Insights into Characteristic Volatiles in Wuyi Rock Teas with Different Cultivars by Chemometrics and Gas Chromatography Olfactometry/Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Tea Samples
2.3. HS-SPME Procedure
2.4. GC–MS Analysis
2.5. GC-O/MS Analysis
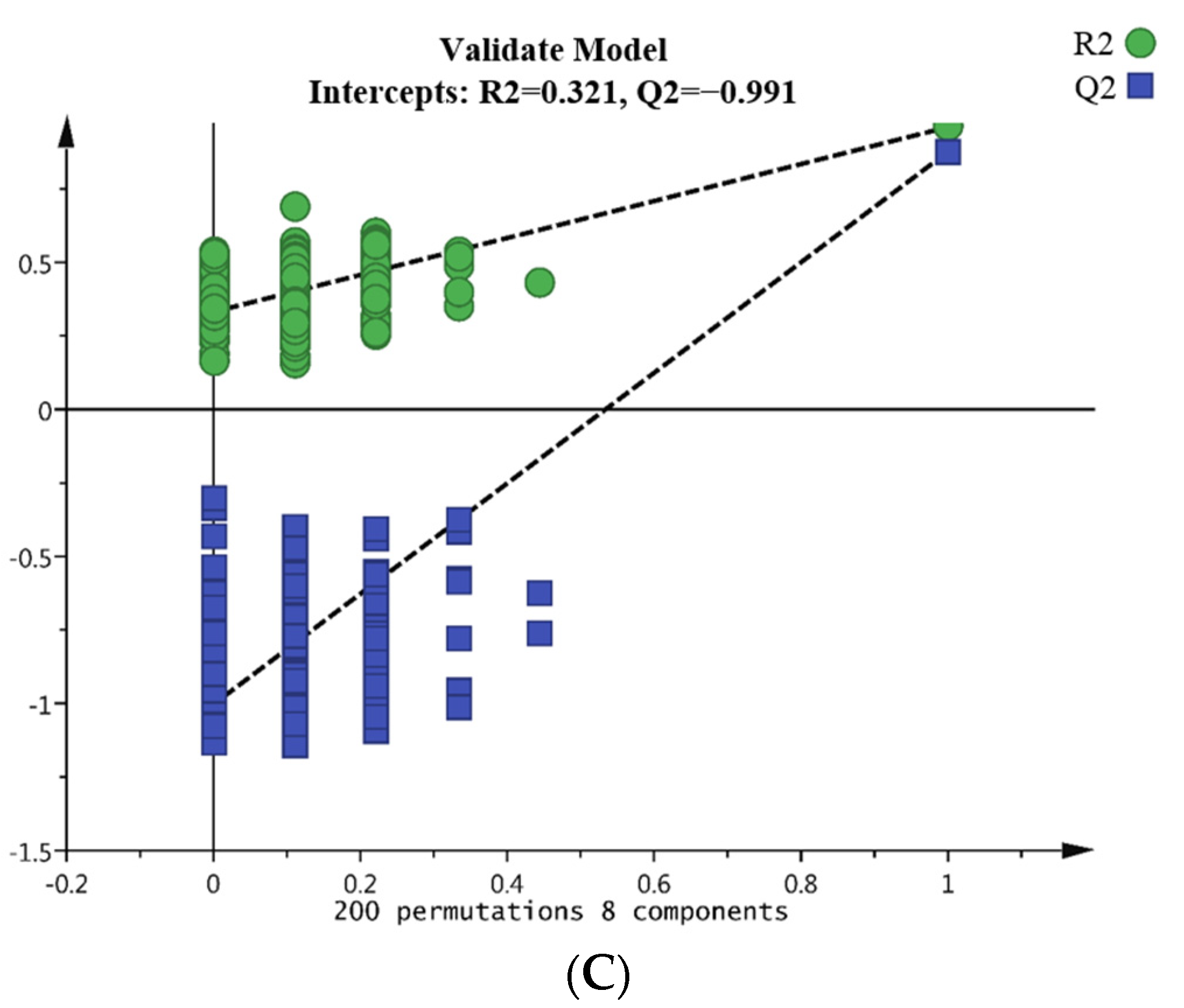
2.6. Data Processing
3. Results and Discussion
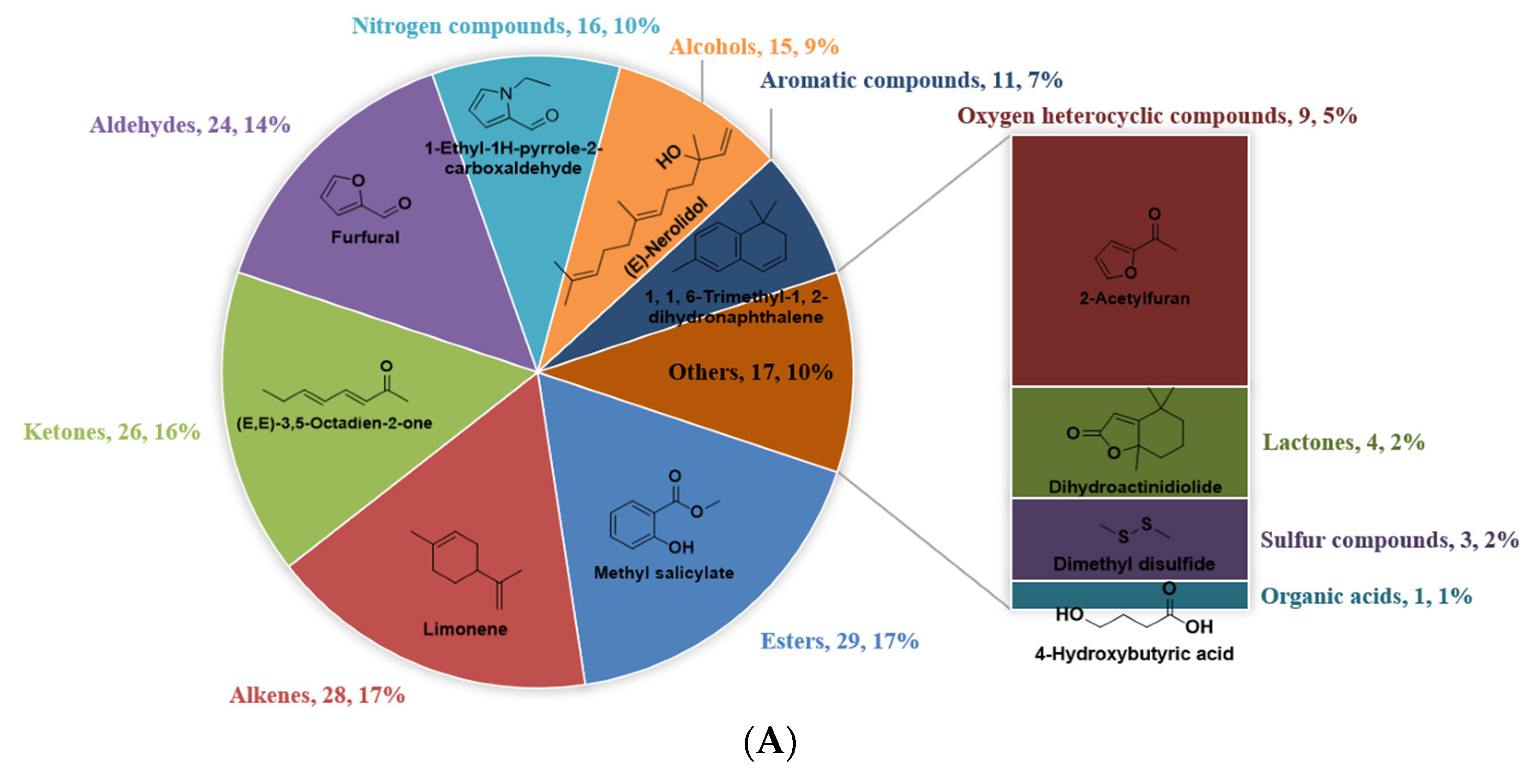
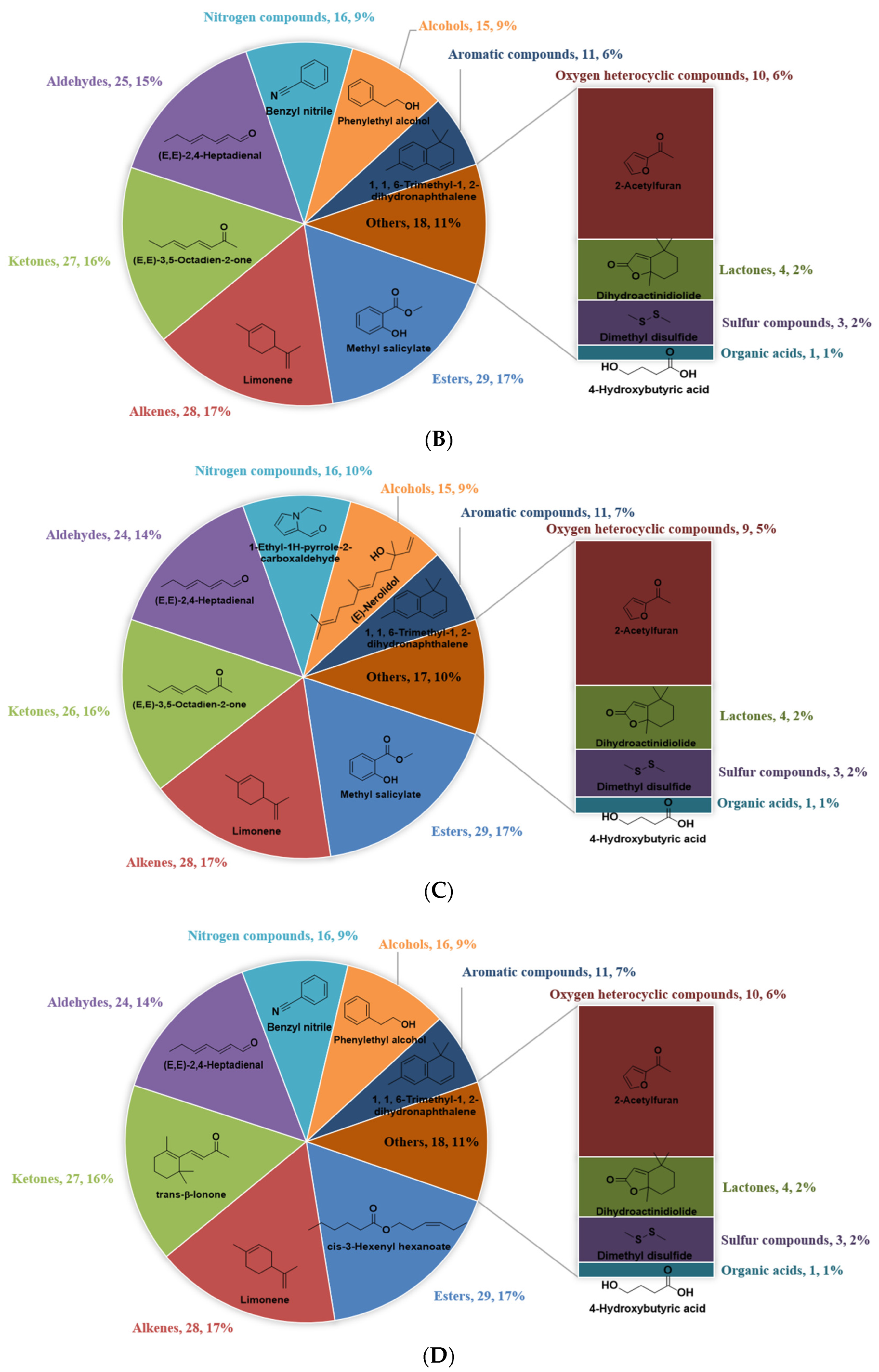
3.1. Identification of VFCs in WRTs with Different Cultivars
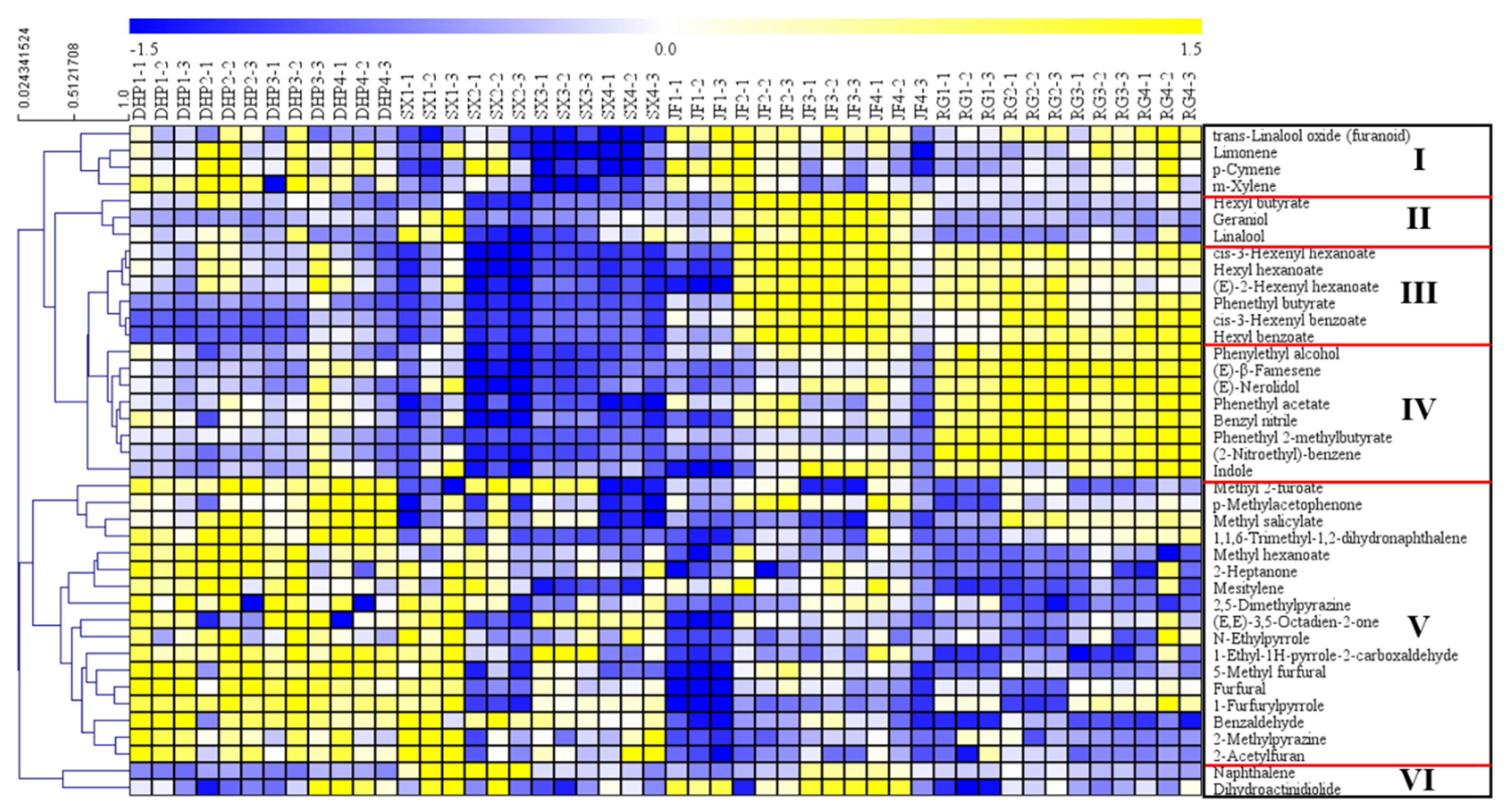
3.2. Screening of Key Differential VFCs among the Four Types of WRTs
3.3. Identification of Aroma-Active Compounds in WRTs with Different Cultivars
3.4. Determination of Characteristic Volatiles in WRTs with Different Cultivars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, L.; Zhou, X.; Su, X.; Yang, Z. Chinese oolong tea: An aromatic beverage produced under multiple stresses. Trends Food Sci. Technol. 2020, 106, 242–253. [Google Scholar] [CrossRef]
- Zhao, F.; Lin, H.; Zhang, S.; Lin, Y.; Yang, J.; Ye, N. Simultaneous determination of caffeine and some selected polyphenols in Wuyi rock tea by high-performance liquid chromatography. J. Agric. Food Chem. 2014, 62, 2772–2781. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ho, C.T.; Wan, X.; Zhu, H.; Liu, Q.; Wen, Z. Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chem. 2021, 341, 128230. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Well. 2015, 4, 9–27. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Yu, M.; Song, H.; Xu, Y.; Lin, Y.; Granvogl, M. Characterization of key aroma-active compounds in rough and moderate fire rougui Wuyi rock tea (Camellia sinensis) by sensory-directed flavor analysis and elucidation of the influences of roasting on aroma. J. Agric. Food Chem. 2022, 70, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, F.; Sun, J.; Ni, L. Dynamic changes of volatile and phenolic components during the whole manufacturing process of Wuyi rock tea (Rougui). Food Chem. 2022, 367, 130624. [Google Scholar] [CrossRef]
- Yang, P.; Song, H.; Lin, Y.; Guo, T.; Wang, L.; Granvogl, M.; Xu, Y. Differences of characteristic aroma compounds in Rougui tea leaves with different roasting temperatures analyzed by switchable GC-O-MS and GC × GC-O-MS and sensory evaluation. Food Funct. 2021, 12, 4797–4807. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Li, P.; Yang, J.; Wang, F.; Kim, E.; Wu, Y.; He, P.; Li, B.; Tu, Y. Chemical characterization of Wuyi rock tea with different roasting degrees and their discrimination based on volatile profiles. RSC Adv. 2021, 11, 12074–12085. [Google Scholar] [CrossRef]
- Guo, X.; Song, C.; Ho, C.T.; Wan, X. Contribution of L-theanine to the formation of 2,5-dimethylpyrazine, a key roasted peanutty flavor in Oolong tea during manufacturing processes. Food Chem. 2018, 263, 18–28. [Google Scholar] [CrossRef]
- Ma, C.; Tan, C.; Li, W.; Chen, L.; Wang, Y.; Chen, X. Identification of the different aroma compounds between conventional and freeze dried Wuyi rock tea (Dangui) using headspace solid phase microextraction. Food Sci. Technol. Res. 2013, 19, 805–811. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y. Clonal Tea Varieties of China; Shanghai Science and Technology Press: Shanghai, China, 2014. [Google Scholar]
- Lin, J.; Zhang, P.; Pan, Z.; Xu, H.; Luo, Y.; Wang, X. Discrimination of oolong tea (Camellia sinensis) varieties based on feature extraction and selection from aromatic profiles analysed by HS-SPME/GC–MS. Food Chem. 2013, 141, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Miao, A.; Chen, W.; Wang, W.; He, X.; Ma, C. Characterization of the volatile compounds profile of the innovative broken oolong-black tea in comparison with broken oolong and broken black tea. Food Control 2021, 129, 108197. [Google Scholar] [CrossRef]
- Guo, X.; Schwab, W.; Ho, C.T.; Song, C.; Wan, X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Tian, C.; Zhou, C.; Zhu, C.; Weng, J.; Sun, Y.; Lin, Y.; Lin, Y.; Lai, Z.; Guo, Y. Non-targeted metabolomics analysis revealed the characteristic non-volatile and volatile metabolites in the Rougui Wuyi rock tea (Camellia sinensis) from different culturing regions. Foods 2022, 11, 1694. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z.; Xiao, Z. Comparison of aroma-active volatiles in Oolong tea infusions using GC−Olfactometry, GC−FPD, and GC−MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Wang, H.; Huang, W.; Li, F.; Wang, L.; Ho, C.; Zhang, Y.; Zhang, L.; Zhai, X.; et al. Decoding the specific roasty aroma Wuyi rock tea (Camellia sinensis: Dahongpao) by the sensomics approach. J. Agric. Food Chem. 2022, 70, 10571–10583. [Google Scholar] [CrossRef]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC×GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.; Shao, C.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.; Tan, J.; Peng, Q.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.; Zhang, Y.; Dai, W.; Guo, L.; Tan, J.; Peng, Q.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, H.; Zhang, Z.; Zeng, J.; Zhang, Y.; Wang, J.; Ma, W.; Wang, M.; Peng, Q.; Lv, H.; et al. Assessment of the contribution of chiral odorants to aroma property of baked green teas using an efficient sequential stir bar sorptive extraction approach. Food Chem. 2021, 365, 130615. [Google Scholar] [CrossRef]
- Wang, M.; Ma, W.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry/(GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, R.; Schieberle, P. Compound identification: A journal of agricultural and food chemistry perspective. J. Agric. Food Chem. 2007, 55, 4625–4629. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemo-metrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, N.; Huang, S.; Fan, X.; Zhang, Y.; Ren, D. Chemical profiling and discrimination of green tea and Pu-erh raw tea based on UPLC–Q–Orbitrap–MS/MS and chemometrics. Food Chem. 2020, 326, 126760. [Google Scholar] [CrossRef]
- Antwi-Baffour, S.; Adjei, J.K.; Agyemang-Yeboah, F.; Annani-Akollor, M.; Kyeremeh, R.; Asare, G.A.; Gyan, B. Proteomic analysis of microparticles isolated from malaria positive blood samples. Proteome Sci. 2017, 15, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Zhou, Y.; Fu, X.; Mei, X.; Cheng, S.; Gui, J.; Dong, F.; Tang, J.; Ma, S.; Yang, Z. Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? Contribution of the stem to oolong tea aroma. Food Chem. 2017, 237, 488–498. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, Y.; Gui, J.; Fu, X.; Mei, X.; Zhen, Y.; Ye, T.; Du, B.; Dong, F.; Watanabe, N.; et al. Formation of volatile tea constituent indole during the oolong tea manufacturing process. J. Agric. Food Chem. 2016, 64, 5011–5019. [Google Scholar] [CrossRef]
- Kumazawa, K.; Masuda, H. Identification of potent odorants in different green tea varieties using flavor dilution technique. J. Agric. Food Chem. 2002, 50, 5660–5663. [Google Scholar] [CrossRef]
- Yan, T.; Lin, J.; Zhu, J.; Ye, N.; Huang, J.; Wang, P.; Jin, S.; Zheng, D.; Yang, J. Aroma analysis of Fuyun 6 and Jinguanyin black tea in the Fu’an area based on E-nose and GC-MS. Eur. Food Res. Technol. 2022, 248, 947–961. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, L.; Granvogl, M.; Ho, C.; Wan, X. Flavor of tea (Camellia sinensis): A review on odorants and analytical techniques. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3867–3909. [Google Scholar] [CrossRef]
- Flaig, M.; Qi, S.; Wei, G.; Yang, X.; Schieberle, P. Characterization of the key odorants in a high-grade Chinese green tea beverage (Camellia sinensis; Jingshan cha) by means of the sensomics approach and elucidation of odorant changes in tea leaves caused by the tea manufacturing process. J. Agric. Food Chem. 2020, 68, 5168–5179. [Google Scholar] [CrossRef] [PubMed]
- Flaig, M.; Qi, S.; Wei, G.; Yang, X.; Schieberle, P. Characterisation of the key aroma compounds in a Longjing green tea infusion (Camellia sinensis) by the sensomics approach and their quantitative changes during processing of the tea leaves. Eur. Food Res. Technol. 2020, 246, 2411–2425. [Google Scholar] [CrossRef]
- Wan, X. Tea Biochemistry, 3rd ed.; Agricultural Press of China: Beijing, China, 2003. [Google Scholar]
- Shao, C.; Zhang, Y.; Lv, H.; Zhang, Z.; Zeng, J.; Peng, Q.; Zhu, Y.; Lin, Z. Aromatic profiles and enantiomeric distributions of chiral odorants in baked green teas with different picking tenderness. Food Chem. 2022, 388, 132969. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wang, R.; Yang, T.; Jiang, L.; Zhang, Z. Functional characterization of salicylic acid carboxyl methyltransferase from Camellia sinensis, providing the aroma compound of methyl salicylate during the withering process of white tea. J. Agric. Food Chem. 2017, 65, 11036–11045. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Zhao, X.; Li, X.; Shan, W.; Wang, X.; Wang, S.; Yu, W.; Yang, Z.; Yu, X. Non-targeted metabolomics analysis reveals dynamic changes of volatile and non-volatile metabolites during oolong tea manufacture. Food Res. Int. 2020, 128, 108778. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]

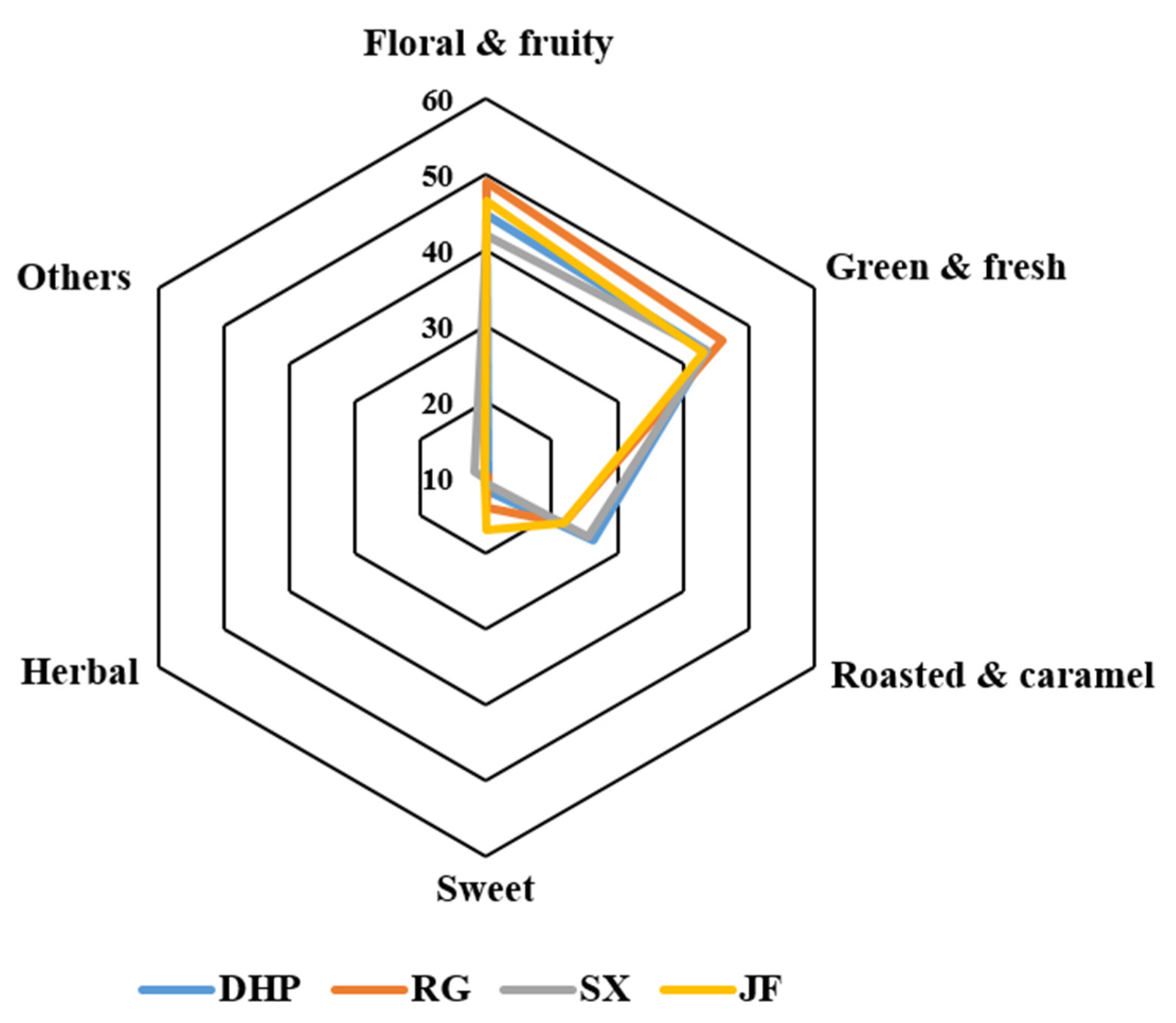
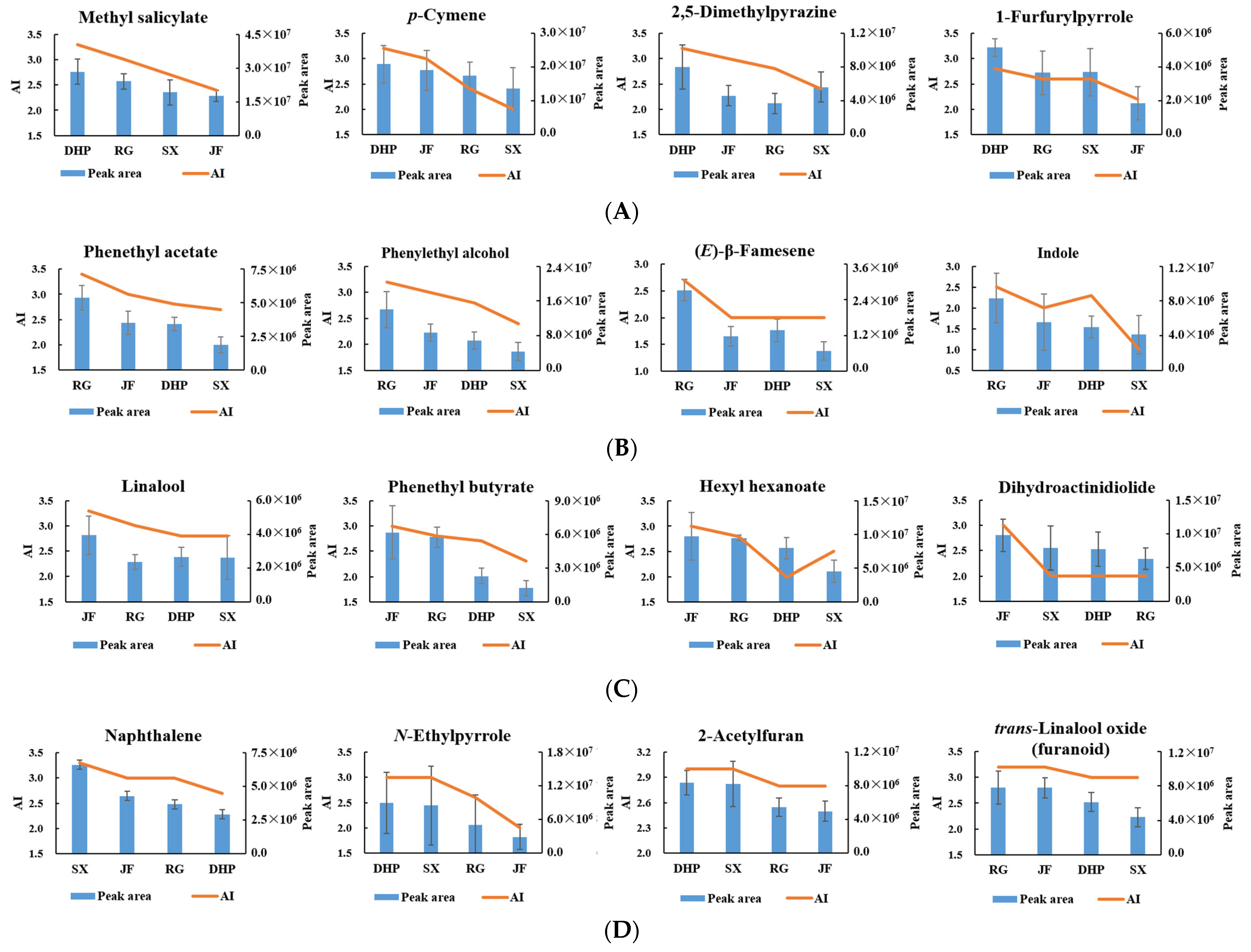
| No | Compound a | Odor Characteristic b | AI | Identification c | |||
|---|---|---|---|---|---|---|---|
| DHP | RG | SX | JF | ||||
| Class A (floral & fruity) | |||||||
| 1 | Benzeneacetaldehyde | Rose-like | 3.4 | 3.0 | 3.0 | 3.0 | MS, RI, O, S |
| 2 | Methyl octanoate | Fruity, sweet, green | 3.2 | 3.0 | 3.0 | 3.0 | MS, RI, O |
| 3 | trans-Linalool oxide (furanoid) * | Floral, sweet | 3.0 | 3.2 | 3.0 | 3.2 | MS, RI, O, S |
| 4 | trans-β-Ocimene | Floral, herbal | 3.0 | 3.0 | 3.0 | 3.3 | MS, RI, O, S |
| 5 | Linalool * | Floral, sweet | 2.8 | 3.0 | 2.8 | 3.3 | MS, RI, O, S |
| 6 | Phenylethyl alcohol * | Floral, sweet | 2.8 | 3.2 | 2.4 | 3.0 | MS, RI, O, S |
| 7 | Phenethyl acetate * | Floral, cucumber, sweet | 2.8 | 3.4 | 2.7 | 3.0 | MS, RI, O |
| 8 | trans-β-Ionone | Rose-like, coconut creamy | 2.8 | 2.8 | 3.0 | 3.0 | MS, RI, O, S |
| 9 | Phenethyl butyrate * | Floral, fruity, sweet | 2.7 | 2.8 | 2.3 | 3.0 | MS, RI, O |
| 10 | Methyl benzoate * | Floral, leather | 2.5 | 2.8 | 3.0 | 2.7 | MS, RI, O, S |
| 11 | (E)-2-Hexenyl hexanoate * | Fruity, floral, sweet | 2.5 | 2.8 | 2.5 | 3.0 | MS, RI, O, S |
| 12 | α-Ionone | Floral, sweet | 2.5 | 2.3 | 1.5 | 1.5 | MS, RI, O, S |
| 13 | Indole * | Floral, leather | 2.3 | 2.5 | 1.0 | 2.0 | MS, RI, O, S |
| 14 | α-Cedrene | Floral, sweet, fruity | 2.3 | 2.8 | 2.3 | 2.4 | MS, RI, O, S |
| 15 | Octanal | Fruity, green, fatty | 2.0 | 3.0 | 2.0 | 2.0 | MS, RI, O, S |
| 16 | Hexyl hexanoate * | Fruity, floral | 2.0 | 2.8 | 2.5 | 3.0 | MS, RI, O, S |
| 17 | (E)-β-Famesene * | Floral, sweet | 2.0 | 2.7 | 2.0 | 2.0 | MS, RI, O, S |
| Class B (green & fresh) | |||||||
| 1 | Methyl salicylate * | Minty, wintergreen-like, herbal | 3.3 | 3.0 | 2.7 | 2.4 | MS, RI, O, S |
| 2 | trans-Alloocimene | Green, fresh, floral | 3.2 | 3.3 | 3.2 | 3.4 | MS, RI, O |
| 3 | (E,E)-2,4-Nonadienal | Green, fruity | 3.2 | 3.2 | 2.8 | 2.8 | MS, RI, O, S |
| 4 | (E,Z)-2,6-Nonadienal | Green, cucumber | 3.2 | 2.8 | 3.3 | 3.3 | MS, RI, O |
| 5 | (E)-2-Octenal | Green, fresh, nut | 3.0 | 3.0 | 3.2 | 2.6 | MS, RI, O, S |
| 6 | Nonanal | Floral, fresh | 2.8 | 3.0 | 2.8 | 3.3 | MS, RI, O, S |
| 7 | Benzyl acetate | Fresh, green | 2.8 | 2.8 | 2.8 | 2.8 | MS, RI, O, S |
| 8 | Propiophenone | Green, fresh | 2.8 | 2.8 | 2.8 | 2.8 | MS, RI, O |
| 9 | Methylheptenone | Green, lemon, floral | 2.8 | 2.7 | 2.7 | 3.2 | MS, RI, O, S |
| 10 | (E)-2-Nonenal | Green, cucumber | 2.6 | 3.0 | 3.0 | 2.5 | MS, RI, O, S |
| 11 | 5-Ethyl-6-methyl-3E-hepten-2-one | Green, fresh | 2.6 | 2.5 | 2.7 | 2.5 | MS, RI, O |
| 12 | 1-Octen-3-ol | Mushroom-like, fresh | 2.5 | 3.0 | 2.7 | 2.6 | MS, RI, O, S |
| 13 | (E)-2-Decenal | Green, fresh | 2.5 | 2.8 | 3.0 | 2.5 | MS, RI, O |
| 14 | Hexanal | Green, grassy | 2.3 | 2.8 | 1.8 | 2.2 | MS, RI, O, S |
| 15 | α-Citral | Citrus, fresh | 2.0 | 2.3 | 2.0 | 2.3 | MS, RI, O |
| 16 | Calamenene | Fresh and cool | 2.0 | 3.0 | 2.0 | 2.0 | MS, RI, O, S |
| Class C (roasted and caramel) | |||||||
| 1 | 2-Methyl-3,5-diethylpyrazine | Nutty | 3.3 | - | 3.3 | - | MS, RI, O |
| 2 | 2,5-Dimethylpyrazine * | Barked, coffee | 3.2 | 2.8 | 2.4 | 3.0 | MS, RI, O, S |
| 3 | 2-Acetylpyrrole | Caramel | 3.0 | 3.0 | 3.0 | 3.2 | MS, RI, O, S |
| 4 | 2-Acetylfuran * | Roasted, cocoa | 3.0 | 2.8 | 3.0 | 2.8 | MS, RI, O, S |
| 5 | N-Ethylpyrrole * | Burnt, barked | 3.0 | 2.6 | 3.0 | 2.0 | MS, RI, O, S |
| 6 | 3-Ethyl-2,5-dimethylpyrazine | Roasted, coffee | 3.0 | 2.4 | 3.2 | 2.8 | MS, RI, O |
| 7 | 2,2’-Methylenebis-furan | Roasted, bitter | 2.8 | 2.8 | 2.4 | 3.0 | MS, RI, O |
| 8 | 1-Furfurylpyrrole * | Roasted, green | 2.8 | 2.6 | 2.6 | 2.2 | MS, RI, O |
| 9 | 2-Methylpyrazine | Roasted, pesticide-like | 2.3 | 2.8 | 2.5 | 2.8 | MS, RI, O, S |
| Class D (sweet) | |||||||
| 1 | Acetophenone | Sweet, floral | 3.4 | 3.2 | 3.2 | 3.2 | MS, RI, O |
| 2 | p-Cymene * | Fragrant and sweet, fresh, rice-like | 3.2 | 2.4 | 2.0 | 3.0 | MS, RI, O |
| 3 | 6-Methyl-3,5-heptadiene-2-one | Sweet, coconut creamy | 3.0 | 3.0 | 3.0 | 2.3 | MS, RI, O, S |
| 4 | γ-Nonanolactone | Coconut creamy, sweet, floral | 2.0 | 2.5 | 2.5 | 2.5 | MS, RI, O, S |
| 5 | Coumarin | Milk-like, sweet, floral | - | 2.8 | - | 3.0 | MS, RI, O, S |
| 6 | 2,2,6-Trimethyl-cyclohexanone | Sweet, cooked rice | - | - | - | 2.8 | MS, RI, O, S |
| Class E (herbal) | |||||||
| 1 | 3,5-Octadien-2-one | Mushroom-like | 3.0 | 2.8 | 3.0 | 2.8 | MS, RI, O, S |
| 2 | 3-Octen-2-one | Herbal, fruity | 2.8 | 2.8 | 3.0 | 2.8 | MS, RI, O |
| 3 | Safranal | Herbal, metallic | 2.5 | 2.0 | 2.3 | 1.7 | MS, RI, O |
| 4 | Dihydroactinidiolide * | Herbal, essential oil | 2.0 | 2.0 | 2.0 | 3.0 | MS, RI, O, S |
| Class F (others) | |||||||
| 1 | Heptanal | Fatty, green, herbal | 2.8 | 2.0 | 2.6 | 2.8 | MS, RI, O, S |
| 2 | Naphthalene * | Pungent, earthy | 2.7 | 3.0 | 3.3 | 3.0 | MS, RI, O |
| 3 | Dimethyl trisulfide | Sulfurous, pickled vegetables | 2.3 | 2.0 | 2.3 | 2.2 | MS, RI, O, S |
| 4 | (E,E)-2,4-Heptadienal | Fatty, slight pungent, green | 2.0 | 3.3 | 3.7 | 2.3 | MS, RI, O, S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Kang, S.; Yan, H.; Xie, D.; Chen, Q.; Lv, H.; Lin, Z.; Zhu, Y. Insights into Characteristic Volatiles in Wuyi Rock Teas with Different Cultivars by Chemometrics and Gas Chromatography Olfactometry/Mass Spectrometry. Foods 2022, 11, 4109. https://doi.org/10.3390/foods11244109
Zhang Y, Kang S, Yan H, Xie D, Chen Q, Lv H, Lin Z, Zhu Y. Insights into Characteristic Volatiles in Wuyi Rock Teas with Different Cultivars by Chemometrics and Gas Chromatography Olfactometry/Mass Spectrometry. Foods. 2022; 11(24):4109. https://doi.org/10.3390/foods11244109
Chicago/Turabian StyleZhang, Yue, Suyoung Kang, Han Yan, Dongchao Xie, Qincao Chen, Haipeng Lv, Zhi Lin, and Yin Zhu. 2022. "Insights into Characteristic Volatiles in Wuyi Rock Teas with Different Cultivars by Chemometrics and Gas Chromatography Olfactometry/Mass Spectrometry" Foods 11, no. 24: 4109. https://doi.org/10.3390/foods11244109





