, B.A.; Boyd, G.; Sites, J.E.; Fan, X.; Sokorai, K.; Jin, T.Z. In-package atmospheric cold plasma treatment of bulk grape tomatoes for microbiological safety and preservation. Food Res. Int. 2018, 108, 378–386. [Google Scholar] [CrossRef]
- Zhou, D.; Li, T.; Cong, K.; Suo, A.; Wu, C. Influence of cold plasma on quality attributes and aroma compounds in fresh-cut cantaloupe during low temperature storage. LWT 2022, 154, 112893. [Google Scholar] [CrossRef]
- Hu, X.; Sun, H.; Yang, X.; Cui, D.; Wang, Y.; Zhuang, J.; Wang, X.; Ma, R.; Jiao, Z. Potential use of atmospheric cold plasma for postharvest preservation of blueberries. Postharvest Biol. Technol. 2021, 179, 111564. [Google Scholar] [CrossRef]
- Limnaios, A.; Pathak, N.; Bovi, G.G.; Fröhling, A.; Valdramidis, V.P.; Taoukis, P.S.; Schlüter, O. Effect of cold atmospheric pressure plasma processing on quality and shelf life of red currants. LWT 2021, 151, 112213. [Google Scholar] [CrossRef]
- Misra, N.; Patil, S.; Moiseev, T.; Bourke, P.; Mosnier, J.; Keener, K.; Cullen, P. In-package atmospheric pressure cold plasma treatment of strawberries. J. Food Eng. 2015, 161, 95. [Google Scholar] [CrossRef]
- Ramazzina, I.; Tappi, S.; Rocculi, P.; Sacchetti, G.; Berardinelli, A.; Marseglia, A.; Rizzi, F. Effect of cold plasma treatment on the functional properties of fresh-cut apples. J. Agric. Food Chem. 2016, 64, 8010–8018. [Google Scholar] [CrossRef]
- Yinxin, L.; Can, Z.; Menglu, H.; Cui, S.; Jinping, C.; Jingyu, W.; Huang, L. Effect of cold atmospheric plasma on the gray mold rot of postharvest mulberry fruit. Food Control 2022, 137, 108906. [Google Scholar] [CrossRef]
- Guyer, L.; Hofstetter, S.S.; Christ, B.; Lira, B.S.; Rossi, M.; Hortensteiner, S. Different mechanisms are responsible for chlorophyll dephytylation during fruit ripening and leaf senescence in tomato. Plant Physiol. 2014, 166, 44–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozukue, N.; Friedman, M. Tomatine, chlorophyll, β-carotene and lycopene content in tomatoes during growth and maturation. J. Sci. Food Agric. 2003, 83, 195–200. [Google Scholar] [CrossRef]
- Barsan, C.; Zouine, M.; Maza, E.; Bian, W.; Egea, I.; Rossignol, M.; Bouyssie, D.; Pichereaux, C.; Purgatto, E.; Bouzayen, M. Proteomic analysis of chloroplast-to-chromoplast transition in tomato reveals metabolic shifts coupled with disrupted thylakoid biogenesis machinery and elevated energy-production components. Plant Physiol. 2012, 160, 708–725. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Fu, M.; Du, Y.; Sun, F.; Chen, Q.; Jin, T.; Zhang, Q.; Liu, B. Improvement of fruit quality and pedicel color of cold stored sweet cherry in response to pre-storage 1-methylciclopropene and chlorine dioxide treatments. Sci. Hortic. 2021, 277, 109806. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, X.; Wang, S.; Zhu, H.; Dun, W.; Zhang, L.; Wang, Y.; Fang, C. 1-Methylcyclopropene affects ethylene synthesis and chlorophyll degradation during cold storage of ‘Comice’ pears. Sci. Hortic. 2020, 260, 108865. [Google Scholar] [CrossRef]
- Ramazzina, I.; Berardinelli, A.; Rizzi, F.; Tappi, S.; Ragni, L.; Sacchetti, G.; Rocculi, P. Effect of cold plasma treatment on physico-chemical parameters and antioxidant activity of minimally processed kiwifruit. Postharvest Biol. Technol. 2015, 107, 55–65. [Google Scholar] [CrossRef]
- Baier, M.; Ehlbeck, J.; Knorr, D.; Herppich, W.B.; Schlüter, O. Impact of plasma processed air (PPA) on quality parameters of fresh produce. Postharvest Biol. Technol. 2015, 100, 120–126. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Wang, J.; Hu, H.; Cui, H. Combined effect of ozone treatment and modified atmosphere packaging on antioxidant defense system of fresh-cut green peppers. J. Food Process. Preserv. 2016, 40, 1145–1150. [Google Scholar] [CrossRef]
- Wu, Z.; Fan, Y.; Qiu, Y.; Hao, X.; Li, S.; Kang, S. Response of yield and quality of greenhouse tomatoes to water and salt stresses and biochar addition in Northwest China. Agric. Water Manag. 2022, 270, 107736. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jiang, W.; Zhao, Y. Fruits and Vegetables Postharvest Physiological and Biochemical Experiment Instruction; China Light Industry Press: Beijing, China, 2007; pp. 54–156. [Google Scholar]
- Wang, C.; Hou, X.; Qi, N.; Li, C.; Luo, Y.; Hu, D.; Li, Y.; Liao, W. An optimized method to obtain high-quality RNA from different tissues in Lilium davidii var. unicolor. Sci. Rep. 2022, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tan, C.; Zou, F.; Sun, Y.; Shang, N.; Wu, W. Impacts of Cold Plasma Technology on Sensory, Nutritional and Safety Quality of Food: A Review. Foods 2022, 11, 2818. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ji, L.; Chen, C.; Dong, C.; Wang, C. Effects of ozone treatment on the storage quality of post-harvest tomato. Int. J. Food Eng. 2018, 14. [Google Scholar] [CrossRef]
- Sammi, S.; Masud, T. Effect of different packaging systems on the quality of tomato (Lycopersicon esculentum var. Rio Grande) fruits during storage. Int. J. Food Sci. Technol. 2009, 44, 918–926. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Yan, Y.; Wang, W.; Zhang, L.; Zong, W. The degradation of Alternaria mycotoxins by dielectric barrier discharge cold plasma. Food Control 2020, 117, 107333. [Google Scholar] [CrossRef]
- Dong, X.Y.; Yang, Y.L. A novel approach to enhance blueberry quality during storage using cold plasma at atmospheric air pressure. Food Bioprocess Technol. 2019, 12, 1409–1421. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Han, C.; Ji, N.; Jin, P.; Zheng, Y. Physiological and metabolomic analysis of cold plasma treated fresh-cut strawberries. J. Agric. Food Chem. 2019, 67, 4043–4053. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The effects of cold plasma-activated water treatment on the microbial growth and antioxidant properties of fresh-cut pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma--A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, M.; Adhikari, B.; Guo, Z. Application of high-pressure argon for improving postharvest quality of cherry tomato. J. Food Process Eng. 2018, 41, e12882. [Google Scholar] [CrossRef]
- Misra, N. Quality of cold plasma treated plant foods. In Cold Plasma in Food and Agriculture; Elsevier: Amsterdam, The Netherlands, 2016; pp. 253–271. [Google Scholar]
- Pankaj, S.K.; Wan, Z.; Keener, K.M. Effects of Cold Plasma on Food Quality: A Review. Foods 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Boudam, M.K.; Moisan, M.; Saoudi, B.; Popovici, C.; Gherardi, N.; Massines, F. Bacterial spore inactivation by atmospheric-pressure plasmas in the presence or absence of UV photons as obtained with the same gas mixture. J. Phys. D Appl. Phys. 2006, 39, 3494. [Google Scholar] [CrossRef]
- Egea, I.; Bian, W.; Barsan, C.; Jauneau, A.; Pech, J.-C.; Latché, A.; Li, Z.; Chervin, C. Chloroplast to chromoplast transition in tomato fruit: Spectral confocal microscopy analyses of carotenoids and chlorophylls in isolated plastids and time-lapse recording on intact live tissue. Ann. Bot. 2011, 108, 291–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Z.; Li, J.; Wu, L.; Guo, J.; Ouyang, L.; Xia, Y.; Huang, X.; Pang, X. Correlation of leaf senescence and gene expression/activities of chlorophyll degradation enzymes in harvested Chinese flowering cabbage (Brassica rapa var. parachinensis). J. Plant Physiol. 2011, 168, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, L.; Limwachiranon, J.; Xie, J.; Luo, Z. Effect of UV-C on ripening of tomato fruits in response to wound. Sci. Hortic. 2016, 213, 104–109. [Google Scholar] [CrossRef]
- Pružinská, A.; Tanner, G.; Anders, I.; Roca, M.; Hörtensteiner, S. Chlorophyll breakdown: Pheophorbide a oxygenase is a Rieske-type iron–sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA 2003, 100, 15259–15264. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.W.; Pruzinská, A.; Hörtensteiner, S.; Ort, D.R. The role of pheophorbide a oxygenase expression and activity in the canola green seed problem. Plant Physiol. 2006, 142, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Rodoni, S.; Schellenberg, M.; Matile, P. Chlorophyll breakdown in senescing barley leaves as correlated with phaeophorbidea oxygenase activity. J. Plant Physiol. 1998, 152, 139–144. [Google Scholar] [CrossRef]
- Wu, C.; Cao, S.; Xie, K.; Chi, Z.; Wang, J.; Wang, H.; Wei, Y.; Shao, X.; Zhang, C.; Xu, F. Melatonin delays yellowing of broccoli during storage by regulating chlorophyll catabolism and maintaining chloroplast ultrastructure. Postharvest Biol. Technol. 2021, 172, 111378. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Zou, D.; Ji, S.; Cheng, S.; Zhou, Q.; Zhou, X.; Li, M.; Wei, B. Chlorine dioxide alleviates the yellowing process of broccoli by regulating chlorophyll degrading enzyme activity and gene expression. J. Food Process. Preserv. 2022, 46, e16985. [Google Scholar] [CrossRef]
- Misra, N.; Pankaj, S.; Segat, A.; Ishikawa, K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, J.H.; Sun, D.W. Activities and conformation changes of food enzymes induced by cold plasma: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 794–811. [Google Scholar] [CrossRef]
- Lv, J.; Ding, S.; Zhang, L.; Xu, D.; Zhang, Y.; Sun, M.; Ge, Y.; Li, J. Low temperature delays degreening of apple fruit by inhibiting pheophorbide a oxygenase (PAO) pathway and chlorophyll oxidation during ripening. J. Food Biochem. 2022, 46, e14173. [Google Scholar] [CrossRef]
- Inze, D.; Van Montagu, M. Oxidative stress in plants. Curr. Opin. Biotechnol. 1995, 6, 153–158. [Google Scholar] [CrossRef]
- Saremnezhad, S.; Soltani, M.; Faraji, A.; Hayaloglu, A.A. Chemical changes of food constituents during cold plasma processing: A review. Food Res. Int. 2021, 147, 110552. [Google Scholar] [CrossRef]
- Wang, H.-T.; Cheng, Y.-J.; Hsiao, J.-T.; Sheu, F.; Kuan, Y.-C. 17-(Allylamino)-17-demethoxygeldanamycin treatment induces the accumulation of heat shock proteins and alleviates senescence in broccoli. Postharvest Biol. Technol. 2022, 186, 111818. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, R.; Wang, T.; Fang, C.; Wang, J. Methyl salicylate delays peel yellowing of ‘Zaosu’pear (Pyrus bretschneideri) during storage by regulating chlorophyll metabolism and maintaining chloroplast ultrastructure. J. Sci. Food Agric. 2019, 99, 4816–4824. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, M.; Bai, L.; Han, X.; Ge, Y.; Wang, W.; Li, J. Effects of 1-methylcyclopropene (1-MCP) on the expression of genes involved in the chlorophyll degradation pathway of apple fruit during storage. Food Chem. 2020, 308, 125707. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Jin, T.; Zhao, H.; Han, C.; Sun, F.; Chen, Q.; Yue, F.; Luo, Z.; Fu, M. Synergistic inhibitory effect of 1-methylcyclopropene (1-MCP) and chlorine dioxide (ClO2) treatment on chlorophyll degradation of green pepper fruit during storage. Postharvest Biol. Technol. 2021, 171, 111363. [Google Scholar] [CrossRef]

| Gene Abbreviation | Primer Sequences (5′-3′) | |
|---|---|---|
| Forward Primer | Reverse Primer | |
| GRAPH | ACCACAAATTGCCTTGCTCCCTTG | ATCAACGGTCTTCTGAGTGGCTGT |
| PAO | GCATTCCGAAATTGGCTTAGAC | GCTAATCCAGCACTTATAATTGC |
| PPH | GTGTCGAATGAACAATGTACC | CCATTGAGAAGTCATTGATCC |
| CLH1 | GGTAGACTTGCTAGTGACCTG | CAAGCTGGCTTGCAACATTCG |
| CLH2 | CTCTAAAATTCTCAGCACTCC | GACCATAATCCTTAGCAAGG |
| CLH3 | CTCATGTTGGGCCAAATTTG | ACCATAAGTTGCCTTTCCTC |
| CLH4 | GCTGAGTTTTTCAACGAGAG | CAGGATCAAGTTTAATAGGAC |
| RCCR | TTTCATACTTGGTAGTTGGGTTCA | GTCCTTTCGCGGAGGTAGAT |
| Color | Treatment | Storage Time | |||||
|---|---|---|---|---|---|---|---|
| 0 Day | 7 Day | 14 Day | 21 Day | 28 Day | 35 Day | ||
| L* | Control | 60.10 ± 0.25 a | 58.22 ± 0.24 b | 55.56 ± 0.23 c | 55.40 ± 0.23 c | 53.28 ± 0.22 d | 51.43 ± 0.21 d |
| 40 kV | 60.10 ± 0.25 a | 57.38 ± 0.23 c | 56.95 ± 0.23 b | 56.08 ± 0.23 b | 55.26 ± 0.23 b | 55.35 ± 0.23 b | |
| 60 kV | 60.10 ± 0.25 a | 58.24 ± 0.25 a | 57.72 ± 0.24 a | 56.23 ± 0.23 a | 56.05 ± 0.26 a | 55.95 ± 0.23 a | |
| 80 kV | 60.10 ± 0.25 a | 55.31 ± 0.23 d | 54.70 ± 0.22 d | 53.54 ± 0.22 d | 54.54 ± 0.22 c | 54.00 ± 0.22 c | |
| a* | Control | −3.44 ± 0.01 a | −1.57 ± 0.01 a | 2.90 ± 0.01 a | 8.62 ± 0.02 a | 9.88 ± 0.04 a | 12.26 ± 0.05 a |
| 40 kV | −3.44 ± 0.01 a | −2.27 ± 0.02 b | 1.89 ± 0.01 b | 5.12 ± 0.02 b | 8.00 ± 0.03 b | 10.54 ± 0.05 c | |
| 60 kV | −3.44 ± 0.01 a | −3.14 ± 0.02 b | −2.77 ± 0.01 d | −1.17 ± 0.01 d | −0.07 ± 0.01 d | 3.09 ± 0.02 d | |
| 80 kV | −3.44 ± 0.01 a | −2.85 ± 0.01 c | 1.24 ± 0.01 c | 4.73 ± 0.02 c | 7.03 ± 0.02 c | 11.57 ± 0.05 b | |
| b* | Control | 19.47 ± 0.08 a | 21.55 ± 0.07 a | 24.21 ± 0.09 b | 20.22 ± 0.09 a | 20.30 ± 0.07 a | 19.76 ± 0.06 b |
| 40 kV | 19.47 ± 0.08 a | 22.75 ± 0.09 a | 24.48 ± 0.10 a | 20.20 ± 0.08 a | 20.44 ± 0.09 a | 20.07 ± 0.08 a | |
| 60 kV | 19.47 ± 0.08 a | 22.66 ± 0.09 a | 24.38 ± 0.10 ab | 20.32 ± 0.08 a | 20.39 ± 0.08 a | 19.94 ± 0.08 ab | |
| 80 kV | 19.47 ± 0.08 a | 22.59 ± 0.08 a | 24.26 ± 0.08 ab | 20.29 ± 0.07 a | 20.32 ± 0.08 b | 19.92 ± 0.07 ab | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, S.; Zhang, N.; Ji, H.; Zhang, X.; Dong, C.; Yu, J.; Yan, S.; Chen, C.; Liang, L. Effects of Atmospheric Cold Plasma Treatment on the Storage Quality and Chlorophyll Metabolism of Postharvest Tomato. Foods 2022, 11, 4088. https://doi.org/10.3390/foods11244088
Jia S, Zhang N, Ji H, Zhang X, Dong C, Yu J, Yan S, Chen C, Liang L. Effects of Atmospheric Cold Plasma Treatment on the Storage Quality and Chlorophyll Metabolism of Postharvest Tomato. Foods. 2022; 11(24):4088. https://doi.org/10.3390/foods11244088
Chicago/Turabian StyleJia, Sitong, Na Zhang, Haipeng Ji, Xiaojun Zhang, Chenghu Dong, Jinze Yu, Shijie Yan, Cunkun Chen, and Liya Liang. 2022. "Effects of Atmospheric Cold Plasma Treatment on the Storage Quality and Chlorophyll Metabolism of Postharvest Tomato" Foods 11, no. 24: 4088. https://doi.org/10.3390/foods11244088
APA StyleJia, S., Zhang, N., Ji, H., Zhang, X., Dong, C., Yu, J., Yan, S., Chen, C., & Liang, L. (2022). Effects of Atmospheric Cold Plasma Treatment on the Storage Quality and Chlorophyll Metabolism of Postharvest Tomato. Foods, 11(24), 4088. https://doi.org/10.3390/foods11244088




