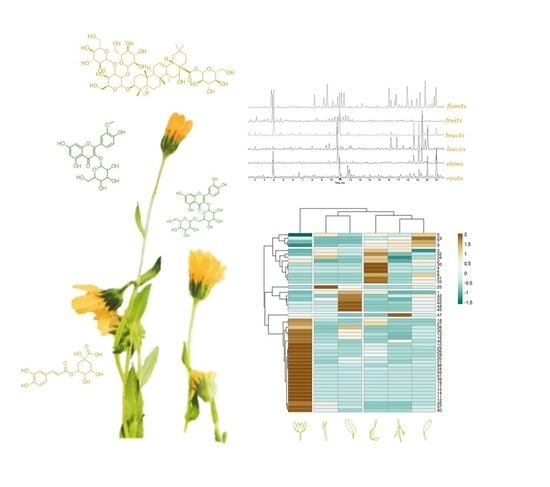
Calendula arvensis (Vaill.) L.: A Systematic Plant Analysis of the Polar Extracts from Its Organs by UHPLC-HRMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Collection, Organ Separation, and Extraction
2.2. UHPLC-ESI-QqTOF-MS and MS/MS Analyses
2.3. Antioxidant Assessment
2.4. Statistical Analysis
3. Results and Discussion
3.1. UHPLC-QqTOF-MS/MS Analysis of Calendula arvensis (Vaill.) L.
3.1.1. Hydroxycinnamic Acids Derivatives
3.1.2. Flavonoids
3.1.3. Triterpene Saponins
3.1.4. Guidelines for the Straightforward Identification of Triterpene Saponins by HR-MS/MS Tools
3.1.5. Other Compounds
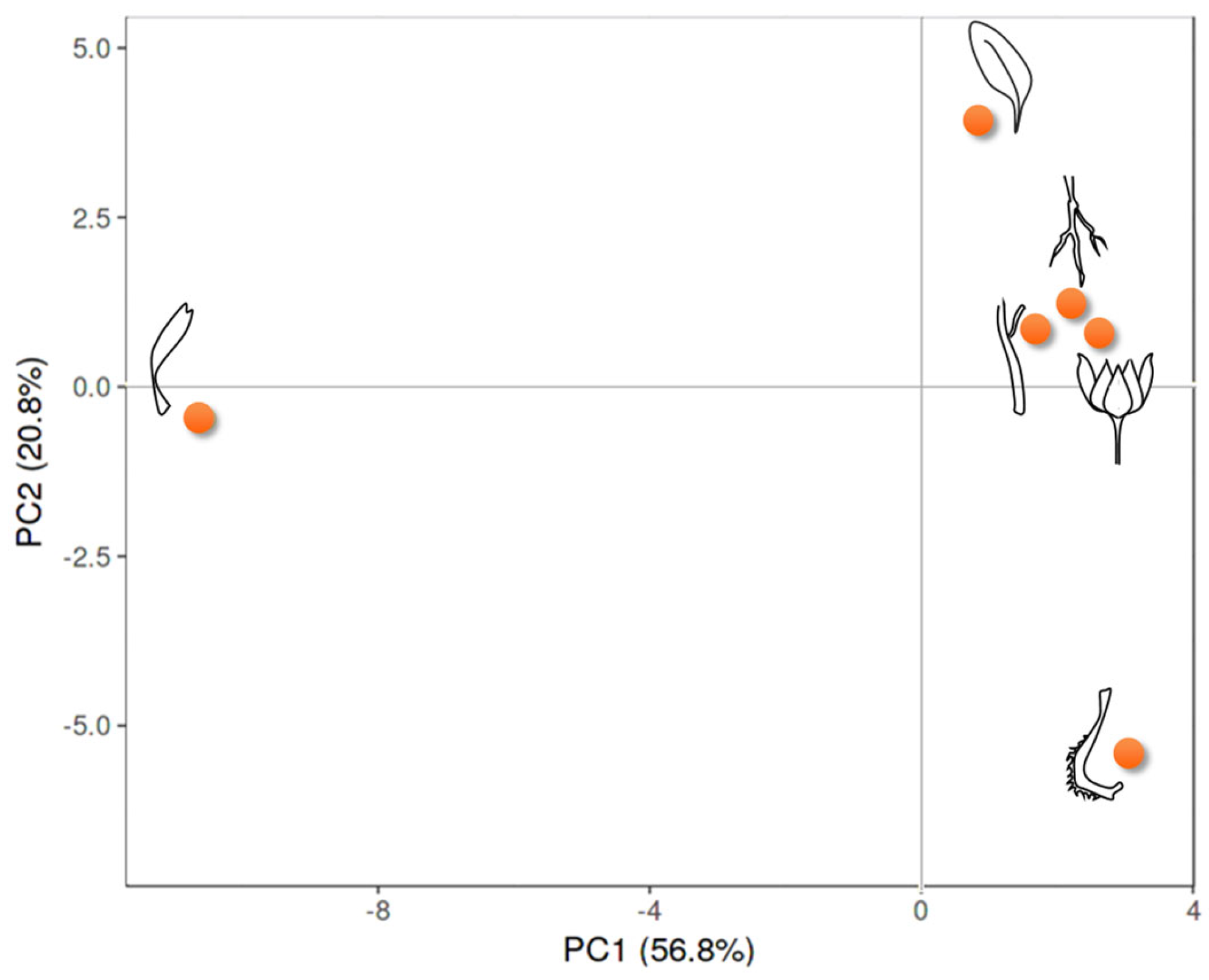
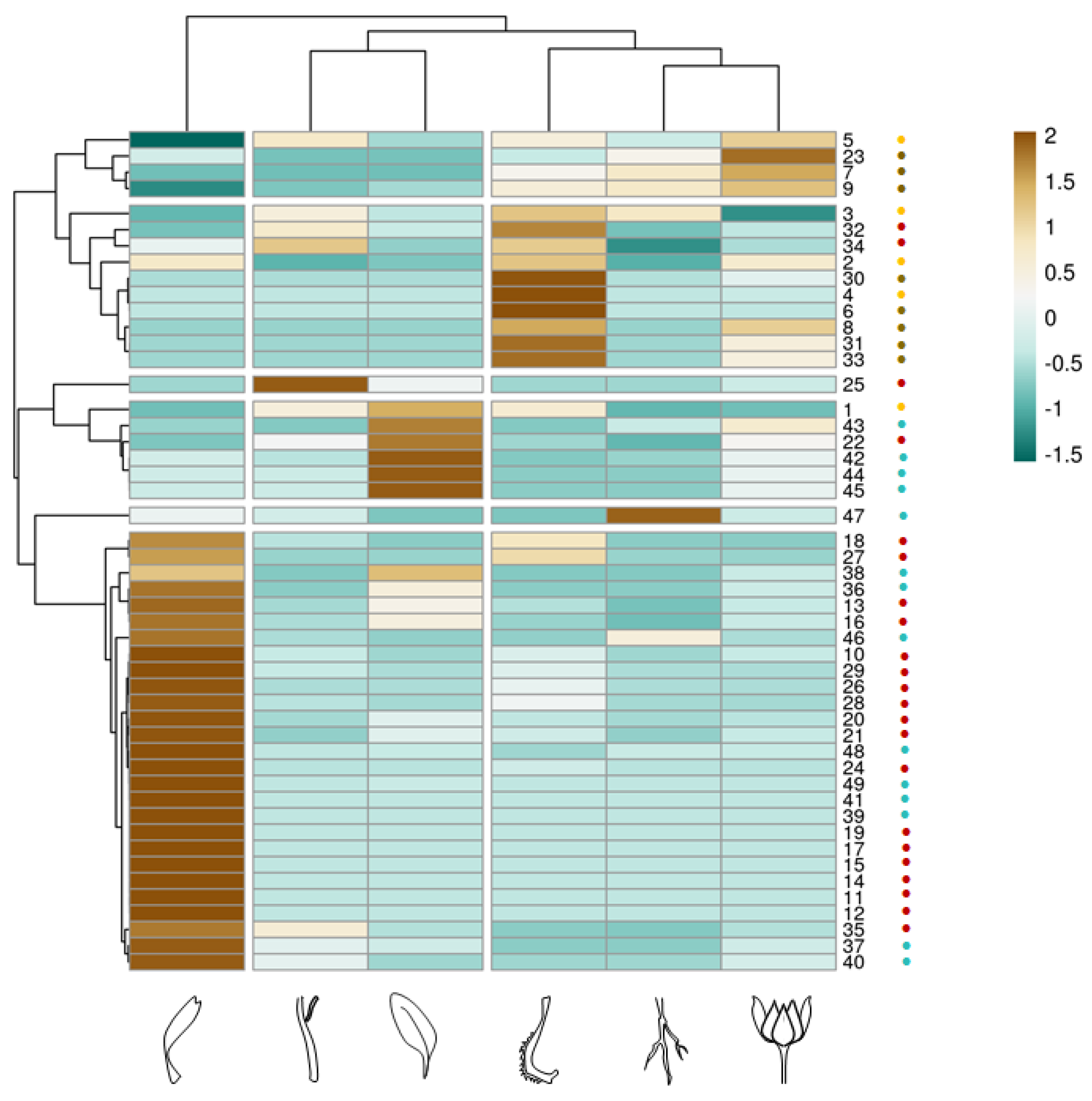
3.2. Multivariate Analysis
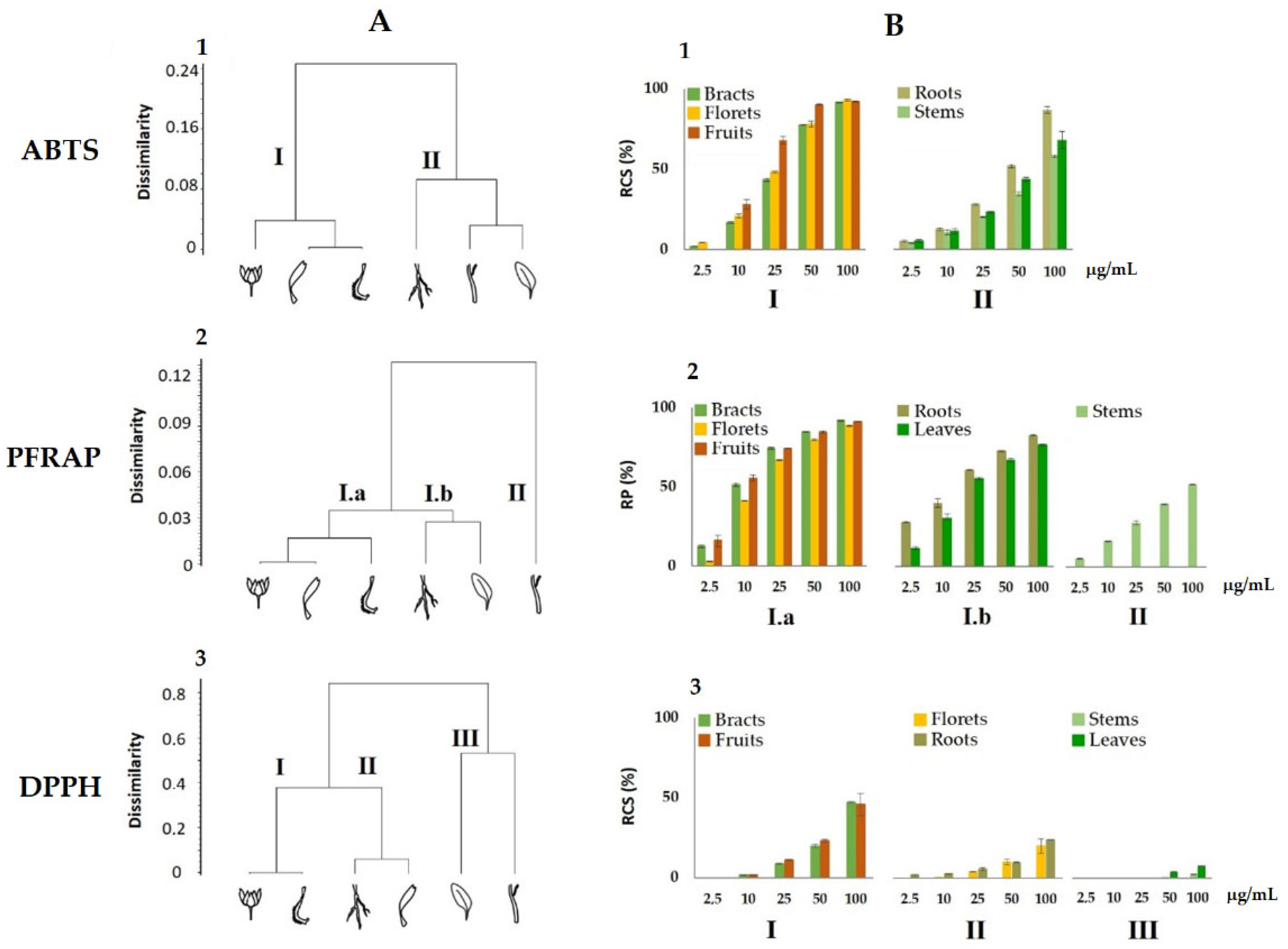
3.3. Antioxidant Activity of Calendula arvensis Alcoholic Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Łuczaj, Ł.; Pieroni, A. Nutritional Ethnobotany in Europe: From Emergency Foods to Healthy Folk Cuisines and Contemporary Foraging Trends. In Mediterranean Wild Edible Plants; Sánchez-Mata, M., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; pp. 33–56. [Google Scholar]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean Wild Edible Plants: Weeds or “New Functional Crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [Green Version]
- Rivera, D.; Obon, C.; Inocencio, C.; Heinrich, M.; Verde, A.; Fajardo, J.; Llorach, R. The Ethnobotanical Study of Local Mediterranean Food Plants as Medicinal Resources in Southern Spain. J. Physiol. Pharmacol. 2005, 56, 97–114. [Google Scholar]
- Guarrera, P.M.; Savo, V. Perceived Health Properties of Wild and Cultivated Food Plants in Local and Popular Traditions of Italy: A Review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Mattalia, G.; Quave, C.L.; Pieroni, A. Traditional Uses of Wild Food and Medicinal Plants among Brigasc, Kyé, and Provençal Communities on the Western Italian Alps. Genet. Resour. Crop. Evol. 2013, 60, 587–603. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and Potential Health Benefits of the Mediterranean Diet: Views from Experts around the World. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Motti, R.; Bonanomi, G.; Lanzotti, V.; Sacchi, R. The Contribution of Wild Edible Plants to the Mediterranean Diet: An Ethnobotanical Case Study Along the Coast of Campania (Southern Italy). Econ. Bot. 2020, 74, 249–272. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Cabrera Loera, R.D.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Díez Marqués, C.; Pardo-de-Santayana, M.; Tardío, J. Wild Vegetables of the Mediterranean Area as Valuable Sources of Bioactive Compounds. Genet. Resour. Crop. Evol. 2012, 59, 431–443. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Pardo-de-Santayana, M.; Tardío, J. Mediterranean Non-Cultivated Vegetables as Dietary Sources of Compounds with Antioxidant and Biological Activity. LWT Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Bacchetta, L.; Visioli, F.; Cappelli, G.; Caruso, E.; Martin, G.; Nemeth, E.; Bacchetta, G.; Bedini, G.; Wezel, A.; van Asseldonk, T.; et al. A Manifesto for the Valorization of Wild Edible Plants. J. Ethnopharmacol. 2016, 191, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Stinca, A.; Musarella, C.M.; Rosati, L.; Laface, V.L.A.; Licht, W.; Fanfarillo, E.; Wagensommer, R.P.; Galasso, G.; Fascetti, S.; Esposito, A.; et al. Italian Vascular Flora: New Findings, Updates and Exploration of Floristic Similarities between Regions. Diversity 2021, 13, 600. [Google Scholar] [CrossRef]
- Paura, B.; Di Marzio, P.; Salerno, G.; Brugiapaglia, E.; Bufano, A. Design a Database of Italian Vascular Alimurgic Flora (AlimurgITA): Preliminary Results. Plants 2021, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.; Lang, T. Sustainable Diets: How Ecological Nutrition Can Transform Consumption and the Food System; Routledge: London, UK; Taylor and Francis Group: New York, NY, USA, 2017. [Google Scholar]
- Dwivedi, S.L.; Lammerts van Bueren, E.T.; Ceccarelli, S.; Grando, S.; Upadhyaya, H.D.; Ortiz, R. Diversifying Food Systems in the Pursuit of Sustainable Food Production and Healthy Diets. Trends Plant. Sci. 2017, 22, 842–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, M.M.C.; Paulino Albuquerque, U. Biodiverse Food Plants: Which Gaps Do We Need to Address to Promote Sustainable Diets? Ethnobio. Conserv. 2020, 9, 9. [Google Scholar] [CrossRef]
- Chivenge, P.; Mabhaudhi, T.; Modi, A.; Mafongoya, P. The Potential Role of Neglected and Underutilised Crop Species as Future Crops under Water Scarce Conditions in Sub-Saharan Africa. Int. J. Environ. Res. 2015, 12, 5685–5711. [Google Scholar] [CrossRef] [Green Version]
- European Commission Biodiversity Strategy for 2030. Available online: https://ec.europa.eu/environment/strategy/biodiversity-strategy-2030_en (accessed on 21 December 2021).
- Kachura, A.; Harris, C.S. An Ethnobotanical Meta-Analysis of North American Medicinal Asteraceae. Botany 2021, 1–11. [Google Scholar] [CrossRef]
- Gras, A.; Hidalgo, O.; D’Ambrosio, U.; Parada, M.; Garnatje, T.; Vallès, J. The Role of Botanical Families in Medicinal Ethnobotany: A Phylogenetic Perspective. Plants 2021, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J.R.; Dikow, R.B.; Siniscalchi, C.M.; Thapa, R.; Watson, L.E.; Funk, V.A. A Fully Resolved Backbone Phylogeny Reveals Numerous Dispersals and Explosive Diversifications throughout the History of Asteraceae. Proc. Natl. Acad. Sci. USA 2019, 116, 14083–14088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, A.C.; Castro, S.; Paiva, J.; Santos, C.; Silveira, P. Taxonomic Revision of the Genus Calendula (Asteraceae) in the Iberian Peninsula and the Balearic Islands. Phytotaxa 2018, 352, 1–91. [Google Scholar] [CrossRef]
- Arora, D.; Rani, A.; Sharma, A. A Review on Phytochemistry and Ethnopharmacological Aspects of Genus Calendula. Phcog. Rev. 2013, 7, 179. [Google Scholar] [CrossRef] [Green Version]
- Ruiz De Clavijo, E. The Reproductive Strategies of the Heterocarpic Annual Calendula arvensis (Asteraceae). Acta Oecol. 2005, 28, 119–126. [Google Scholar] [CrossRef]
- Arévalo-Precioso, M.L. Las plantas recolectadas y sinantrópicas. In Estudio de Los Restos Paleo Botànicos 2; Revista ArqueoMurcia: Revista Electrónica de Arqueología de la Región de Murcia; IBADER: Lugo, Spain, 2004; Volume 2. [Google Scholar]
- Heyn, C.C.; Joel, A. Reproductive Relationships between Annual Species of Calendula (Compositae). Plant Syst. Evol. 1983, 143, 311–329. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia 3; Edagricole: Bologna, Italy, 2018. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia 4; Edagricole: Bologna, Italy, 2019. [Google Scholar]
- Meikle, R.D. Flora Europea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1976; Volume 4. [Google Scholar]
- Licata, M.; Tuttolomondo, T.; Leto, C.; Virga, G.; Bonsangue, G.; Cammalleri, I.; Gennaro, M.C.; La Bella, S. A Survey of Wild Plant Species for Food Use in Sicily (Italy)—Results of a 3-Year Study in Four Regional Parks. J. Ethnobiol. Ethnomed. 2016, 12, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belabbes, R.; Dib, M.E.A.; Djabou, N.; Ilias, F.; Tabti, B.; Costa, J.; Muselli, A. Chemical Variability, Antioxidant and Antifungal Activities of Essential Oils and Hydrosol Extract of Calendula arvensis L. from Western Algeria. Chem. Biodivers. 2017, 14, e1600482. [Google Scholar] [CrossRef]
- Passalacqua, N.G.; Guarrera, P.M.; De Fine, G. Contribution to the Knowledge of the Folk Plant Medicine in Calabria Region (Southern Italy). Fitoterapia 2007, 78, 52–68. [Google Scholar] [CrossRef]
- Maccioni, S.; Flamini, G.; Cioni, P.L.; Bedini, G.; Guazzi, E. Ricerche Etnobotaniche In Liguria. La Riviera Spezzina (Liguria Orientale). Atti Soc. Tosc. Sci. Nat. Mem. Serie B 2008, 115, 77–82. [Google Scholar]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast Proliferation and Migration in Wound Healing by Phytochemicals: Evidence for a Novel Synergic Outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abudunia, A.-M.; Marmouzi, I.; Faouzi, M.E.A.; Ramli, Y.; Taoufik, J.; El Madani, N.; Essassi, E.M.; Salama, A.; Khedid, K.; Ansar, M.; et al. Anticandidal, Antibacterial, Cytotoxic and Antioxidant Activities of Calendula arvensis Flowers. J. Mycol. Med. 2017, 27, 90–97. [Google Scholar] [CrossRef]
- Abutaha, N.; Nasr, F.A.; Mohammed, A.Z.; Semlali, A.; Al-Mekhlafi, F.A.; Wadaan, M.A. Calendula arvensis L. as an Anti-Cancer Agent against Breast Cancer Cell Lines. Mol. Biol. Rep. 2019, 46, 2187–2196. [Google Scholar] [CrossRef]
- Servi, H.; Vatansever, C.; Doğan, A.; Majeed, V. Antibacterial Activity and Essential Oil Composition of Calendula arvensis L. Int. J. Second. Metab. 2020, 7, 229–236. [Google Scholar] [CrossRef]
- Paolini, J.; Barboni, T.; Desjobert, J.M.; Djabou, N.; Muselli, A.; Costa, J. Chemical Composition, Intraspecies Variation and Seasonal Variation in Essential Oils of Calendula arvensis L. Biochem. Syst. Ecol. 2010, 38, 865–874. [Google Scholar] [CrossRef]
- Kırmızıbekmez, H.; Bassarello, C.; Piacente, S.; Pizza, C.; Çalış, İ. Triterpene Saponins from Calendula arvensis. Z. Naturforsc. B 2006, 61, 1170–1173. [Google Scholar] [CrossRef]
- Faustino, M.V.; Pinto, D.C.G.A.; Gonçalves, M.J.; Salgueiro, L.; Silveira, P.; Silva, A.M.S. Calendula, L. Species Polyphenolic Profile and in Vitro Antifungal Activity. J. Funct. Foods 2018, 45, 254–267. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic Composition of Cynara cardunculus L. Organs, and Their Biological Activities. C. R. Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Riffault, L.; Destandau, E.; Pasquier, L.; André, P.; Elfakir, C. Phytochemical Analysis of Rosa hybrida Cv. ‘Jardin de Granville’ by HPTLC, HPLC-DAD and HPLC-ESI-HRMS: Polyphenolic Fingerprints of Six Plant Organs. Phytochemistry 2014, 99, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Kim, J.S.; Hyun, T.K. Polyphenolic Composition and Anti-Melanoma Activity of White Forsythia (Abeliophyllum Distichum Nakai) Organ Extracts. Plants 2020, 9, 757. [Google Scholar] [CrossRef]
- Rivas-Martinéz, S.; Penas, A.; Dìaz, T.E. Bioclimatic & Biogeographic Maps of Europe. Available online: https://webs.ucm.es/info/cif/form/maps.htm (accessed on 20 December 2021).
- Di Gennaro, A.; Aronne, G.; De Mascellis, R.; Vingiani, S.; Sarnataro, M.; Abalsamo, P.; Cona, F.; Vitelli, L.; Arpaia, I. Sistemi di Terre della CAMPANIA. Monografia e Carta 1:250.000, Con Legenda; Università degli Studi di Napoli Federico II: Napoli, Italy, 2002. [Google Scholar]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.-P.; Marciano, S.; Bauer, R.; Monaco, P. Seasonal Variation in Phenolic Composition and Antioxidant and Anti-Inflammatory Activities of Calamintha nepeta (L.) Savi. Food Res. Int. 2015, 69, 121–132. [Google Scholar] [CrossRef]
- Formato, M.; Piccolella, S.; Zidorn, C.; Pacifico, S. UHPLC-HRMS Analysis of Fagus sylvatica (Fagaceae) Leaves: A Renewable Source of Antioxidant Polyphenols. Antioxidants 2021, 10, 1140. [Google Scholar] [CrossRef]
- Di Maro, A.; Pacifico, S.; Fiorentino, A.; Galasso, S.; Gallicchio, M.L.; Guida, V.; Severino, V.; Parente, A. Raviscanina wild asparagus (Asparagus acutifolius L.): A nutritionally valuable crop with antioxidant and antiproliferative properties. Food Res. Int. 2013, 53, 180–188. [Google Scholar] [CrossRef]
- Podani, J. SYN-TAX 2000:Computer Programs for Data Analysis in Ecology and Systematics; User’s Manual; Scientia: Budapest, Hungary, 2001; p. 452. [Google Scholar]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Dal Poggetto, F.; Catauro, M. New Insights into Phenol and Polyphenol Composition of Stevia rebaudiana Leaves. J. Pharm. Biomed. 2019, 163, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Yan, X.; Ono, H.; Yoshida, M.; Nagata, T.; Nakanishi, T. Caffeic Acid Derivatives in the Roots of Yacon (Smallanthus sonchifolius). J. Agric. Food Chem. 2003, 51, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.K.; Dudkowski, Ł.; Bazylko, A.; Kaźmierski, S.; Kiss, A.K. Caffeic Acid Derivatives Isolated from the Aerial Parts of Galinsoga parviflora and Their Effect on Inhibiting Oxidative Burst in Human Neutrophils. Phytochem. Lett. 2016, 16, 303–310. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yagi, M.; Takabe, W.; Sakata, Y.; Yonei, Y. Anti-Glycative Effect of Yogurt: Prevention of Advanced Glycation End Product Formation. Glycative Stress Res. 2017, 4, 25–31. [Google Scholar]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; Garcia, P.A.; Castro, M.Á.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Characterization and Bioactive Properties of Two Aromatic Plants: Calendula officinalis L. (Flowers) and Mentha cervina L. (Leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal-Ollivier, E.; Elias, R.; Faure, F.; Babadjamian, A.; Crespin, F.; Balansard, G.; Boudon, G. Flavonol Glycosides from Calendula officinalis Flowers. Planta Med. 1989, 55, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I. Calendosides I–IV, New Quercetin and Isorhamnetin Rhamnoglucosides from Calendula officinalis. Chem. Nat. Compd. 2014, 50, 633–637. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. New Isorhamnetin Glycosides and Other Phenolic Compounds from Calendula officinalis. Chem. Nat. Compd. 2013, 49, 833–840. [Google Scholar] [CrossRef]
- De Tommasi, N.; Conti, C.; Stein, M.; Pizza, C. Structure and in Vitro Antiviral Activity of Triterpenoid Saponins from Calendula arvensis. Planta Med. 1991, 57, 250–253. [Google Scholar] [CrossRef]
- Liang, Q.; Qian, H.; Yao, W. Identification of Flavonoids and Their Glycosides by High-Performance Liquid Chromatography with Electrospray Ionization Mass Spectrometry and with Diode Array Ultraviolet Detection. Eur. J. Mass Spectrom. 2005, 11, 93–101. [Google Scholar] [CrossRef]
- Choi, S.-J.; Tai, B.H.; Cuong, N.M.; Kim, Y.-H.; Jang, H.-D. Antioxidative and Anti-Inflammatory Effect of Quercetin and Its Glycosides Isolated from Mampat (Cratoxylum formosum). Food Sci. Biotechnol. 2012, 21, 587–595. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible Flowers as Sources of Phenolic Compounds with Bioactive Potential. Int. Food Res. J. 2018, 105, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Mohammad Sadaka, M.W. A Novel Eudesmane Glycosides Sesquiterpene from Calendula officinalis L. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 3, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Akobirshoeva, A.; Zilfikarov, I.N.; Vennos, C. Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations. Int. J. Mol. Sci. 2017, 18, 1685. [Google Scholar] [CrossRef] [Green Version]
- Szakiel, A.; Ruszkowski, D.; Janiszowska, W. Saponins in Calendula officinalis L.—Structure, Biosynthesis, Transport and Biological Activity. Phytochem. Rev. 2005, 4, 151–158. [Google Scholar] [CrossRef]
- Lehbili, M.; Alabdul Magid, A.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Abedini, A.; Morjani, H.; Sarazin, T.; Gangloff, S.C.; Kabouche, Z. Oleanane-Type Triterpene Saponins from Calendula stellata. Phytochemistry 2017, 144, 33–42. [Google Scholar] [CrossRef]
- Pizza, C.; Zhong-Liang, Z.; de Tommasi, N. Plant Metabolites. Triterpenoid Saponins from Calendula arvensis. J. Nat. Prod. 1987, 50, 927–931. [Google Scholar] [CrossRef]
- Huang, S.P.; Hsu, H.C.; Liew, C.Y.; Tsai, S.T.; Ni, C.K. Logically Derived Sequence Tandem Mass Spectrometry for Structural Determination of Galactose Oligosaccharides. Glycoconj. J. 2021, 38, 177–189. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Kishi, A.; Kageura, T.; Matsuda, H. Medicinal Flowers. III. Marigold. (1): Hypoglycemic, Gastric Emptying Inhibitory, and Gastroprotective Principles and New Oleanane-Type Triterpene Oligoglycosides, Calendasaponins A, B, C, and D, from Egyptian Calendula officinalis. Chem. Pharm. Bull. 2001, 49, 863–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Li, W.; Fu, H.; Zhang, Q.; Koike, K. Pancreatic Lipase-Inhibiting Triterpenoid Saponins from Fruits of Acanthopanax senticosus. Chem. Pharm. Bull. 2007, 55, 1087–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ChemSpider|Search and Share Chemistry. Available online: http://www.chemspider.com/ (accessed on 21 September 2021).
- Chemli, R.; Babadjamian, A.; Faure, R.; Boukef, K.; Balansard, G.; Vidal, E. Arvensoside A and B, Triterpenoid Saponins from Calendula arvensis. Phytochemistry 1987, 26, 1785–1788. [Google Scholar] [CrossRef]
- Vecherko, L.P.; Zinkevich, É.P.; Kogan, L.M. The Structure of Calenduloside F from the Roots of Calendula officinalis. Chem. Nat. Compd. 1973, 9, 532–533. [Google Scholar] [CrossRef]
- Jan, N.; Majeed, U.; Andrabi, K.I.; John, R. Cold Stress Modulates Osmolytes and Antioxidant System in Calendula officinalis. Acta Physiol. Plant 2018, 40, 73. [Google Scholar] [CrossRef]
- Calvano, C.D.; Cataldi, T.R.I.; Kögel, J.F.; Monopoli, A.; Palmisano, F.; Sundermeyer, J. Structural Characterization of Neutral Saccharides by Negative Ion MALDI Mass Spectrometry Using a Superbasic Proton Sponge as Deprotonating Matrix. J. Am. Soc. Mass Spectrom. 2017, 28, 1666–1675. [Google Scholar] [CrossRef]
- Richards, A.B.; Krakowka, S.; Dexter, L.B.; Schmid, H.; Wolterbeek, A.P.M.; Waalkens-Berendsen, D.H.; Shigoyuki, A.; Kurimoto, M. Trehalose: A Review of Properties, History of Use and Human Tolerance, and Results of Multiple Safety Studies. Food Chem. Toxicol. 2002, 40, 871–898. [Google Scholar] [CrossRef]
- Matysik, G.; Wójciak-Kosior, M.; Paduch, R. The Influence of Calendulae officinalis Flos Extracts on Cell Cultures, and the Chromatographic Analysis of Extracts. J. Pharm. Biomed. 2005, 38, 285–292. [Google Scholar] [CrossRef] [PubMed]
- International Herb Association. Available online: https://iherb.org/ (accessed on 21 December 2021).
- Ercetin, T.; Senol, F.S.; Erdogan Orhan, I.; Toker, G. Comparative Assessment of Antioxidant and Cholinesterase Inhibitory Properties of the Marigold Extracts from Calendula arvensis L. and Calendula officinalis L. Ind. Crops Prod. 2012, 36, 203–208. [Google Scholar] [CrossRef]
- Ak, G.; Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Cakır, O.; Bensari, S.; Yılmaz, M.A.; Gallo, M.; Montesano, D. A Comparative Bio-Evaluation and Chemical Profiles of Calendula officinalis L. Extracts Prepared via Different Extraction Techniques. Appl. Sci. 2020, 10, 5920. [Google Scholar] [CrossRef]
- Abudunia, A.-M.; Marmouzi, I.; Kharbach, M.; Jemli, M.E.; Sayah, K.; Bouyahya, A.; Al-kaf, A.; Alyahawi, A.; Ansar, M.; Bouklouze, A.; et al. Hypoglycemic Effect of Calendula Arvensis Flowers Is Mediated by Digestive Enzyme Inhibition. Curr. Bioact. Compd. 2020, 16, 588–592. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Weed Risk Assessment for Calendula arvensis L. (Asteraceae)-Field Marigold. 2016. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/weeds/downloads/wra/Calendula-arvensis.pdf (accessed on 12 November 2021).



| Peak n° | tR (min) | Formula | RDB | [M−H]− (m/z) Found | [M−H]− (m/z) calcd. | ppm | MS/MS (m/z) | Tentative Assignment |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.311 | C4H6O5 | 2.0 | 133.0140 | 133.0142 | −1.9 | 133.0139, 115.0036 (100) | Malic acid |
| 2 | 0.317 | C7H12O6 | 2.0 | 191.0557 | 191.0561 | −2.2 | 191.0556 (100), 111.0083, 93.0344, 85.0294 | Quinic acid |
| 3 | 0.322 | C12H22O11 | 2.0 | 341.1108 377.0860 [M + Cl−]− | 341.1089 | −2.2 | 341.1077, 179.0562, 119.0348, 113.0247, 89.0247 | Dihexose |
| 4 | 1.174 | C7H6O4 | 5.0 | 153.0195 | 153.0193 | 1.1 | 109.0287, 108.0335 | Dihydroxybenzoic acid |
| 5 | 1.824 | C12H14O8 | 6.0 | 285.0619 | 285.0616 | 1.1 | 285.0605, 153.0190, 152.0111, 108.0214 | Pentosyl dihydroxybenzoic acid |
| 6 | 2.080 | C15H18O9 | 7.0 | 341.0880 | 341.0878 | 0.6 | 251.0570, 179.0354, 161.0252 (100), 135.0456 | Caffeoyl hexose |
| 7 | 3.799 | C16H18O9 | 8.0 | 353.0886 707.1848 | 353.0878 | 2.2 | 191.0559 (100) | 5-O-Caffeoylquinic acid (1) |
| 8 | 4.627 | C16H18O9 | 8.0 | 353.0877 | 353.0878 | −0.3 | 191.0561 (100) | 5-O-Caffeoylquinic acid (2) |
| 9 | 6.582 | C17H20O9 | 8.0 | 367.1038 | 367.1035 | 0.9 | 193.0513, 191.0560 (100), 173.0453 | 5-O-Feruloyl quinic acid |
| 10 | 8.179 | C27H30O17 | 13.0 | 625.1414 | 625.1410 | 0.6 | 625.1422 (100), 301.0346, 300.0269, 271.0237, 255.0294 | Quercetin-3-O-dihexoside |
| 11 | 8.771 | C26H28O16 | 13 | 595.1301 | 595.1305 | −0.6 | 595.1318, 301.0349, 300.0253, 271.0234, 255.0291 | Quercetin-3-O-hexosylpentoside |
| 12 | 9.116 | C27H30O16 | 13.0 | 609.1472 | 609.1461 | 1.8 | 609.1477, 301.0348, 300.0270 (100), 271.0235, 255.0292 | Quercetin-3-O-hexosyldeoxyhexoside |
| 13 | 9.386 | C21H20O12 | 12.0 | 463.0882 | 463.0882 | 0 | 463.0905, 301.0358 300.0281 (100), 271.0253, 255.0300 | Quercetin-3-O-hexoside (1) |
| 14 | 9.326 | C26H28O16 | 13.0 | 595.1309 | 595.1305 | 0.7 | 595.1303, 463.0916 (<5%), 301.0340, 300.0260 (100), 271.0226, 255.0306 | Quercetin-3-O-hexosylpentoside |
| 15 | 9.473 | C27H30O16 | 13.0 | 609.1473 | 609.1461 | 1.8 | 609.1484, 301.0253 300.0275 (100), 271.0246, 255.0295 | Rutin |
| 16 | 9.692 | C21H20O12 | 12.0 | 463.0883 | 463.0882 | 0.2 | 463.0879, 301.0341 300.0267 (100), 271.0238, 255. 0285 | Quercetin-3-O-hexoside (2) |
| 17 | 10.024 | C27H30O15 | 13.0 | 593.1507 | 593.1512 | −0.8 | 593.1536 (100), 285.0395, 284.0314, 255.0287 | Kaempferol-3-O-hexosyldeoxyhexoside |
| 18 | 10.024 | C28H32O17 | 13.0 | 639.1578 | 639.1567 | 1.8 | 639.1594, 315.0507 (100), 300.0267, 314.0426, 299.0187, 271.0237, 255.0287 | Isorhamnetin-3-O-dihexoside |
| 19 | 10.885 | C27H30O15 | 13.0 | 593.1527 | 593.1512 | 2.5 | 593.1444, 285.0403, 284.0323 (100), 255.0298, 227.0335 | Kaempferol-3-O-rutinoside |
| 20 | 10.338 | C21H20O11 | 12.0 | 447.0927 | 447.0933 | −1.3 | 447.0925, 285.0388, 284.0316 (100), 255.0283, 227.0333 | Keampferol-3-O-hexoside (1) |
| 21 | 10.974 | C21H20O11 | 12.0 | 447.0930 | 447.0933 | −0.6 | 447.0915, 285.0391, 284.0314 (100), 255.0280, 227.0334 | Keampferol-3-O-hexoside (2) |
| 22 | 10.424 | C23H22O13 | 13.0 | 505.0997 | 505.0988 | 1.9 | 505.1005, 463.0879, 301.0351, 300. 0273 (100), 271.0253, 255.0287; 243.0315; 151.0027 | Quercetin-3-O-acetylhexoside (1) |
| 23 | 10.615 | C25H24O12 | 14.0 | 515.1197 | 515.1195 | 0.4 | 353.0878, 191.0561 (100), 179.0346, 135.0450 | Dicaffeoyl quinic acid |
| 24 | 10.872 | C27H30O15 | 13.0 | 593.1515 | 593.1512 | 0.5 | 593.1436, 285.0404 (100), 284.0324, 255.0290 | Kaempferol-7-O-rutinoside |
| 25 | 10.920 | C23H22O13 | 13.0 | 505.0998 | 505.0988 | 2.0 | 505.1008, 445.0825, 301.0345, 300.0271, 271.0264, 255.0293, 174.9562 | Quercetin-3-O-acetylhexoside (2) |
| 26 | 11.013 | C28H32O16 | 13.0 | 623.1634 | 623.1618 | 2.6 | 623.1645, 315.0512, 314.0437 (100), 300.0275, 299.0199, 243.0300 | Isorhamnetin-3-O-hexosyldeoxyhexoside (1) |
| 27 | 11.247 | C22H22O12 | 12.0 | 477.1043 | 477.1029 | 0.9 | 477.1037, 315.0497, 314.0422 (100), 300.0258, 299.0181, 285.0386, 271.0234, 243.0287, 242.0207 | Isorhamnetin-3-O-hexoside (1) |
| 28 | 11.366 | C28H32O16 | 13.0 | 623.1629 | 623.1618 | 1.8 | 623.1656, 315.0514 (100), 314.0436, 299.0200, 271.0253, 255.0297, 243.0298 | Isorhamnetin-3-O-hexosyldeoxyhexoside (2) |
| 29 | 11.442 | C22H22O12 | 12.0 | 477.1043 | 477.1029 | 0.9 | 477.1053, 315.0501, 314.0430 (100), 299.0193, 285.0396, 271.0244, 243.0291, 242.0217 | Isorhamnetin-3-O-hexoside (2) |
| 30 | 11.617 | C33H28O17 | 20 | 695.1270 | 695.1254 | 2.3 | 695.1256, 533.0958, 371.0620, 209.0299 (100), 191.0186 | Tricaffeoyl citric acid (1) |
| 31 | 11.833 | C33H28O17 | 20 | 695.1267 | 695.1254 | 1.9 | 695.1305, 533.0978, 371.0633, 353.0513, 209.0303 (100), 101.0191, 85.0293 | Tricaffeoyl citric acid (2) |
| 32 | 11.963 | C24H24O13 | 13.0 | 519.1155 | 519.1148 | 2.1 | 519.1174, 315.0507, 314.0432 (100), 300.0279, 299.0192, 285.0397, 271.0241 | Isorhamnetin-3-O-acetylhexoxide |
| 33 | 12.026 | C33H28O17 | 20 | 695.1268 | 695.1254 | 2.1 | 695.1284, 533.0977, 371.0616, 353.0487, 209.0297 (100), 191.0298 | Tricaffeoyl citric acid (3) |
| 34 | 12.164 | C24H24O13 | 13.0 | 519.1163 | 519.1148 | 3.6 | 519.1161, 315.0499, 314.0423 (100), 300.0264, 299.0192, 285.0394, 271.0237 | Isorhamnetin-3-O-acetylhexoxide |
| 35 | 13.538 | C15H10O7 | 11.0 | 301.0354 | 301.0354 | 0.1 | 301.0349, 273.0401, 245.0472, 178.9986, 151.0037 (100), 107.0136 | Quercetin |
| 36 | 15.960 | C54H88O24 | 11.0 | [M + HCOO−]− 1165.5691 [M + Cl−]− 1155.5410 | 1119.5593 | n.c. | 957.5162, 837.4726, 795.4604 (100), 777.4502, 733.4599, 633.4058, 615.3949, 505.3716, 471.3505, 407.3335, 161.0449, 119.0346, 113.0243 | 3-O-trihexosyl 28-O-echinocystic acid hexosyl ester |
| 37 | 16.229 | C48H76O20 | 11.0 | 971.4901 | 971.4857 | 4.5 | 971.4929, 851.4468, 809.4365 (100), 747.4366, 647.3833, 585.3834, 513.3617, 471.3478, 409.3477, 407.3328, 157.0152, 119.0344, 113.0240 | 3-O-(hexosyl)hexuronidyl 28-O-echinocystic acid hexosyl ester |
| 38 | 16.603 | C48H78O19 | 10.0 | [M + HCOO−]− 1003.5116 | 957.5065 | n.c. | 795.4572 (100), 733.4632, 633.4036, 615.3947, 471.3478, 161.0433, 101.0245 | 3-O-dihexosyl 28-O-echinocystic acid hexosyl ester |
| 39 | 16.758 | C42H66O15 | 10.0 | 809.4365 | 809.4388 | 4.5 | 809.4373, 689.3982, 647.3858, 585.3823, 539.3798, 471.3512, 425.3427, 407.3340 (100), 391.3033, 245.1536, 113.0244 | 3-O-hexuronidyl 28-O-hexoxyl echinocystic acid |
| 40 | 17.088 | C42H66O15 | 10.0 | 809.4369 | 809.4388 | 3.8 | 809.4419, 647.3859, 603.3941, 485.3646, 471.3508 (100), 469.3355, 453.3399, 439.3254, 393.3168, 113.0243 | 3-O-hexuronidyl 28-O-mesembryanthemoidigenic hexosyl ester |
| 41 | 17.939 | C48H74O45 | 12.0 | 953.4785 | 953.4752 | 3.6 | 809.4382, 689.3939, 647.3846 (100), 585.3841, 539.3767, 471.3505, 407.3328, 409.3488, 391.3006, 113.0242 | 3-O-(hydroxymethylglutarylhexosyl)hexuronidyl echinocystic acid |
| 42 | 18.377 | C54H88O23 | 11.0 | [M + HCOO−]− 1149.5758 | 1103.5644 | 1.9 | 941.5228, 779.4672, 617.4126, 599.4016, 551.3785, 455.3568 (100) | 3-O-trihexosyl 28-O-oleanonic acid hexosyl ester |
| 43 | 18.900 | C48H76O19 | 11.0 | 955.4946 | 955.4908 | 4.0 | 955.5031, 793.4472, 731.4451, 631.3906, 571.3697, 569.3895, 551.3790 (100), 497.3666, 483.3509, 455.3565, 453.3405, 437.3444, 407.3332 | 3-O-(hexosyl)hexuronidyl 28-O-oleanonic acid hexosyl ester |
| 44 | 19.211 | C48H78O18 | 10.0 | [M + HCOO−]− 987.5207 | 941.5115 | n.c. | 779.4669, 617.4121 (100), 599.4011, 455.3558 | 3-O-dihexosyl 28-O-oleanonic acid hexosyl ester |
| 45 | 19.273 | C42H68O13 | 9.0 | [M + HCOO−]− 825.4661 | 779.4587 | n.c | 617.4100, 599.4001 (100), 455.3538 | 3-O-hexosyl 28-O-oleanonic acid hexosyl ester |
| 46 | 19.778 | C42H66O14 | 10.0 | 793.4417 | 793.4418 | 4.7 | 793.4419, 631.3922, 569.3895, 497.3670, 455.3562 (100), 437.3439 | 3-O-hexuronidyl 28-O-oleanonic acid hexosyl ester |
| 47 | 20.713 | C44H68O15 | 11.0 | 835.4520 | 835.4485 | 4.1 | 793.4436, 631.3912, 569.3898 (100), 551.3805, 497.3677, 455.3556, 437.3447 | 3-O-hexuronidyl 28-O-oleanonic acid acetylhexosyl ester |
| 48 | 20.888 | C48H74O18 | 12.0 | 937.4846 | 937.4802 | 4.7 | 793.4457, 673.4011, 631.3909, 569.3899 (100), 551.3770, 497.3672, 455.3559, 437.3435 | 3-O-(hydroxymethylglutaryl)hexuronidyl 28-O-oleanonic acid hexosyl ester |
| 49 | 21.821 | C42H64O14 | 11.0 | 791.4258 | 791.4223 | 4.4 | 647.3850, 571.3670, 471.3516, 469.3603, 407.3341 (100), 391.3022, 116.0116, 113.0239 | 3-O-(hydroxymethylglutaryl)hexuronidyl echynocistic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorentino, M.; Gravina, C.; Piccolella, S.; Pecoraro, M.T.; Formato, M.; Stinca, A.; Pacifico, S.; Esposito, A. Calendula arvensis (Vaill.) L.: A Systematic Plant Analysis of the Polar Extracts from Its Organs by UHPLC-HRMS. Foods 2022, 11, 247. https://doi.org/10.3390/foods11030247
Fiorentino M, Gravina C, Piccolella S, Pecoraro MT, Formato M, Stinca A, Pacifico S, Esposito A. Calendula arvensis (Vaill.) L.: A Systematic Plant Analysis of the Polar Extracts from Its Organs by UHPLC-HRMS. Foods. 2022; 11(3):247. https://doi.org/10.3390/foods11030247
Chicago/Turabian StyleFiorentino, Marika, Claudia Gravina, Simona Piccolella, Maria Tommasina Pecoraro, Marialuisa Formato, Adriano Stinca, Severina Pacifico, and Assunta Esposito. 2022. "Calendula arvensis (Vaill.) L.: A Systematic Plant Analysis of the Polar Extracts from Its Organs by UHPLC-HRMS" Foods 11, no. 3: 247. https://doi.org/10.3390/foods11030247
APA StyleFiorentino, M., Gravina, C., Piccolella, S., Pecoraro, M. T., Formato, M., Stinca, A., Pacifico, S., & Esposito, A. (2022). Calendula arvensis (Vaill.) L.: A Systematic Plant Analysis of the Polar Extracts from Its Organs by UHPLC-HRMS. Foods, 11(3), 247. https://doi.org/10.3390/foods11030247