Dairy Products: A Potential Source of Multidrug-Resistant Enterococcus faecalis and Enterococcus faecium Strains
Abstract
:1. Introduction
2. Materials and Methods
3. Results
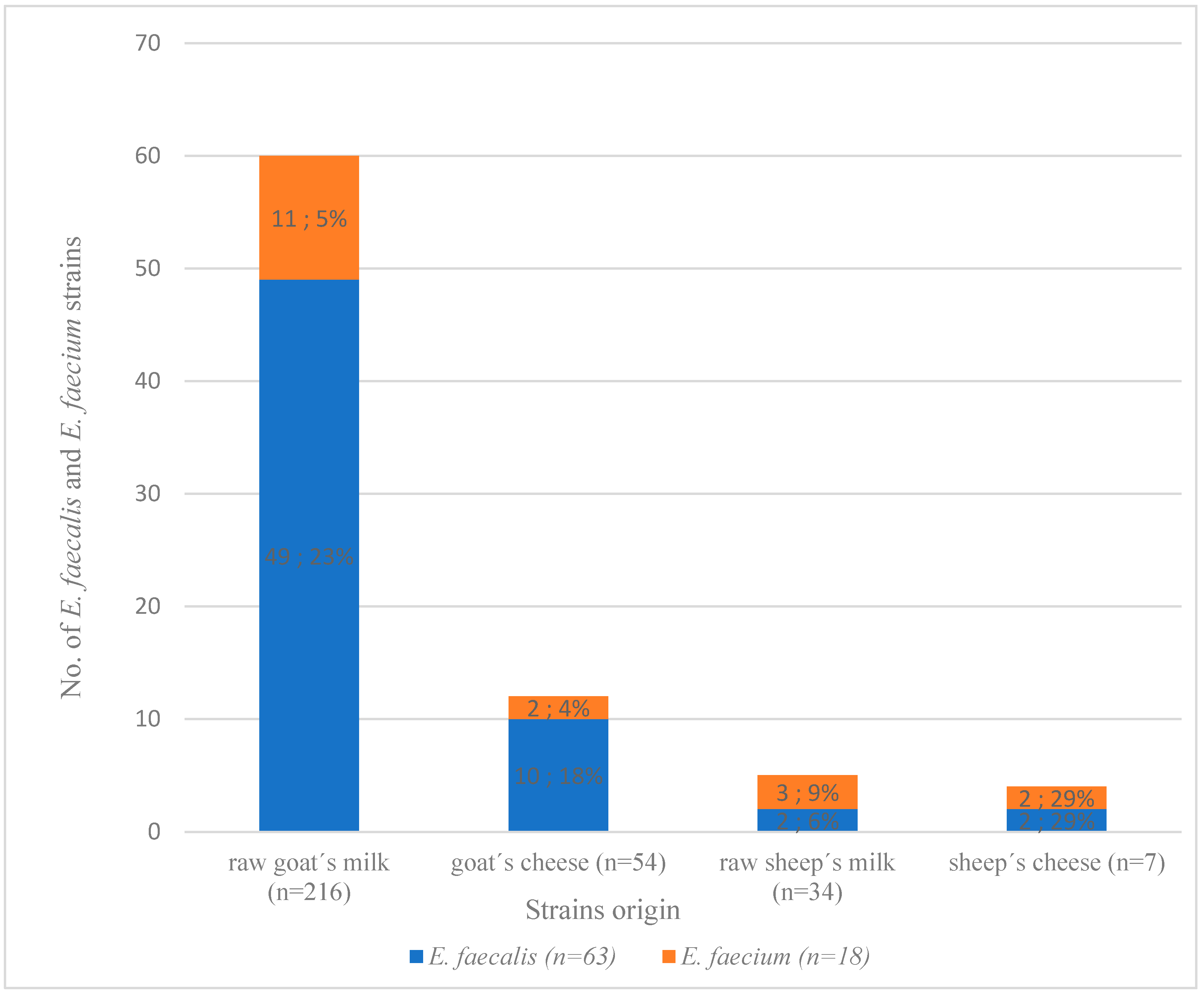
3.1. Identification of E. faecium and E. faecalis from Dairy Products
3.2. Antimicrobial Resistance of Enterococcus faecalis and Enterococcus faecium
3.3. Detection of Virulence and Resistance Genes among Enterococcus faecium and Enterococcus faecalis Isolates
3.4. Connection between Resistance Genes and Phenotypical Resistance to Antimicrobials
3.5. Connection between Virulence Genes and Multidrug-Resistant Enterococcus faecalis and Enterococcus faecium Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terzić-Vidojević, A.; Veljović, K.; Popović, N.; Tolinački, M.; Golić, N. Enterococci from raw-milk cheeses: Current knowledge on safety, technological, and probiotic concerns. Foods 2021, 10, 2753. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Zeyni, B.; Rastyani, S.; Jafari, R.; Shamloo, F.; Tabar, Z.; Arabestani, M.R. Presence of virulence factors and antibiotic resistances in Enterococcus spp. collected from dairy products and meat. Der Pharm. Lett. 2016, 8, 138–145. [Google Scholar]
- Giraffa, G. Functionality of enterococci in dairy products. Int. J. Food Microbiol. 2003, 88, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Výrostková, J.; Regecová, I.; Dudriková, E.; Marcinčák, S.; Vargová, M.; Kováčová, M.; Maľová, J. Antimicrobial Resistance of Enterococcus sp. isolated from Sheep and Goat Cheeses. Foods 2021, 10, 1844. [Google Scholar] [CrossRef] [PubMed]
- Foulquié Moreno, M.R.; Sarantinopoulos, P.; Tsakalidou, E.; De Vuyst, L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006, 106, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Serio, A.; Paparella, A.; Chavez-López, C.; Corsetti, A.; Suzzi, G. Enterococcus populations in pecorino abruzzese cheese: Biodiversity and safety aspects. J. Food Prot. 2007, 70, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Martín-Platero, A.M.; Valdivia, E.; Maqueda, M.; Martínez-Bueno, M. Characterization and safety evaluation of enterococci isolated from Spanish goats’ milk cheeses. Int. J. Food Microbiol. 2009, 132, 24–32. [Google Scholar] [CrossRef]
- Ruiz, P.; Pérez-Martín, F.; Seseña, S.; Palop, M.L. Seasonal diversity and safety evaluation of enterococci population from goat milk in a farm. Dairy Sci. Technol. 2016, 96, 359–375. [Google Scholar] [CrossRef] [Green Version]
- El-Zamkan, M.A.; Mohamed, H.M.A. Antimicrobial resistance, virulence genes and biofilm formation in Enterococcus species isolated from milk of sheep and goat with subclinical mastitis. PLoS ONE 2021, 16, e0259584. [Google Scholar] [CrossRef]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic Acid Bacteria in Raw-Milk Cheeses: From Starter Cultures to Probiotic Functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef]
- Mariam, S.H. A sampling survey of enterococci within pasteurized, fermented dairy products and their virulence and antibiotic resistance properties. PLoS ONE 2021, 16, e0254390. [Google Scholar] [CrossRef] [PubMed]
- Hammad, A.M.; Hassan, H.A.; Shimamoto, T. Prevalence, antibiotic resistance and virulence of Enterococcus spp. in Egyptian fresh raw milk cheese. Food Control 2015, 50, 815–820. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Łaniewska-Trokenheim, Ł. Virulence factors of Enterococcus spp. presented in food. LWT 2017, 75, 670–676. [Google Scholar] [CrossRef]
- Sava, I.G.; Heikens, E.; Huebner, J. Pathogenesis and immunity in enterococcal infections. Clin. Microbiol. Infect. 2010, 16, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Dutka-Malen, S.; Evers, S.; Courvalin, P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 1995, 33, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Agersø, Y.; Jensen, L.B.; Givskov, M.; Roberts, M.C. The identification of a tetracycline resistance gene tet(M), on a Tn916-like transposon, in the Bacillus cereus group. FEMS Microbiol. Lett. 2002, 214, 251–256. [Google Scholar] [CrossRef]
- de Vries, L.E.; Vallès, Y.; Agersø, Y.; Vaishampayan, P.A.; García-Montaner, A.; Kuehl, J.V.; Christensen, H.; Barlow, M.; Francinoet, P.M. The Gut as Reservoir of Antibiotic Resistance: Microbial Diversity of Tetracycline Resistance in Mother and Infant. PLoS ONE 2011, 6, e21644. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.B.; Frimodt-Moeller, N.; Aarestrup, F.M. Presence of erm gene classes in Gram-positive bacteria of animal and human origin in Denmark. FEMS Microbiol. Lett. 1999, 170, 151–158. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Jensen, L.B.; Aarestrup, F.M. Detection of the satA gene and transferability of virginiamycin resistance in Enterococcus faecium from food-animals. FEMS Microbiol. Lett. 1998, 168, 145–151. [Google Scholar] [CrossRef]
- Aarestrup, F.M. Glycopeptide susceptibility among Danish Enterococcus faecium and Enterococcus faecalis isolates of animal and human origin and PCR identification of genes within the vanA cluster. Antimicrob. Agents Chemother. 1996, 40, 1938–1940. [Google Scholar] [CrossRef] [PubMed]
- EURL-AR Primer List. Available online: https://www.eurl-ar.eu/CustomerData/Files/Folders/25-resourcer/459_primerliste-til-web-07-11-2018.pdf (accessed on 20 February 2020).
- Shafeek, M.; El-Malt, L.; Abdel Hameed, K.; El-Zamkan, M. Some virulence genes of pathogenic enterococci isolated from raw milk and some milk products. SVU-Int. J. Vet. Sci. 2018, 1, 102–113. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing: Breakpoint tables for interpretation of MICs and zone diameters, Version 11.0. EUCAST, Växjö. 2019. Available online: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/ (accessed on 22 October 2022).
- CLSI. M100 Performance Standars for Antimicrobial Susceptibility Testing, 29th ed.; Clinical and Laboratory Standards Institute: Pennsylvania, PA, USA, 2019. [Google Scholar]
- Yoon, S.; Lee, Y.J. Molecular characteristics of Escherichia coli from bulk tank milk in Korea. J. Vet. Sci. 2022, 23, e9. [Google Scholar] [CrossRef] [PubMed]
- Jahansepas, A.; Aghazadeh, M.; Rezaee, M.A.; Heidarzadeh, S.; Mardaneh, J.; Mohammadzadeh, A.; Pouresmaeil, O. Prevalence, antibiotic resistance and virulence of Enterococcus spp. isolated from traditional cheese types. Ethiop. J. Health Sci. 2022, 32, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, R.; Todaro, M.; Scatassa, M.L.; Franciosi, E.; Corona, O.; Mancuso, I.; Di Gerlando, R.; Cardamone, C.; Settanni, L. Transformation of raw ewes’ milk applying “Grana” type pressed cheese technology: Development of extra-hard “Gran Ovino” cheese. Int. J. Food Microbiol. 2019, 307, 108277. [Google Scholar] [CrossRef]
- Palmeri, M.; Mancuso, I.; Gaglio, R.; Arcuri, L.; Barreca, S.; Barbaccia, P.; Scatassa, M.L. Identification and evaluation of antimicrobial resistance of enterococci isolated from raw ewes’ and cows’ milk collected in western Sicily: A preliminary investigation. Ital. J. Food Saf. 2020, 9, 220–225. [Google Scholar] [CrossRef]
- Bulajić, S.; Tambur, Z.; Opačić, D.; Miljković-Selimović, B.; Doder, R.; Cenić-Milošević, D. Characterization of antibiotic resistance phenotypes and resistance genes in Enterococcus spp. isolated from cheeses. Arch. Biol. Sci. 2015, 67, 139–146. [Google Scholar] [CrossRef]
- Silvetti, T.; Morandi, S.; Brasca, M. Does Enterococcus faecalis from traditional raw milk cheeses serve as a reservoir of antibiotic resistance and pathogenic traits? Foodborne Pathog. Dis. 2019, 16, 359–367. [Google Scholar] [CrossRef]
- Gaglio, R.; Couto, N.; Marques, C.; de Fatima Silva Lopes, M.; Moschetti, G.; Pomba, C.; Settanni, L. Evaluation of antimicrobial resistance and virulence of enterococci from equipment surfaces, raw materials, and traditional cheeses. Int. J. Food Microbiol. 2016, 236, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, A.S.; Omar, N.B.; Abriouel, H.; López, R.L.; Ortega, E.; Cañamero, M.M.; Gálvez, A. Risk factors in enterococci isolated from foods in Morocco: Determination of antimicrobial resistance and incidence of virulence traits. Food Chem. Toxicol. 2008, 46, 2648–2652. [Google Scholar] [CrossRef]
- Gomes, B.C.; Esteves, C.T.; Palazzo, I.C.; Darini, A.L.; Felis, G.E.; Sechi, L.A.; Franco, B.D.; De Martinis, E.C. Prevalence and characterization of Enterococcus spp. isolated from Brazilian foods. Food Microbiol. 2008, 25, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Youn, H.Y.; Kang, H.J.; Moon, J.S.; Jang, Y.S.; Song, K.Y.; Seo, K.H. Prevalence and virulence characteristics of Enterococcus faecalis and Enterococcus faecium in bovine mastitis milk compared to bovine normal raw milk in south Korea. Animals 2022, 12, 1407. [Google Scholar] [CrossRef] [PubMed]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; García-Solache, M. Ready-to-eat dairy products as a source of multidrug-resistant Enterococcus strains: Phenotypic and genotypic characteristics. J. Dairy Sci. 2020, 103, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Różańska, H.; Lewtak-Piłat, A.; Kubajka, M.; Weiner, M. Occurrence of enterococci in mastitic cow’s milk and their antimicrobial resistance. J. Vet. Res. 2019, 63, 93–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, W.; Neubauer, H.; Tomaso, H.; El Hofy, F.I.; Monecke, S.; Abd El-Tawab, A.A.; Hotzel, H. Characterization of enterococci-and ESBL-producing Escherichia coli isolated from milk of bovides with mastitis in Egypt. Pathogens 2021, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Jamet, E.; Akary, E.; Poisson, M.A.; Chamba, J.F.; Bertrand, X.; Serror, P. Prevalence and characterization of antibiotic resistant Enterococcus faecalis in French cheeses. Food Microbiol. 2012, 31, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Erbas, G.; Parin, U.; Turkyilmaz, S.; Ucan, N.; Ozturk, M.; Kaya, O. Distribution of antibiotic resistance genes in Enterococcus spp. isolated from mastitis bovine milk. Acta Vet. Brno 2016, 66, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Cui, P.; Feng, L.; Zhang, L.; He, J.; An, T.; Fu, X.; Li, C.; Zhao, X.; Zhai, Y.; Li, H.; et al. Antimicrobial resistance, virulence genes, and biofilm formation capacity among Enterococcus species from yaks in Aba Tibetan Autonomous Prefecture, China. Front. Microbiol. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Shang, X.; Wang, X.; Yan, Z.; Li, H.; Li, J. Short communication: Antimicrobial resistance and virulence genes of Enterococcus faecalis isolated from subclinical bovine mastitis cases in China. J. Dairy Sci. 2019, 102, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, E.; Ladero, V.; Chico, I.; Maldonado-Barragán, A.; López, M.; Martín, V.; Fernández, L.; Fernández, M.; Álvarez, M.A.; Torres, C.; et al. Antibiotic resistance, virulence determinants and production of biogenic amines among Enterococci from ovine, feline, canine, porcine and human milk. BMC Microbiol. 2013, 13, 288. [Google Scholar] [CrossRef] [Green Version]
- İspirli, H.; Demirbaş, F.; Dertli, E. Characterization of functional properties of Enterococcus spp. isolated from Turkish white cheese. LWT 2017, 75, 358–365. [Google Scholar] [CrossRef]
- Tendolkar, P.M.; Baghdayan, A.S.; Gilmore, M.S.; Shankar, N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 2004, 72, 6032–6039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gołaś-Prądzyńska, M.; Rola, J.G. Occurrence and antimicrobial resistance of enterococci isolated from goat’s milk. J. Vet. Res. 2021, 65, 449–455. [Google Scholar] [CrossRef] [PubMed]
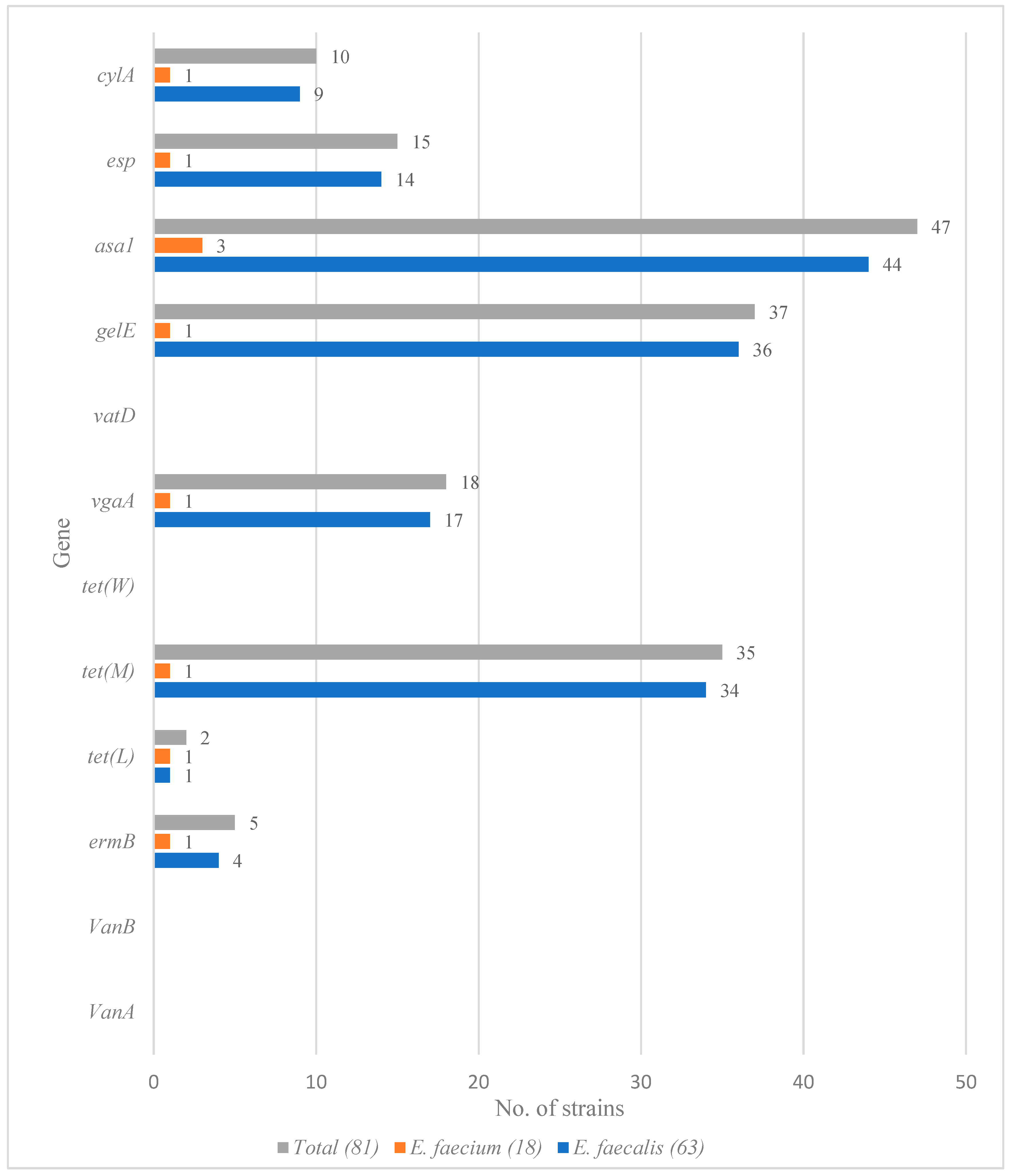
| Antimicrobial Substance | E. faecium | E. faecalis |
|---|---|---|
| MIC (μg/mL) R is > | MIC (μg/mL) R is > | |
| Ampicillin, AMP | 4 | 4 |
| Chloramphenicol, CHL | 32 | 32 |
| Ciprofloxacin, CIP | 4 | 4 |
| Daptomycin, DAP | 8 | 4 |
| Erythromycin, ERY | 4 | 4 |
| Gentamicin, GEN | 32 | 32 |
| Linezolid, LZD | 4 | 4 |
| Quinupristin/Dalfopristin (Synercid), SYN | 4 | Intrinsically resistant |
| Teicoplanin, TEI | 2 | 2 |
| Tetracycline, TET | 4 | 4 |
| Tigecycline, TGC | 0.25 | 0.5 |
| Vancomycin, VAN | 4 | 4 |
| Kanamycin, KAN | 1024 * | 1024 * |
| Lincomycin, LIN | 8 * | 8 * |
| Nitrofurantoin, NIT | 64 | 64 |
| Penicillin, PEN | 16 * | 16 * |
| Streptomycin, STR | 512 | 512 |
| Tylosin, TYL | 32 * | 32 * |
| Material | E. faecalis Isolates (n = 63) | E. faecium Isolates (n = 18) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw Goat’s Milk (49) | Goat’s Cheese (10) | Raw Sheep’s Milk (2) | Sheep’s Cheese (2) | Total (63) | Raw Goat’s Milk (11) | Goat’s Cheese (2) | Raw Sheep’s Milk (3) | Sheep’s Cheese (2) | Total (18) | |
| Antimicrobial substance | ||||||||||
| Ampicillin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chloramphenicol | 3 (6%) | 0 | 0 | 0 | 3 (5%) | 1 (9%) | 0 | 0 | 0 | 1 (5%) |
| Ciprofloxacin | 1 (2%) | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 0 | 0 | 0 |
| Daptomycin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Erythromycin | 5 (10%) | 1 (10%) | 0 | 0 | 6 (9%) | 5 (45%) | 1 (50%) | 0 | 0 | 6 (33%) |
| Gentamicin | 0 | 0 | 2 (100%) | 1 (50%) | 3 (5%) | 1 (9%) | 0 | 3 (100%) | 1 (50%) | 5 (28%) |
| Linezolid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quinupristin/Dalfopristin | 49 (100%) | 10 (100%) | 2 (100%) | 2 (100%) | 63 (100%) | 4 (36%) | 2 (100%) | 0 | 0 | 6 (33%) |
| Teicoplanin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tetracycline | 34 (69%) | 6 (60%) | 0 | 0 | 40 (63%) | 1 (9%) | 0 | 0 | 0 | 1 (5%) |
| Tigecycline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vancomycin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kanamycin | 8 (16%) | 1 (10%) | 0 | 0 | 9 (14%) | 2 (18%) | 0 | 1 (33%) | 0 | 3 (17%) |
| Lincomycin | 48 (98%) | 10 (100%) | 2 (100%) | 2 (100%) | 62 (98%) | 8 (73%) | 2 (100%) | 3 (100%) | 1 (50%) | 14 (78%) |
| Nitrofurantoin | 0 | 0 | 0 | 0 | 0 | 1 (9%) | 1 (50%) | 0 | 0 | 2 (11%) |
| Penicillin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Streptomycin | 9 (18%) | 1 (10%) | 0 | 0 | 10 (16%) | 1 (9%) | 0 | 0 | 0 | 1 (5%) |
| Tylosin | 6 (12%) | 1 (10%) | 0 | 0 | 7 (11%) | 1 (9%) | 1 (50%) | 0 | 0 | 2 (11%) |
| Virulence Genes | E. faecalis (63) | E. faecium (18) | Total (81) |
|---|---|---|---|
| gelE + asa1 | 15 (24%) | 0 | 15 (18%) |
| gelE + esp | 8 (13%) | 0 | 8 (10%) |
| asa1 + cylA | 5 (8%) | 0 | 5 (6%) |
| gelE + asa1 + esp | 2 (3%) | 0 | 2 (2%) |
| asa1 + esp + cylA | 1 (2%) | 0 | 1 (1%) |
| gelE + asa1 + cylA | 1 (2%) | 0 | 1 (1%) |
| gelE + asa1 + esp + cylA | 2 (3%) | 1 (5%) | 3 (4%) |
| Antimicrobial Substance | Profile | E. faecalis (n = 63) | E. faecium (n = 18) |
|---|---|---|---|
| Tetracycline | R | 40 | 1 |
| tet(M) | 33 | 0 | |
| tet(L) | 0 | 0 | |
| tet(M) + tet(L) | 1 | 1 | |
| Erythromycin | R | 6 | 6 |
| ermB | 2 | 1 | |
| vgaA | 1 | 0 | |
| ermB + vgaA | 1 | 0 | |
| Quinupristin/Dalfopristin | R | 63 | 6 |
| ermB | 3 | 1 | |
| vgaA | 16 | 0 | |
| ermB + vgaA | 1 | 0 |
| Number of Antimicrobial Substance Groups | MDR Strain Resistance Profile | Virulence Gene Profile | |
|---|---|---|---|
| E. faecalis (n = 63) | 3 | TET + LIN + STR (1 *) | asa1 |
| ERY + TET + LIN (2 *) | asa1 (2 *) | ||
| TET + KAN + LIN (1 *) | asa1 + esp + cylA | ||
| TET + LIN + STR + KAN (5 *) | gelE + esp (3 *) | ||
| gelE (1 *) | |||
| esp (1 *) | |||
| 4 | TET + LIN + STR + CIP (1 *) | gelE + asa1 + esp + cylA | |
| CHL + ERY + TET + LIN (1 *) | gelE + asa1 | ||
| ERY + TET + KAN + LIN + STR (1 *) | gelE + asa1 | ||
| 5 | CHL + ERY + TET + KAN + LIN + STR (2 *) | gelE + esp (2 *) | |
| E. faecium (n = 18) | 3 | ERY + LIN + NIT (1 *) | - |
| 6 | CHL + ERY + GEN + TET + KAN + LIN + STR (1 *) | gelE + asa1 + esp + cylA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołaś-Prądzyńska, M.; Łuszczyńska, M.; Rola, J.G. Dairy Products: A Potential Source of Multidrug-Resistant Enterococcus faecalis and Enterococcus faecium Strains. Foods 2022, 11, 4116. https://doi.org/10.3390/foods11244116
Gołaś-Prądzyńska M, Łuszczyńska M, Rola JG. Dairy Products: A Potential Source of Multidrug-Resistant Enterococcus faecalis and Enterococcus faecium Strains. Foods. 2022; 11(24):4116. https://doi.org/10.3390/foods11244116
Chicago/Turabian StyleGołaś-Prądzyńska, Marlena, Magdalena Łuszczyńska, and Jolanta Grażyna Rola. 2022. "Dairy Products: A Potential Source of Multidrug-Resistant Enterococcus faecalis and Enterococcus faecium Strains" Foods 11, no. 24: 4116. https://doi.org/10.3390/foods11244116
APA StyleGołaś-Prądzyńska, M., Łuszczyńska, M., & Rola, J. G. (2022). Dairy Products: A Potential Source of Multidrug-Resistant Enterococcus faecalis and Enterococcus faecium Strains. Foods, 11(24), 4116. https://doi.org/10.3390/foods11244116






