Prevalence and Virulence Determinants of Staphylococcus aureus in Wholesale and Retail Pork in Wuhan, Central China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Identification from Raw Pork
2.2. Antimicrobial Resistance Testing
2.3. Detection of Methicillin Resistance Genes
2.4. Multilocus Sequence Typing (MLST)
2.5. Whole-Genome Sequencing and Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Prevalence of S. aureus and MRSA in Wholesale and Retail Pork
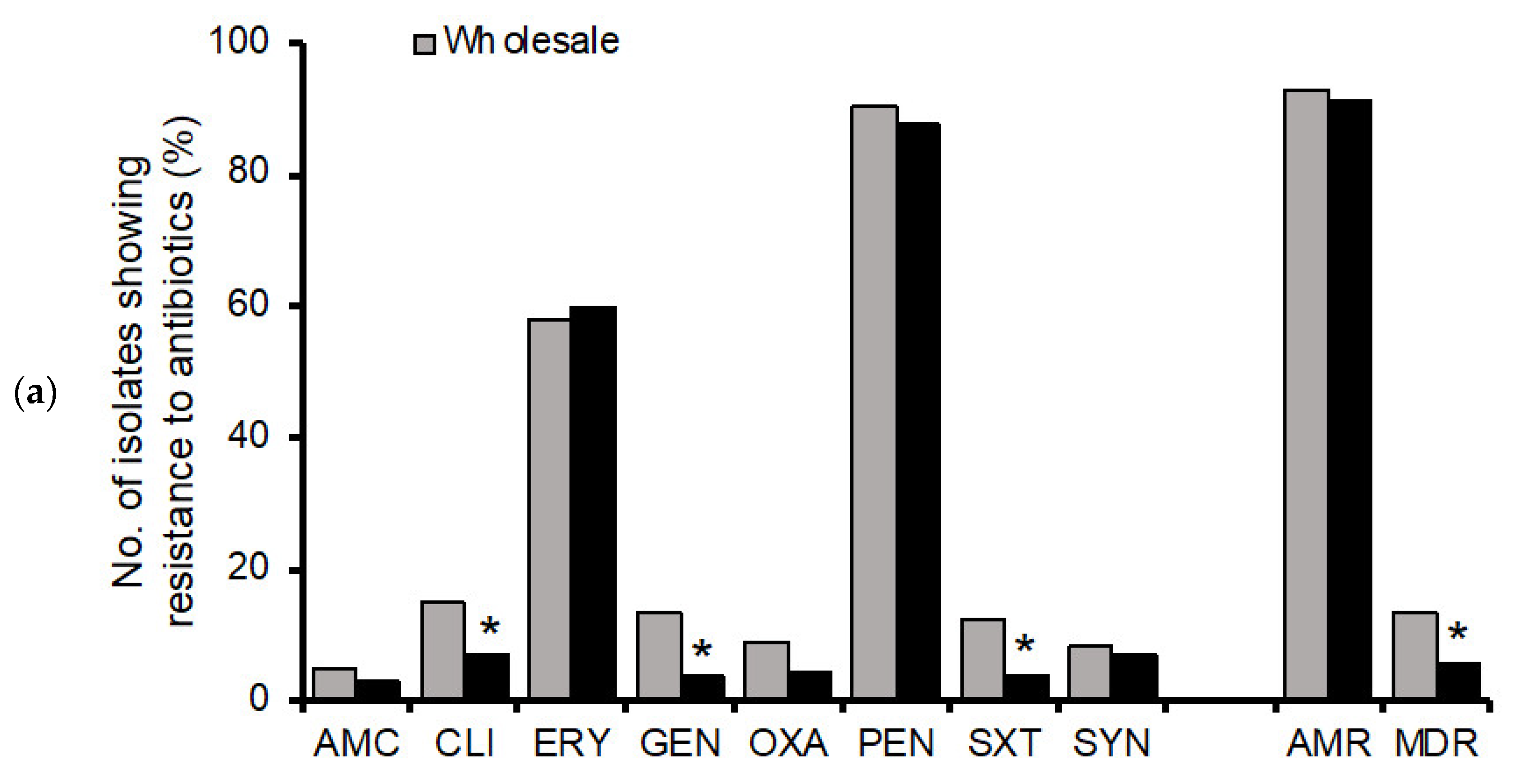
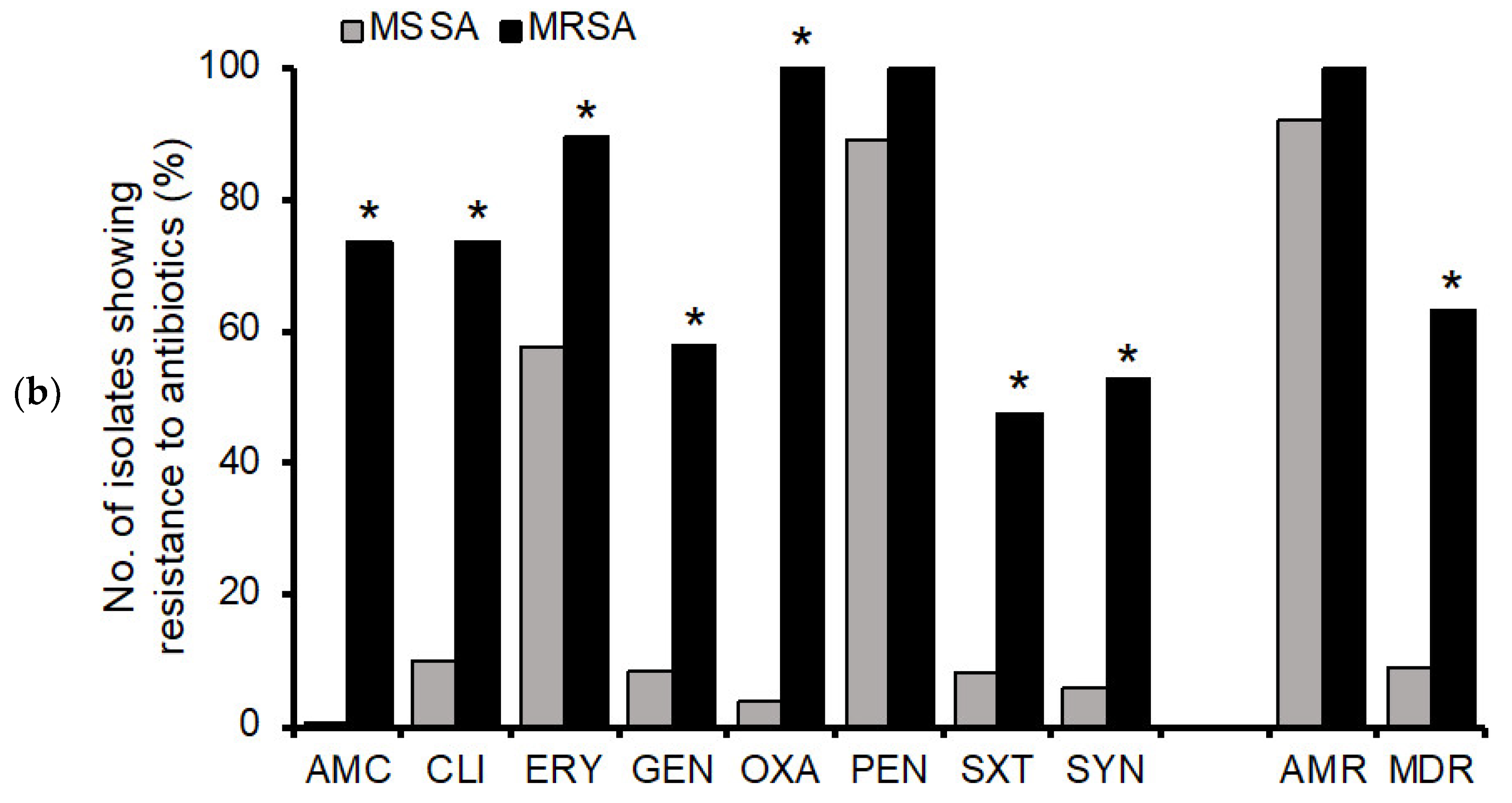
3.2. Antimicrobial Resistance Profiles
3.3. Multilocus Sequence Typing (MLST) of the S. aureus Isolates
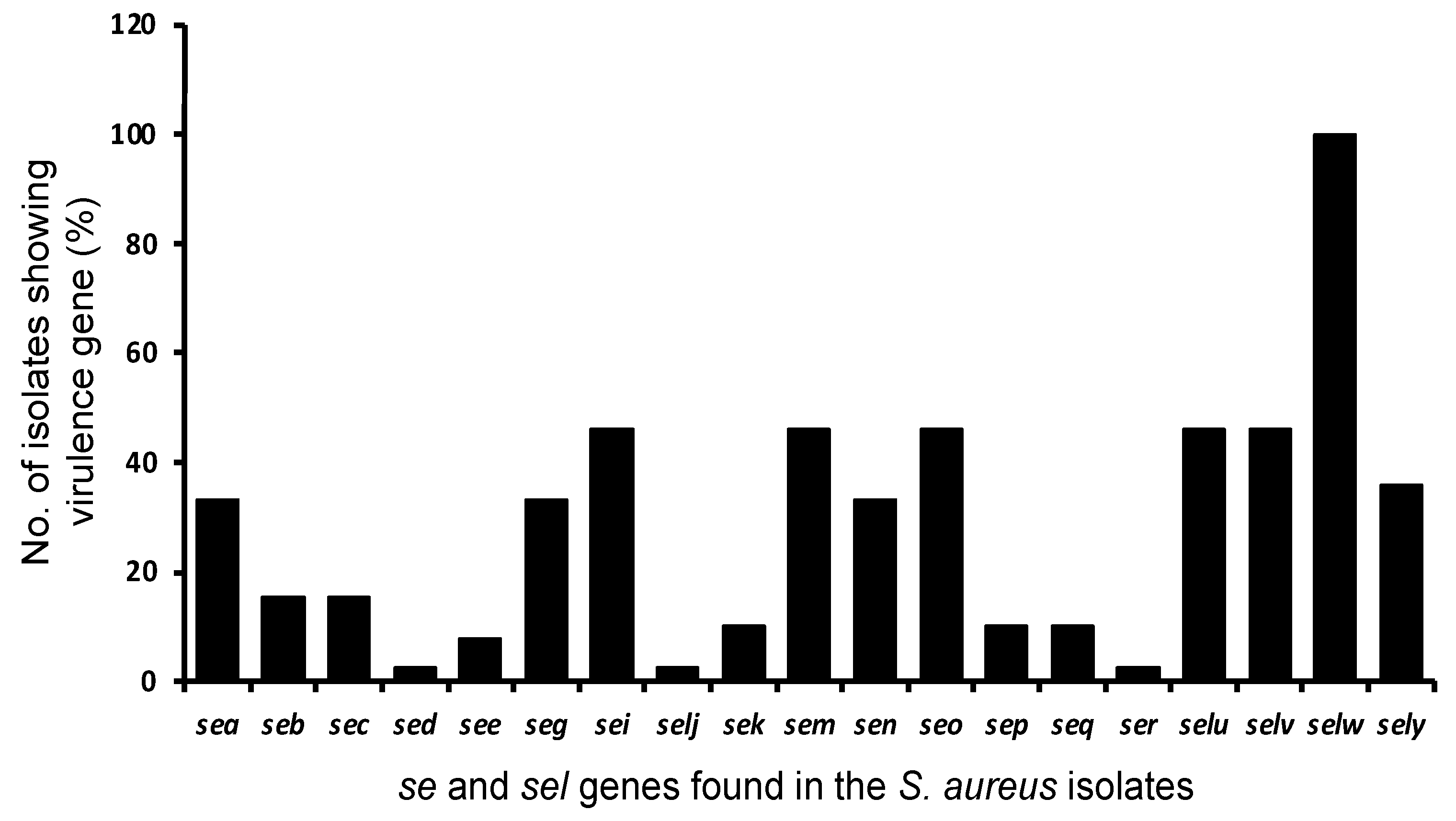
3.4. Genotypic Characteristics of the S. aureus Isolates
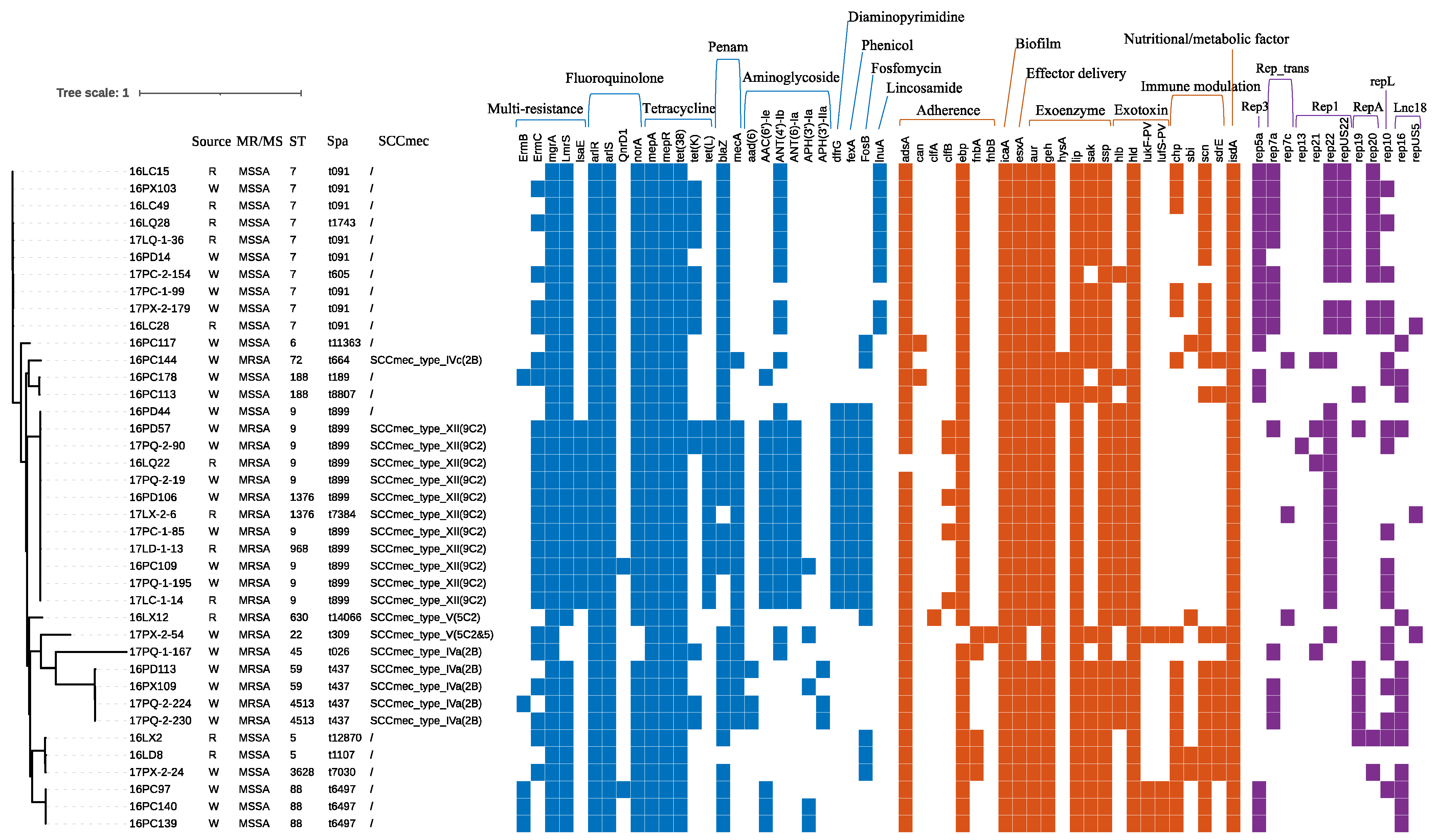
3.5. Whole-Genome Sequencing Analysis of S. aureus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollitt, E.J.G.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus infection dynamics. PLoS Pathog. 2018, 14, e1007112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittl, S.; Brodard, I.; Heim, D.; Andina-Pfister, P.; Overesch, G. Methicillin-resistant Staphylococcus aureus strains in swiss pigs and their relation to isolates from farmers and veterinarians. Appl. Environ. Microbiol. 2020, 86, e01865-19. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.L.; Li, S.; Fang, R.; Ono, H.K. Update on molecular diversity and multipathogenicity of staphylococcal superantigen toxins. Anim. Dis. 2021, 1, 69–83. [Google Scholar] [CrossRef]
- Zeleny, R.; Emteborg, H.; Charoud-Got, J.; Schimmel, H.; Nia, Y.; Mutel, I.; Ostyn, A.; Herbin, S.; Hennekinne, J.A. Development of a reference material for Staphylococcus aureus enterotoxin A in cheese: Feasibility study, processing, homogeneity and stability assessment. Food Chem. 2015, 168, 241–246. [Google Scholar] [CrossRef]
- Hu, D.L.; Nakane, A. Mechanisms of staphylococcal enterotoxin-induced emesis. Eur. J. Pharmacol. 2014, 722, 95–107. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ono, H.K.; Shimojima, Y.; Kubota, H.; Kato, R.; Kakuda, T.; Hirose, S.; Hu, D.L.; Nakane, A.; Takai, S.; et al. A novel staphylococcal enterotoxin SE02 involved in a staphylococcal food poisoning outbreak that occurred in Tokyo in 2004. Food Microbiol. 2020, 92, 103588. [Google Scholar] [CrossRef]
- Umeda, K.; Ono, H.K.; Wada, T.; Motooka, D.; Nakamura, S.; Nakamura, H.; Hu, D.L. High production of egc2-related staphylococcal enterotoxins caused a food poisoning outbreak. Int. J. Food Microbiol. 2021, 357, 109366. [Google Scholar] [CrossRef]
- Ono, H.K.; Hirose, S.; Naito, I.; Sato’o, Y.; Asano, K.; Hu, D.L.; Omoe, K.; Nakane, A. The emetic activity of staphylococcal enterotoxins, SEK, SEL, SEM, SEN and SEO in a small emetic animal model, the house musk shrew. Microbiol. Immunol. 2017, 61, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Sato’o, Y.; Omoe, K.; Naito, I.; Ono, H.K.; Nakane, A.; Sugai, M.; Yamagishi, N.; Hu, D.L. Molecular epidemiology and identification of a Staphylococcus aureus clone causing food poisoning outbreaks in Japan. J. Clin. Microbiol. 2014, 52, 2637–2640. [Google Scholar] [CrossRef]
- Hu, D.L.; Omoe, K.; Inoue, F.; Kasai, T.; Yasujima, M.; Shinagawa, K.; Nakane, A. Comparative prevalence of superantigenic toxin genes in meticillin-resistant and meticillin-susceptible Staphylococcus aureus isolates. J. Med. Microbiol. 2008, 57, 1106–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlemann, A.C.; Porcella, S.F.; Trivedi, S.; Sullivan, S.B.; Hafer, C.; Kennedy, A.D.; Barbian, K.D.; McCarthy, A.J.; Street, C.; Hirschberg, D.L.; et al. Identification of a highly transmissible animal-independent Staphylococcus aureus ST398 clone with distinct genomic and cell adhesion properties. mBio 2012, 3, e00027-12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.L.; Maina, E.K.; Omoe, K.; Inoue, F.; Yasujima, M.; Nakane, A. Superantigenic toxin genes coexist with specific staphylococcal cassette chromosome mec genes in methicillin-resistant Staphylococcus aureus. Tohoku J. Exp. Med. 2011, 225, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Jiang, N.; Ke, Y.; Fessler, A.T.; Wang, Y.; Schwarz, S.; Wu, C. Characterization of pig-associated methicillin-resistant Staphylococcus aureus. Vet. Microbiol. 2017, 201, 183–187. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, C.J. Detection and phylogeny of Staphylococcus aureus sequence type 398 in Taiwan. J. Biomed. Sci. 2020, 27, 15. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wu, C.; Wang, X.; Meng, J. Prevalence and characterization of methicillin susceptible Staphylococcus aureus ST398 isolates from retail foods. Int. J. Food Microbiol. 2015, 196, 94–97. [Google Scholar] [CrossRef]
- Aires-de-Sousa, M. Methicillin-resistant Staphylococcus aureus among animals: Current overview. Clin. Microbiol. Infect. 2017, 23, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Chuang, Y.Y.; Huang, Y.C. Livestock-associated meticillin-resistant Staphylococcus aureus in Asia: An emerging issue? Int. J. Antimicrob. Agents 2015, 45, 334–340. [Google Scholar] [CrossRef]
- David, M.Z.; Siegel, J.; Lowy, F.D.; Zychowski, D.; Taylor, A.; Lee, C.J.; Boyle-Vavra, S.; Daum, R.S. Asymptomatic carriage of sequence type 398, spa type t571 methicillin-susceptible Staphylococcus aureus in an urban jail: A newly emerging, transmissible pathogenic strain. J. Clin. Microbiol. 2013, 51, 2443–2447. [Google Scholar] [CrossRef]
- Waters, A.E.; Contente-Cuomo, T.; Buchhagen, J.; Liu, C.M.; Watson, L.; Pearce, K.; Foster, J.T.; Bowers, J.; Driebe, E.M.; Engelthaler, D.M.; et al. Multidrug-resistant Staphylococcus aureus in US meat and poultry. Clin. Infect. Dis. 2011, 52, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Larsen, J.; Kjeldgaard, J.; Andersen, P.S.; Skov, R.; Ingmer, H. Methicillin-resistant and -susceptible Staphylococcus aureus from retail meat in Denmark. Int. J. Food Microbiol. 2017, 249, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Groves, M.D.; O’Sullivan, M.V.; Brouwers, H.J.; Chapman, T.A.; Abraham, S.; Trott, D.J.; Al Jassim, R.; Coombs, G.W.; Skov, R.L.; Jordan, D. Staphylococcus aureus ST398 detected in pigs in Australia. J. Antimicrob. Chemother. 2014, 69, 1426–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, D.A.; Bakker, S.; Coombs, G.W.; Tan, H.; Monecke, S.; Heffernan, H. Emergence and molecular characterization of clonal complex 398 (CC398) methicillin-resistant Staphylococcus aureus (MRSA) in New Zealand. J. Antimicrob. Chemother. 2014, 69, 1428–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neela, V.; Mohd Zafrul, A.; Mariana, N.S.; van Belkum, A.; Liew, Y.K.; Rad, E.G. Prevalence of ST9 methicillin-resistant Staphylococcus aureus among pigs and pig handlers in Malaysia. J. Clin. Microbiol. 2009, 47, 4138–4140. [Google Scholar] [CrossRef] [Green Version]
- Larsen, J.; Imanishi, M.; Hinjoy, S.; Tharavichitkul, P.; Duangsong, K.; Davis, M.F.; Nelson, K.E.; Larsen, A.R.; Skov, R.L. Methicillin-resistant Staphylococcus aureus ST9 in pigs in Thailand. PLoS ONE 2012, 7, e31245. [Google Scholar] [CrossRef] [Green Version]
- Kadlec, K.; Schwarz, S. Identification of the novel dfrK-carrying transposon Tn559 in a porcine methicillin-susceptible Staphylococcus aureus ST398 strain. Antimicrob. Agents Chemother. 2010, 54, 3475–3477. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Yu, X.; Tao, X.; Zhang, J.; Zhang, B.; Dong, R.; Xue, C.; Grundmann, H.; Zhang, J. Staphylococcus aureus ST398 from slaughter pigs in northeast China. Int. J. Med. Microbiol. 2014, 304, 379–383. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, M.; Zhao, C.; Zhang, Q.; Li, L.; Zhang, Y.; Luo, Y.; Liu, Y. Whole-genome epidemiology and characterization of methicillin-susceptible Staphylococcus aureus ST398 from retail pork and bulk tank milk in Shandong, China. Front. Microbiol. 2021, 12, 764105. [Google Scholar] [CrossRef]
- Mediavilla, J.R.; Chen, L.; Uhlemann, A.C.; Hanson, B.M.; Rosenthal, M.; Stanak, K.; Koll, B.; Fries, B.C.; Armellino, D.; Schilling, M.E.; et al. Methicillin-susceptible Staphylococcus aureus ST398, New York and New Jersey, USA. Emerg. Infect. Dis. 2012, 18, 700–702. [Google Scholar] [CrossRef]
- Zhou, L.J.; Ying, G.G.; Liu, S.; Zhang, R.Q.; Lai, H.J.; Chen, Z.F.; Pan, C.G. Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. Sci. Total Environ. 2013, 444, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, X.; Yan, H. Genotypic characteristics and correlation of epidemiology of Staphylococcus aureus in healthy pigs, diseased pigs, and environment. Antibiotics 2020, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Taneike, I.; Mimura, D.; Iwakura, N.; Nakayama, T.; Emura, T.; Kitatsuji, M.; Fujimoto, A.; Yamamoto, T. Gene sequences and specific detection for Panton-Valentine leukocidin. Biochem. Biophys. Res. Commun. 2005, 328, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Paterson, G.K.; Larsen, A.R.; Robb, A.; Edwards, G.E.; Pennycott, T.W.; Foster, G.; Mot, D.; Hermans, K.; Baert, K.; Peacock, S.J.; et al. The newly described mecA homologue, mecALGA251, is present in methicillin-resistant Staphylococcus aureus isolates from a diverse range of host species. J. Antimicrob. Chemother. 2012, 67, 2809–2813. [Google Scholar] [CrossRef]
- Paterson, G.K.; Harrison, E.M.; Holmes, M.A. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014, 22, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Kondo, Y.; Ito, T.; Ma, X.X.; Watanabe, S.; Kreiswirth, B.N.; Etienne, J.; Hiramatsu, K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: Rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 2007, 51, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Chairat, S.; Gharsa, H.; Lozano, C.; Gomez-Sanz, E.; Gomez, P.; Zarazaga, M.; Boudabous, A.; Torres, C.; Ben Slama, K. Characterization of Staphylococcus aureus from raw meat samples in Tunisia: Detection of clonal lineage ST398 from the african continent. Foodborne Pathog. Dis. 2015, 12, 686–692. [Google Scholar] [CrossRef]
- Ronco, T.; Klaas, I.C.; Stegger, M.; Svennesen, L.; Astrup, L.B.; Farre, M.; Pedersen, K. Genomic investigation of Staphylococcus aureus isolates from bulk tank milk and dairy cows with clinical mastitis. Vet. Microbiol. 2018, 215, 35–42. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.D. Accurate confidence intervals for binomial proportion and Poisson rate estimation. Comput. Biol. Med. 2003, 33, 509–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Bai, Y.; Xu, J.; Carter, M.Q.; Shi, C.; Shi, X. Genetic diversity and virulence potential of Staphylococcus aureus isolates from raw and processed food commodities in Shanghai. Int. J. Food Microbiol. 2015, 195, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buyukcangaz, E.; Velasco, V.; Sherwood, J.S.; Stepan, R.M.; Koslofsky, R.J.; Logue, C.M. Molecular typing of Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) isolated from animals and retail meat in North Dakota, United States. Foodborne Pathog. Dis. 2013, 10, 608–617. [Google Scholar] [CrossRef]
- Ivbule, M.; Miklasevics, E.; Cupane, L.; Berzina, L.; Balins, A.; Valdovska, A. Presence of methicillin-resistant Staphylococcus aureus in slaughterhouse environment, pigs, carcasses, and workers. J. Vet. Res. 2017, 61, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Hanson, B.M.; Dressler, A.E.; Harper, A.L.; Scheibel, R.P.; Wardyn, S.E.; Roberts, L.K.; Kroeger, J.S.; Smith, T.C. Prevalence of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) on retail meat in Iowa. J. Infect. Public Health 2011, 4, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Lassok, B.; Tenhagen, B.A. From pig to pork: Methicillin-resistant Staphylococcus aureus in the pork production chain. J. Food Prot. 2013, 76, 1095–1108. [Google Scholar] [CrossRef]
- Ge, B.; Mukherjee, S.; Hsu, C.H.; Davis, J.A.; Tran, T.T.T.; Yang, Q.; Abbott, J.W.; Ayers, S.L.; Young, S.R.; Crarey, E.T.; et al. MRSA and multidrug-resistant Staphylococcus aureus in U.S. retail meats, 2010–2011. Food Microbiol. 2017, 62, 289–297. [Google Scholar] [CrossRef]
- Jackson, C.R.; Davis, J.A.; Barrett, J.B. Prevalence and characterization of methicillin-resistant Staphylococcus aureus isolates from retail meat and humans in Georgia. J. Clin. Microbiol. 2013, 51, 1199–1207. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, K.; Wang, X.; Donabedian, S.; Zervos, M.; de Rocha, L.; Zhang, Y. Methicillin-resistant Staphylococcus aureus in retail meat, Detroit, Michigan, USA. Emerg. Infect. Dis. 2011, 17, 1135–1137. [Google Scholar] [CrossRef]
- Tanomsridachchai, W.; Changkaew, K.; Changkwanyeun, R.; Prapasawat, W.; Intarapuk, A.; Fukushima, Y.; Yamasamit, N.; Flav Kapalamula, T.; Nakajima, C.; Suthienkul, O.; et al. Antimicrobial resistance and molecular characterization of methicillin-resistant Staphylococcus aureus isolated from slaughtered pigs and pork in the central region of Thailand. Antibiotics 2021, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, M.; Sreevatsan, S.; Davies, P.R. Prevalence and characterization of Staphylococcus aureus in growing pigs in the USA. PLoS ONE 2015, 10, e0143670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrieling, M.; Tuffs, S.W.; Yebra, G.; van Smoorenburg, M.Y.; Alves, J.; Pickering, A.C.; Park, J.Y.; Park, N.; Heinrichs, D.E.; Benedictus, L.; et al. Population analysis of Staphylococcus aureus reveals a cryptic, highly prevalent superantigen SElW that contributes to the pathogenesis of bacteremia. mBio 2020, 11, e02082-20. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.L.; Ono, H.K.; Isayama, S.; Okada, R.; Okamura, M.; Lei, L.C.; Liu, Z.S.; Zhang, X.C.; Liu, M.Y.; Cui, J.C.; et al. Biological characteristics of staphylococcal enterotoxin Q and its potential risk for food poisoning. J. Appl. Microbiol. 2017, 122, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
| Pork Source (Markets) | Season | No. of Samples | S. aureus | MRSA | MSSA | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Isolates | Prevalence (%) | 95% CI * (%) | No. of Isolates | Prevalence (%) | No. of Isolates | Prevalence (%) | |||
| Wholesale | Spring | 576 | 85 | 14.8 β,γ | 12.0–17.9 | 3 | 3.5 | 82 | 96.5 |
| Summer | 594 | 71 | 12.0 γ | 9.5–14.8 | 2 | 2.8 | 69 | 97.2 | |
| Autumn | 661 | 106 | 16.0 β | 13.3–19.1 | 6 | 5.7 | 100 | 94.3 | |
| Winter | 522 | 94 | 18.0 α,β | 14.8–21.6 | 3 | 3.2 | 91 | 96.8 | |
| Total | 2353 | 356 | 15.1 | 13.7–16.6 | 14 | 3.9 | 342 | 96.1 | |
| Retail | Spring | 153 | 37 | 24.2 b | 17.6–31.8 | 1 | 2.7 | 36 | 97.3 |
| Summer | 194 | 31 | 16.0 b | 11.1–21.9 | 1 | 3.2 | 30 | 96.8 | |
| Autumn | 186 | 32 | 17.2 b | 12.1–23.4 | 2 | 6.3 | 30 | 93.8 | |
| Winter | 181 | 62 | 34.3 a | 27.4–41.7 | 1 | 1.6 | 61 | 98.4 | |
| Total | 714 | 162 | 22.7 | 19.7–25.9 | 5 | 3.1 | 157 | 96.9 | |
| Total | 3067 | 518 | 16.9 | 15.6–18.3 | 19 | 3.7 | 499 | 96.3 | |
| MLST | No. of S. aureus | No. from Markets | No. of MRSA | No. of MSSA | ||||
|---|---|---|---|---|---|---|---|---|
| Type | (n = 518, %) | Wholesale | Retail | (n = 19, %) | (n = 499, %) | |||
| ST7 | 298 | 57.5 | 200 | 98 | - | - | 298 | 59.7 |
| ST5 | 46 | 9.1 | 22 | 24 | - | - | 46 | 9.2 |
| ST3055 | 22 | 4.3 | 20 | 2 | - | - | 22 | 4.4 |
| ST188 | 19 | 3.7 | 15 | 4 | - | - | 19 | 3.8 |
| ST9 | 17 | 3.3 | 14 | 3 | 8 | 42.1 | 9 | 1.8 |
| ST15 | 14 | 2.7 | 11 | 3 | - | - | 14 | 2.8 |
| ST25 | 14 | 2.7 | 14 | - | - | - | 14 | 2.8 |
| ST1 | 10 | 1.9 | 6 | 4 | - | - | 10 | 2 |
| ST6 | 10 | 1.9 | 8 | 2 | - | - | 10 | 2 |
| ST88 | 10 | 1.9 | 10 | - | - | - | 10 | 2 |
| ST1640 | 10 | 1.9 | - | 10 | - | - | 10 | 2 |
| ST72 | 7 | 1.4 | 3 | 4 | 1 | 5.3 | 6 | 1.2 |
| ST2250 | 6 | 1.2 | 5 | 1 | - | - | 6 | 1.2 |
| ST630 | 5 | 1 | 1 | 4 | 1 | 5.3 | 4 | 0.8 |
| ST1281 | 5 | 1 | 5 | - | - | - | 5 | 1 |
| ST59 | 4 | 0.8 | 4 | - | 2 | 10.5 | 2 | 0.4 |
| ST398 | 4 | 0.8 | 4 | - | - | - | 4 | 0.8 |
| ST672 | 3 | 0.6 | 2 | 1 | - | - | 3 | 0.6 |
| ST338 | 2 | 0.4 | 2 | - | - | - | 2 | 0.4 |
| ST1376 | 2 | 0.4 | 1 | 1 | 2 | 10.5 | - | - |
| ST1920 | 2 | 0.4 | 2 | - | - | - | 2 | 0.4 |
| ST4513 | 2 | 0.4 | 2 | - | 2 | 10.5 | - | - |
| ST22 | 1 | 0.2 | 1 | - | 1 | 5.3 | - | - |
| ST45 | 1 | 0.2 | 1 | - | 1 | 5.3 | - | - |
| ST968 | 1 | 0.2 | 1 | - | 1 | 5.3 | - | - |
| ST1821 | 1 | 0.2 | - | 1 | - | - | 1 | 0.2 |
| ST1921 | 1 | 0.2 | 1 | - | - | - | 1 | 0.2 |
| ST2315 | 1 | 0.2 | 1 | - | - | - | 1 | 0.2 |
| S. aureus | MLST | Spa | SCCmec | SE or SEl Genes |
|---|---|---|---|---|
| MRSA | ST9 | t899 | XII(9C2) | sea, selw |
| t899 | XII(9C2) | sea, selw | ||
| t899 | XII(9C2) | seb, sec, seg, sei, sem, sen, seo, selu, selv, selw | ||
| t899 | XII(9C2) | seg, sei, sem, sen, seo, selu, selv, selw | ||
| t899 | XII(9C2) | sei, sem, seo, selu, selv, selw, sey | ||
| t899 | XII(9C2) | seb, sec, selw | ||
| t899 | XII(9C2) | sea, sec, sek, sep, seq, selw, sey | ||
| t899 | XII(9C2) | sea, selw | ||
| ST22 | t309 | V(5C2&5) | seg, sei, sem, sen, seo, selu, selv, selw, sey | |
| ST45 | t026 | IVa(2B) | sea, selw | |
| ST59 | t437 | IVa(2B) | sei, sem, seo, selu, selv, selw | |
| t437 | IVa(2B) | sea, selw | ||
| ST72 | t664 | IVc(2B) | sei, sem, seo, selu, selv, selw, sey | |
| ST630 | t14066 | V(5C2) | seb, sec, sek, sep, seq, selw, sey | |
| ST968 | t899 | XII(9C2) | seg, sei, sem, sen, seo, selu, selv, selw, sey | |
| ST1376 | t899 | XII(9C2) | sea, selw | |
| t7384 | XII(9C2) | selw | ||
| ST4513 | t437 | IVa(2B) | sea, selw | |
| t437 | IVa(2B) | sea, selw | ||
| MSSA | ST5 | t12870 | - | seg, sei, sem, sen, seo, selu, selv, selw |
| t1107 | - | sea, see, sek, sep, seq, selw | ||
| ST6 | t11363 | - | sei, sem, seo, selu, selv, selw | |
| ST7 | t065 | - | seg, sei, sem, sen, seo, selu, selv, selw | |
| t091 | - | sea, selw | ||
| t091 | - | seg, sei, sem, sen, seo, selu, selv, selw, sly | ||
| t091 | - | sea, see, selw | ||
| t091 | - | sea, seb, sec, sed, see, seg, sei, selj, sem, sen, seo, ser, selu, selv, selw | ||
| t091 | - | sei, sem, seo, selu, selv, selw, sey | ||
| t091 | - | seg, sei, sem, sen, seo, selu, selv, selw | ||
| t091 | - | selw | ||
| t091 | - | selw | ||
| t1743 | - | seb, sec, sek, sep, seq, selw, sey | ||
| ST9 | t899 | - | sea, selw | |
| ST88 | t6497 | - | selw | |
| t6497 | - | seg, sei, sem, sen, seo, selu, selv, selw, sey | ||
| t6497 | - | seg, sei, sem, sen, seo, selu, selv, selw, sey | ||
| ST188 | t189 | - | seg, sei, sem, sen, seo, selu, selv, selw, sey | |
| t8807 | - | seg, sei, sem, sen, seo, selu, selv, selw, sey | ||
| ST3628 | t7030 | - | selw |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Liu, X.; Chen, X.; Zou, G.; Huang, Q.; Meng, X.; Pei, X.; Chen, Z.; Zhou, R.; Hu, D.; et al. Prevalence and Virulence Determinants of Staphylococcus aureus in Wholesale and Retail Pork in Wuhan, Central China. Foods 2022, 11, 4114. https://doi.org/10.3390/foods11244114
Zhu Z, Liu X, Chen X, Zou G, Huang Q, Meng X, Pei X, Chen Z, Zhou R, Hu D, et al. Prevalence and Virulence Determinants of Staphylococcus aureus in Wholesale and Retail Pork in Wuhan, Central China. Foods. 2022; 11(24):4114. https://doi.org/10.3390/foods11244114
Chicago/Turabian StyleZhu, Zhihao, Xiaoying Liu, Xingyu Chen, Geng Zou, Qi Huang, Xianrong Meng, Xiaoying Pei, Zhou Chen, Rui Zhou, Dongliang Hu, and et al. 2022. "Prevalence and Virulence Determinants of Staphylococcus aureus in Wholesale and Retail Pork in Wuhan, Central China" Foods 11, no. 24: 4114. https://doi.org/10.3390/foods11244114







