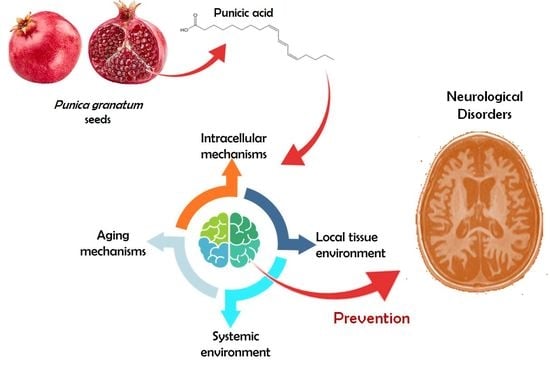
Punicic Acid and Its Role in the Prevention of Neurological Disorders: A Review
Abstract
:1. Introduction
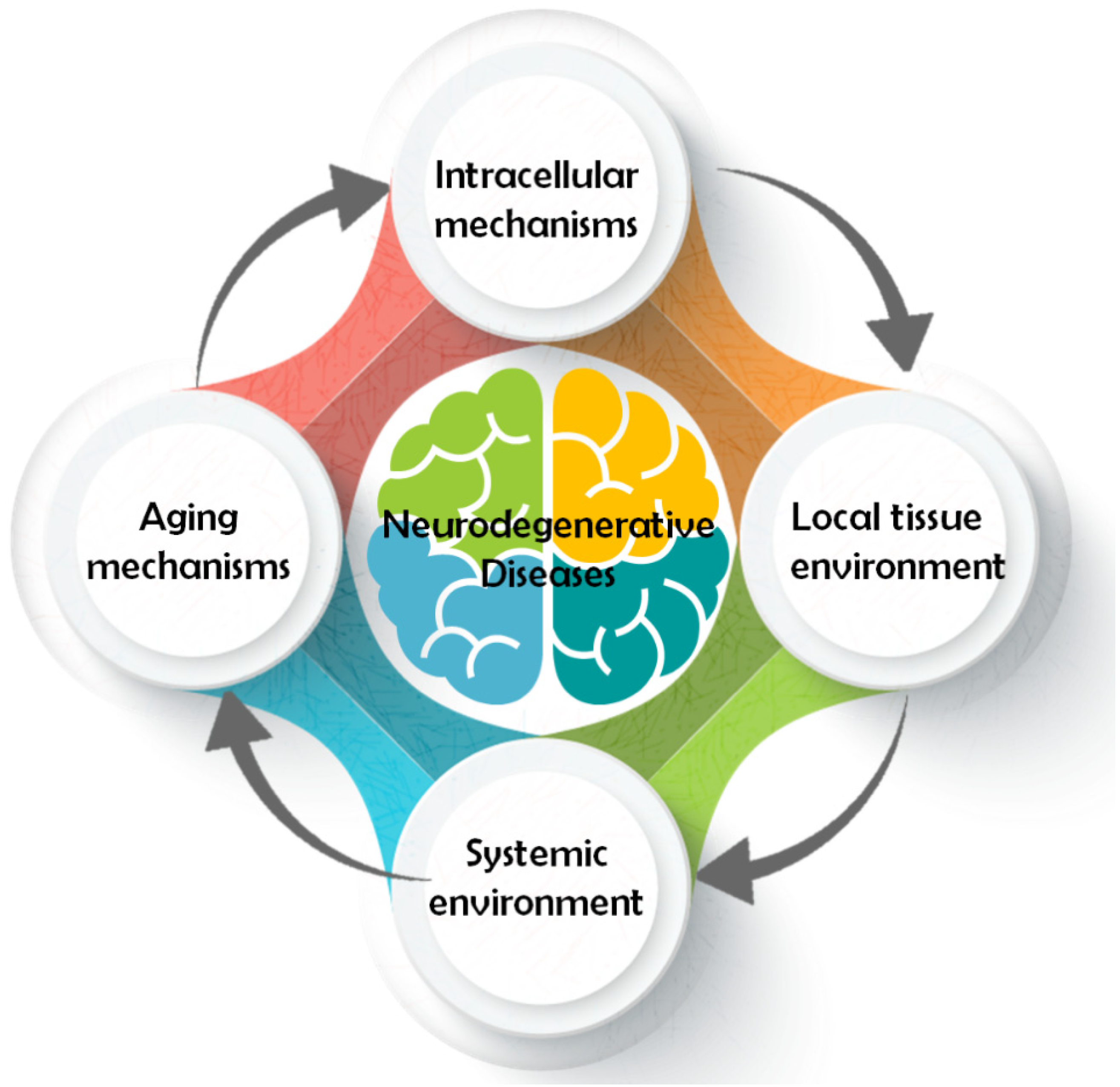
2. Main Pathways Involved in Neurological Disease
2.1. Intracellular Mechanism
2.2. Local Tissue Environment
2.3. Systemic Environment
2.4. Aging Mechanism
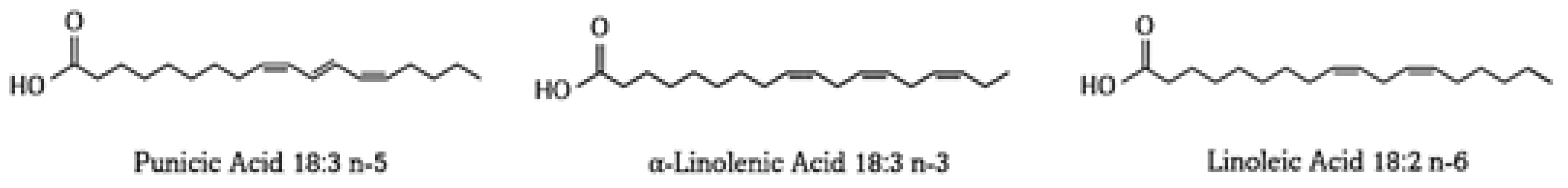
3. Punicic Acid
3.1. Punicic Acid Metabolism
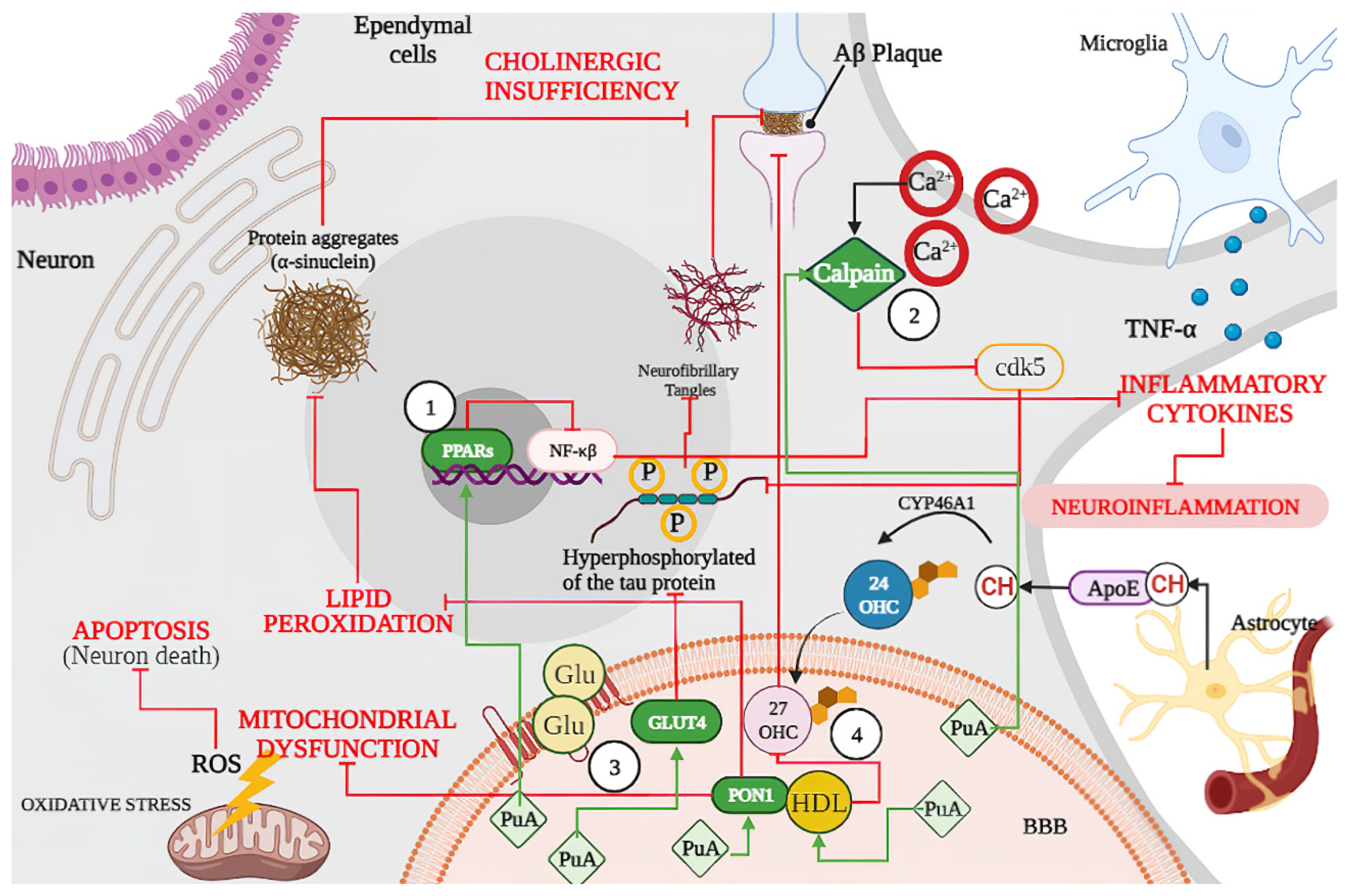
3.2. Punicic Acid Effects on Neurodegenerative Disease
3.2.1. Punicic Acid Increases Expression of Peroxisome Proliferators Activated Receptors (PPARs)
3.2.2. Punicic Acid Participation in Calpain Hyperactivation Inhibition
3.2.3. Punicic Acid Induced a Higher Expression of GLUT4
3.2.4. Effect of Punicic Acid over HDL and PON1
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wyss-Coray, T. Ageing, Neurodegeneration and Brain Rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Checkoway, H.; Lundin, J.I.; Kelada, S.N. Neurodegenerative Diseases. IARC Sci. Publ. 2011, 163, 407–419. [Google Scholar]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The Global Prevalence of Dementia: A Systematic Review and Metaanalysis. Alzheimers Dement. 2013, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Song, B.-J.; Essa, M.M.; Khan, M. Pomegranate: An Ideal Fruit for Human Health. Int. J. Nutr. Pharm. Neurol. Dis. 2015, 5, 141. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and Its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Jalal, H.; Pal, M.A.; Hamdani, H.; Rovida, M.; Khan, N.N. Antioxidant Activity of Pomegranate Peel and Seed Powder Extracts. J. Pharmacogn. Phytochem. 2018, 7, 992–997. [Google Scholar]
- Kýralan, M.; Gölükcü, M.; Tokgöz, H. Oil and Conjugated Linolenic Acid Contents of Seeds from Important Pomegranate Cultivars (Punica Granatum L.) Grown in Turkey. J. Am. Oil Chem. Soc. 2009, 86, 985–990. [Google Scholar] [CrossRef]
- Peng, Y. Comparative Analysis of the Biological Components of Pomegranate Seed from Different Cultivars. Int. J. Food Prop. 2019, 22, 784–794. [Google Scholar] [CrossRef] [Green Version]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Effects of Enzymatic Pretreatment of Seeds on the Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity of Pomegranate Seed Oil. Molecules 2021, 26, 4575. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Talaat, I.M.; Elrashidy, F.H.; Hegazy, A.Y.; Sultan, A.S. Therapeutic Role of Punica Granatum (Pomegranate) Seed Oil Extract on Bone Turnover and Resorption Induced in Ovariectomized Rats. J. Nutr. Health Aging 2017, 21, 1299–1306. [Google Scholar] [CrossRef]
- Mandal, A.; Bhatia, D.; Bishayee, A. Anti-Inflammatory Mechanism Involved in Pomegranate-Mediated Prevention of Breast Cancer: The Role of NF-ΚB and Nrf2 Signaling Pathways. Nutrients 2017, 9, 436. [Google Scholar] [CrossRef] [Green Version]
- Zamora-López, K.; Noriega, L.G.; Estanes-Hernández, A.; Escalona-Nández, I.; Tobón-Cornejo, S.; Tovar, A.R.; Barbero-Becerra, V.; Pérez-Monter, C. Punica Granatum L.-Derived Omega-5 Nanoemulsion Improves Hepatic Steatosis in Mice Fed a High Fat Diet by Increasing Fatty Acid Utilization in Hepatocytes. Sci. Rep. 2020, 10, 15229. [Google Scholar] [CrossRef]
- Mohamed, S.S.; Fayed, A.-H.M. Anti-Obesity Synergistic Effect of Pomegranate Seed Oil (PSO) and Arabic Gum (AG) in Albino Rats. Int. J. Vet. Sci. 2020, 9, 84–89. [Google Scholar]
- Aruna, P.; Venkataramanamma, D.; Singh, A.K.; Singh, R.P. Health Benefits of Punicic Acid: A Review: Health Benefits of Punicic Acid. Compr. Rev. Food Sci. Food Saf. 2016, 15, 16–27. [Google Scholar] [CrossRef]
- Bedel, H.A.; Turgut, N.T.; Kurtoglu, A.U.; Usta, C. Effects of Nutraceutical Punicic Acid. Pharm. Sci. 2017, 79, 328–334. [Google Scholar] [CrossRef]
- Gutierrez Alvarez, A.; Yachelevich, N.; Kohn, B.; Brar, P.C. Genotype—Phenotype Correlation in an Adolescent Girl with Pathogenic PPARy Genetic Variation That Caused Severe Hypertriglyceridemia and Early Onset Type 2 Diabetes. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Dhar Dubey, K.K.; Sharma, G.; Kumar, A. Conjugated Linolenic Acids: Implication in Cancer. J. Agric. Food Chem. 2019, 67, 6091–6101. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holic, R.; Xu, Y.; Caldo, K.M.P.; Singer, S.D.; Field, C.J.; Weselake, R.J.; Chen, G. Bioactivity and Biotechnological Production of Punicic Acid. Appl. Microbiol. Biotechnol. 2018, 102, 3537–3549. [Google Scholar] [CrossRef]
- Ramanan, V.K.; Saykin, A.J. Pathways to Neurodegeneration: Mechanistic Insights from GWAS in Alzheimer’s Disease, Parkinson’s Disease, and Related Disorders. Am. J. Neurodegener. Dis. 2013, 2, 145–175. [Google Scholar]
- Fan, J.; Dawson, T.M.; Dawson, V.L. Cell Death Mechanisms of Neurodegeneration. In Neurodegenerative Diseases; Beart, P., Robinson, M., Rattray, M., Maragakis, N.J., Eds.; Advances in Neurobiology; Springer International Publishing: Cham, Switzerland, 2017; Volume 15, pp. 403–425. ISBN 978-3-319-57191-1. [Google Scholar]
- Plaza-Zabala, A.; Sierra-Torre, V.; Sierra, A. Autophagy and Microglia: Novel Partners in Neurodegeneration and Aging. Int. J. Mol. Sci. 2017, 18, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial Dysfunction in Neurodegenerative Diseases and Drug Targets via Apoptotic Signaling. Mitochondrion 2019, 49, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, L.E.; Moore, M.E.; Sarraf, S.A.; Pickrell, A.M. Ubiquitin and Receptor-Dependent Mitophagy Pathways and Their Implication in Neurodegeneration. J. Mol. Biol. 2020, 432, 2510–2524. [Google Scholar] [CrossRef]
- Ilic, K.; Mlinac-Jerkovic, K.; Jovanov-Milosevic, N.; Simic, G.; Habek, N.; Bogdanovic, N.; Kalanj-Bognar, S. Hippocampal Expression of Cell-Adhesion Glycoprotein Neuroplastin Is Altered in Alzheimer’s Disease. J. Cell Mol. Med. 2019, 23, 1602–1607. [Google Scholar] [CrossRef]
- Tehran, D.A.; Kuijpers, M.; Haucke, V. Presynaptic Endocytic Factors in Autophagy and Neurodegeneration. Curr. Opin. Neurobiol. 2018, 48, 153–159. [Google Scholar] [CrossRef]
- O’Carroll, A.; Coyle, J.; Gambin, Y. Prions and Prion-like Assemblies in Neurodegeneration and Immunity: The Emergence of Universal Mechanisms across Health and Disease. Semin. Cell Dev. Biol. 2020, 99, 115–130. [Google Scholar] [CrossRef]
- Nichols, M.R.; St-Pierre, M.; Wendeln, A.; Makoni, N.J.; Gouwens, L.K.; Garrad, E.C.; Sohrabi, M.; Neher, J.J.; Tremblay, M.; Combs, C.K. Inflammatory Mechanisms in Neurodegeneration. J. Neurochem. 2019, 149, 562–581. [Google Scholar] [CrossRef] [Green Version]
- Castellanos, D.B.; Martín-Jiménez, C.A.; Rojas-Rodríguez, F.; Barreto, G.E.; González, J. Brain Lipidomics as a Rising Field in Neurodegenerative Contexts: Perspectives with Machine Learning Approaches. Front. Neuroendocrinol. 2021, 61, 100899. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The Role of Brain Vasculature in Neurodegenerative Disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Pluvinage, J.V.; Wyss-Coray, T. Systemic Factors as Mediators of Brain Homeostasis, Ageing and Neurodegeneration. Nat. Rev. Neurosci. 2020, 21, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Aromolaran, K.A.; Zukin, R.S. The Emerging Field of Epigenetics in Neurodegeneration and Neuroprotection. Nat. Rev. Neurosci. 2017, 18, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, T.; Savall, A.; Gutierrez, M.Z.; Pinton, S. Neurotrophic Factors in Alzheimer’s and Parkinson’s Diseases: Implications for Pathogenesis and Therapy. Neural Regen. Res. 2017, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, X.; Wang, F.; Wang, F.; Geng, X. Role of Senescence and Neuroprotective Effects of Telomerase in Neurodegenerative Diseases. Rejuvenation Res. 2020, 23, 150–158. [Google Scholar] [CrossRef]
- Ross, C.A.; Truant, R. A Unifying Mechanism in Neurodegeneration. Nature 2017, 541, 34–35. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Role of Mitochondrial ROS in the Brain: From Physiology to Neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A.; Nabavi, S.M. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 2020, 19, 114–126. [Google Scholar] [CrossRef]
- Magalhães, C.A.; Ferreira, C.N.; Loures, C.M.G.; Fraga, V.G.; Chaves, A.C.; Oliveira, A.C.R.; de Souza, L.C.; de P. F. Resende, E.; Carmona, K.C.; Guimarães, H.C.; et al. Leptin, HsCRP, TNF-α and IL-6 Levels from Normal Aging to Dementia: Relationship with Cognitive and Functional Status. J. Clin. Neurosci. 2018, 56, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Paouri, E.; Tzara, O.; Kartalou, G.-I.; Zenelak, S.; Georgopoulos, S. Peripheral Tumor Necrosis Factor-Alpha (TNF-α) Modulates Amyloid Pathology by Regulating Blood-Derived Immune Cells and Glial Response in the Brain of AD/TNF Transgenic Mice. J. Neurosci. 2017, 37, 5155–5171. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, T.; Yamaguchi, K.; Matsui, K.; Sano, T.; Kubota, T.; Hashimoto, T.; Mano, A.; Yamada, K.; Matsuo, Y.; Kubota, N.; et al. Differential Effects of Diet- and Genetically-Induced Brain Insulin Resistance on Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 15. [Google Scholar] [CrossRef]
- Folch, J.; Olloquequi, J.; Ettcheto, M.; Busquets, O.; Sánchez-López, E.; Cano, A.; Espinosa-Jiménez, T.; García, M.L.; Beas-Zarate, C.; Casadesús, G.; et al. The Involvement of Peripheral and Brain Insulin Resistance in Late Onset Alzheimer’s Dementia. Front. Aging Neurosci. 2019, 11, 236. [Google Scholar] [CrossRef]
- Corsaro, A.; Thellung, S.; Villa, V.; Nizzari, M.; Florio, T. Role of Prion Protein Aggregation in Neurotoxicity. Int. J. Mol. Sci. 2012, 13, 8648–8669. [Google Scholar] [CrossRef] [Green Version]
- Binyamin, O.; Nitzan, K.; Frid, K.; Ungar, Y.; Rosenmann, H.; Gabizon, R. Brain Targeting of 9c,11t-Conjugated Linoleic Acid, a Natural Calpain Inhibitor, Preserves Memory and Reduces Aβ and P25 Accumulation in 5XFAD Mice. Sci. Rep. 2019, 9, 18437. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Stachowiak, A.; Mamun, A.A.; Tzvetkov, N.T.; Takeda, S.; Atanasov, A.G.; Bergantin, L.B.; Abdel-Daim, M.M.; Stankiewicz, A.M. Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Front. Aging Neurosci. 2018, 10, 04. [Google Scholar] [CrossRef] [PubMed]
- Shafei, M.A.; Harris, M.; Conway, M.E. Divergent Metabolic Regulation of Autophagy and MTORC1—Early Events in Alzheimer’s Disease? Front. Aging Neurosci. 2017, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Fusco, S.; Grassi, C. Brain Insulin Resistance and Hippocampal Plasticity: Mechanisms and Biomarkers of Cognitive Decline. Front. Neurosci. 2019, 13, 788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsillach, J.; Adorni, M.P.; Zimetti, F.; Papotti, B.; Zuliani, G.; Cervellati, C. HDL Proteome and Alzheimer’s Disease: Evidence of a Link. Antioxidants 2020, 9, 1224. [Google Scholar] [CrossRef] [PubMed]
- Zimetti, F.; Adorni, M.P.; Marsillach, J.; Marchi, C.; Trentini, A.; Valacchi, G.; Cervellati, C. Connection between the Altered HDL Antioxidant and Anti-Inflammatory Properties and the Risk to Develop Alzheimer’s Disease: A Narrative Review. Oxidative Med. Cell. Longev. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Balakrishnan, J.; Kannan, S.; Govindasamy, A. Structured form of DHA Prevents Neurodegenerative Disorders: A Better Insight into the Pathophysiology and the Mechanism of DHA Transport to the Brain. Nutr. Res. 2021, 85, 119–134. [Google Scholar] [CrossRef]
- Hammouda, S.; Ghzaiel, I.; Khamlaoui, W.; Hammami, S.; Mhenni, S.Y.; Samet, S.; Hammami, M.; Zarrouk, A. Genetic Variants in FADS1 and ELOVL2 Increase Level of Arachidonic Acid and the Risk of Alzheimer’s Disease in the Tunisian Population. Prostaglandins Leukot. Essent. Fat. Acids 2020, 160, 102159. [Google Scholar] [CrossRef]
- Xu, Y.; Mietkiewska, E.; Shah, S.; Weselake, R.J.; Chen, G. Punicic Acid Production in Brassica Napus. Metab. Eng. 2020, 62, 20–29. [Google Scholar] [CrossRef]
- HMDB: The Human Metabolome Database Metabocard for Punicic Acid (HMDB0030963). Available online: https://hmdb.ca/metabolites/HMDB0030963#links (accessed on 1 January 2021).
- National Center for Biotechnology Information PubChem Compound Summary for CID 5281126, Punicic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Punicic-acid (accessed on 1 January 2022).
- Shabbir, M.A.; Khan, M.R.; Saeed, M.; Pasha, I.; Khalil, A.A.; Siraj, N. Punicic Acid: A Striking Health Substance to Combat Metabolic Syndromes in Humans. Lipids Health Dis. 2017, 16, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Wang, L.; He, M.; Zhang, Y.; Wang, H. Nanodispersions of Monoglycerides of Punicic Acid: A Potential Nutrient Precursor with Higher Oxidative Stability and Cytotoxicity. RSC Adv. 2014, 4, 43392–43398. [Google Scholar] [CrossRef]
- Adu-Frimpong, M.; Omari-Siaw, E.; Mukhtar, Y.M.; Xu, X.; Yu, J. Formulation of Pomegranate Seed Oil: A Promising Approach of Improving Stability and Health-Promoting Properties. Eur. J. Lipid Sci. Technol. 2018, 120, 1800177. [Google Scholar] [CrossRef]
- Cortez-Trejo, M.C.; Wall-Medrano, A.; Gaytán-Martínez, M.; Mendoza, S. Microencapsulation of Pomegranate Seed Oil Using a Succinylated Taro Starch: Characterization and Bioaccessibility Study. Food Biosci. 2021, 41, 100929. [Google Scholar] [CrossRef]
- Comunian, T.A.; Grassmann Roschel, G.; da Silva Anthero, A.G.; de Castro, I.A.; Dupas Hubinger, M. Influence of Heated, Unheated Whey Protein Isolate and Its Combination with Modified Starch on Improvement of Encapsulated Pomegranate Seed Oil Oxidative Stability. Food Chem. 2020, 326, 126995. [Google Scholar] [CrossRef]
- Mizrahi, M.; Friedman-Levi, Y.; Larush, L.; Frid, K.; Binyamin, O.; Dori, D.; Fainstein, N.; Ovadia, H.; Ben-Hur, T.; Magdassi, S.; et al. Pomegranate Seed Oil Nanoemulsions for the Prevention and Treatment of Neurodegenerative Diseases: The Case of Genetic CJD. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1353–1363. [Google Scholar] [CrossRef]
- Ferrari Cervi, V.; Parcianello Saccol, C.; Henrique Marcondes Sari, M.; Cristóvão Martins, C.; Saldanha da Rosa, L.; Dias Ilha, B.; Zovico Soares, F.; Luchese, C.; Antunes Wilhelm, E.; Cruz, L. Pullulan Film Incorporated with Nanocapsules Improves Pomegranate Seed Oil Anti-Inflammatory and Antioxidant Effects in the Treatment of Atopic Dermatitis in Mice. Int. J. Pharm. 2021, 609, 121144. [Google Scholar] [CrossRef]
- Adu-Frimpong, M.; Firempong, C.K.; Omari-Siaw, E.; Wang, Q.; Mukhtar, Y.M.; Deng, W.; Yu, Q.; Xu, X.; Yu, J. Preparation, Optimization, and Pharmacokinetic Study of Nanoliposomes Loaded with Triacylglycerol-bound Punicic Acid for Increased Antihepatotoxic Activity. Drug Dev. Res. 2019, 80, 230–245. [Google Scholar] [CrossRef]
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; Sahafi, S.M. Formulation and Characterization of Novel Nanostructured Lipid Carriers Made from Beeswax, Propolis Wax and Pomegranate Seed Oil. Food Chem. 2018, 244, 83–92. [Google Scholar] [CrossRef]
- Talkar, S.S.; Kharkar, P.B.; Patravale, V.B. Docetaxel Loaded Pomegranate Seed Oil Based Nanostructured Lipid Carriers: A Potential Alternative to Current Formulation. AAPS PharmSciTech 2020, 21, 295. [Google Scholar] [CrossRef]
- Mirsafaei, R.; Varshosaz, J.; Mirsattari, S.N. Folate-Targeted Polyacrylamide/Punicic Acid Nanomicelles for Flutamide Delivery in Prostate Cancer: Characterization, In Vitro Biological Evaluation, and Its DFT Study. Recent Pat. Nanotechnol. 2020, 14, 360–374. [Google Scholar] [CrossRef]
- Fayaz, G.; Goli, S.A.H.; Kadivar, M.; Valoppi, F.; Barba, L.; Balducci, C.; Conte, L.; Calligaris, S.; Nicoli, M.C. Pomegranate Seed Oil Organogels Structured by Propolis Wax, Beeswax, and Their Mixture. Eur. J. Lipid Sci. Technol. 2017, 119, 1700032. [Google Scholar] [CrossRef]
- Modaresi, J.; Fathi Nasri, M.H.; Rashidi, L.; Dayani, O.; Kebreab, E. Short Communication: Effects of Supplementation with Pomegranate Seed Pulp on Concentrations of Conjugated Linoleic Acid and Punicic Acid in Goat Milk. J. Dairy Sci. 2011, 94, 4075–4080. [Google Scholar] [CrossRef] [Green Version]
- Pamisetty, A.; Kumar, K.A.; Indrani, D.; Singh, R.P. Rheological, Physico-Sensory and Antioxidant Properties of Punicic Acid Rich Wheat Bread. J. Food Sci. Technol. 2020, 57, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhove, C.P.; Moyano, A.; Castro-Gómez, P.; Fontecha, J.; Sáez, G.; Zárate, G.; Pizarro, P.L. Comparative Study of Pomegranate and Jacaranda Seeds as Functional Components for the Conjugated Linolenic Acid Enrichment of Yogurt. LWT 2019, 111, 401–407. [Google Scholar] [CrossRef]
- Chojnacka, A.; Gładkowski, W.; Gliszczyńska, A.; Niezgoda, N.; Kiełbowicz, G.; Wawrzeńczyk, C. Synthesis of Structured Phosphatidylcholine Containing Punicic Acid by the Lipase-Catalyzed Transesterification with Pomegranate Seed Oil. Catal. Commun. 2016, 75, 60–64. [Google Scholar] [CrossRef]
- Calvano, C.D.; Losito, I.; Cataldi, T. Editorial to the Special Issue “Lipidomics and Neurodegenerative Diseases”. Int. J. Mol. Sci. 2021, 22, 1270. [Google Scholar] [CrossRef]
- Pereira de Melo, I.L.; de Oliveira e Silva, A.M.; Yoshime, L.T.; Gasparotto Sattler, J.A.; Teixeira de Carvalho, E.B.; Mancini-Filho, J. Punicic Acid Was Metabolised and Incorporated in the Form of Conjugated Linoleic Acid in Different Rat Tissues. Int. J. Food Sci. Nutr. 2019, 70, 421–431. [Google Scholar] [CrossRef]
- Yuan, G.; Sinclair, A.J.; Xu, C.; Li, D. Incorporation and Metabolism of Punicic Acid in Healthy Young Humans. Mol. Nutr. Food Res. 2009, 53, 1336–1342. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Kawakami, Y.; Abe, R.; Nakagawa, K.; Koba, K.; Imamura, J.; Iwata, T.; Ikeda, I.; Miyazawa, T. Conjugated Linolenic Acid Is Slowly Absorbed in Rat Intestine, but Quickly Converted to Conjugated Linoleic Acid. J. Nutr. 2006, 136, 2153–2159. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.-F.; Yuan, J.-Q.; Li, D. Punicic Acid from Trichosanthes Kirilowii Seed Oil Is Rapidly Metabolized to Conjugated Linoleic Acid in Rats. J. Med. Food 2009, 12, 416–422. [Google Scholar] [CrossRef]
- Mele, M.C.; Cannelli, G.; Carta, G.; Cordeddu, L.; Melis, M.P.; Murru, E.; Stanton, C.; Banni, S. Metabolism of C9,T11-Conjugated Linoleic Acid (CLA) in Humans. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Fa, M.; Diana, A.; Carta, G.; Cordeddu, L.; Melis, M.; Murru, E.; Sogos, V.; Banni, S. Incorporation and Metabolism of C9,T11 and T10,C12 Conjugated Linoleic Acid (CLA) Isomers in Rat Brain. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2005, 1736, 61–66. [Google Scholar] [CrossRef]
- Queiroz, M.P.; da S. Lima, M.; Barbosa, M.Q.; de Melo, M.F.F.T.; de M. S. Bertozzo, C.C.; de Oliveira, M.E.G.; Bessa, R.J.B.; Alves, S.P.A.; Souza, M.I.A.; Queiroga, R.; et al. Effect of Conjugated Linoleic Acid on Memory and Reflex Maturation in Rats Treated During Early Life. Front. Neurosci. 2019, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Murru, E.; Carta, G.; Manca, C.; Sogos, V.; Pistis, M.; Melis, M.; Banni, S. Conjugated Linoleic Acid and Brain Metabolism: A Possible Anti-Neuroinflammatory Role Mediated by PPARα Activation. Front. Pharmacol. 2021, 11, 587140. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eckel, R.H. What Are Lipoproteins Doing in the Brain? Trends Endocrinol. Metab. 2014, 25, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazinet, R.P.; Layé, S. Polyunsaturated Fatty Acids and Their Metabolites in Brain Function and Disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Ringseis, R.; Wen, G.; Saal, D.; Eder, K. Conjugated Linoleic Acid Isomers Reduce Cholesterol Accumulation in Acetylated LDL-Induced Mouse RAW264.7 Macrophage-Derived Foam Cells. Lipids 2008, 43, 913–923. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, R.; Yang, T.; Xu, N.; Chen, J.; Gao, Y.; Stetler, R.A. Fatty Acid Transporting Proteins: Roles in Brain Development, Aging, and Stroke. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 35–45. [Google Scholar] [CrossRef]
- Anusree, S.S.; Sindhu, G.; Preetha Rani, M.R.; Raghu, K.G. Insulin Resistance in 3T3-L1 Adipocytes by TNF-α Is Improved by Punicic Acid through Upregulation of Insulin Signalling Pathway and Endocrine Function, and Downregulation of Proinflammatory Cytokines. Biochimie 2018, 146, 79–86. [Google Scholar] [CrossRef]
- Hontecillas, R.; O’Shea, M.; Einerhand, A.; Diguardo, M.; Bassaganya-Riera, J. Activation of PPAR γ and α by Punicic Acid Ameliorates Glucose Tolerance and Suppresses Obesity-Related Inflammation. J. Am. Coll. Nutr. 2009, 28, 184–195. [Google Scholar] [CrossRef]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Hamishehkar, H.; Mobasseri, M.; Ebrahimzadeh, V.; Alipour, M.; Alipour, B. Effect of Pomegranate Seed Oil Supplementation on the GLUT-4 Gene Expression and Glycemic Control in Obese People with Type 2 Diabetes: A Randomized Controlled Clinical Trial. J. Cell Physiol. 2019, 234, 19621–19628. [Google Scholar] [CrossRef] [PubMed]
- Dorantes-Morales, A.; Estrada-Luna, D.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Luna-Luna, M.; Flores-Castillo, C.; Vargas-Alarcón, G.; Fragoso, J.M.; Pérez-Méndez, Ó.; Carreón-Torres, E. Microencapsulated Pomegranate Modifies the Composition and Function of High-Density Lipoproteins (HDL) in New Zealand Rabbits. Molecules 2020, 25, 3297. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Luna, D.; Carreón-Torres, E.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Dorantes-Morales, A.; Luna-Luna, M.; Vargas-Barrón, J.; Mejía, A.M.; Fragoso, J.M.; Carvajal-Aguilera, K.; et al. Microencapsulated Pomegranate Reverts High-Density Lipoprotein (HDL)-Induced Endothelial Dysfunction and Reduces Postprandial Triglyceridemia in Women with Acute Coronary Syndrome. Nutrients 2019, 11, 1710. [Google Scholar] [CrossRef] [Green Version]
- Frid, K.; Binyamin, O.; Usman, A.; Gabizon, R. Delay of GCJD Aggravation in Sick TgMHu2ME199K Mice by Combining NPC Transplantation and Nano-PSO Administration. Neurobiol. Aging 2020, 95, 231–239. [Google Scholar] [CrossRef]
- Strosznajder, A.K.; Wójtowicz, S.; Jeżyna, M.J.; Sun, G.Y.; Strosznajder, J.B. Recent Insights on the Role of PPAR-β/δ in Neuroinflammation and Neurodegeneration, and Its Potential Target for Therapy. Neuromol. Med. 2021, 23, 86–98. [Google Scholar] [CrossRef]
- Jamwal, S.; Blackburn, J.K.; Elsworth, J.D. PPARγ/PGC1α Signaling as a Potential Therapeutic Target for Mitochondrial Biogenesis in Neurodegenerative Disorders. Pharmacol. Ther. 2021, 219, 107705. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Das, D.; Kommaddi, R.P.; Diwakar, L.; Gowaikar, R.; Rupanagudi, K.V.; Bennett, D.A.; Ravindranath, V. Isoform-Specific Hyperactivation of Calpain-2 Occurs Presymptomatically at the Synapse in Alzheimer’s Disease Mice and Correlates with Memory Deficits in Human Subjects. Sci. Rep. 2018, 8, 13119. [Google Scholar] [CrossRef]
- Chen, H.-H.; Liu, P.; Auger, P.; Lee, S.-H.; Adolfsson, O.; Rey-Bellet, L.; Lafrance-Vanasse, J.; Friedman, B.A.; Pihlgren, M.; Muhs, A.; et al. Calpain-Mediated Tau Fragmentation Is Altered in Alzheimer’s Disease Progression. Sci. Rep. 2018, 8, 16725. [Google Scholar] [CrossRef]
- Weber, J.J.; Haas, E.; Maringer, Y.; Hauser, S.; Casadei, N.L.P.; Chishti, A.H.; Riess, O.; Hübener-Schmid, J. Calpain-1 Ablation Partially Rescues Disease-Associated Hallmarks in Models of Machado-Joseph Disease. Hum. Mol. Genet. 2020, 29, 892–906. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate Seed Oil in Food Industry: Extraction, Characterization, and Applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Lee, E.; Eom, J.-E.; Kim, H.-L.; Baek, K.H.; Jun, K.-Y.; Kim, H.-J.; Lee, M.; Mook-Jung, I.; Kwon, Y. Effect of Conjugated Linoleic Acid, μ-Calpain Inhibitor, on Pathogenesis of Alzheimer’s Disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Brain Glucose Transporters: Role in Pathogenesis and Potential Targets for the Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 8142. [Google Scholar] [CrossRef] [PubMed]
- Głuchowska, K.; Pliszka, M.; Szablewski, L. Expression of Glucose Transporters in Human Neurodegenerative Diseases. Biochem. Biophys. Res. Commun. 2021, 540, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gil-Iturbe, E.; Solas, M.; Cuadrado-Tejedo, M.; García-Osta, A.; Escoté, X.; Ramírez, M.J.; Lostao, M.P. GLUT12 Expression in Brain of Mouse Models of Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 798–805. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Hu, X.; Huang, X.; Chen, G.-X. Current Understanding of Glucose Transporter 4 Expression and Functional Mechanisms. World J. Biol. Chem. 2020, 11, 76–98. [Google Scholar] [CrossRef]
- Salazar, J.G.; Marsillach, J.; Reverte, I.; Mackness, B.; Mackness, M.; Joven, J.; Camps, J.; Colomina, M.T. Paraoxonase-1 and -3 Protein Expression in the Brain of the Tg2576 Mouse Model of Alzheimer’s Disease. Antioxidants 2021, 10, 339. [Google Scholar] [CrossRef]
- Su, N.-D.; Liu, X.-W.; Kim, M.R.; Jeong, T.-S.; Sok, D.-E. Protective Action of CLA against Oxidative Inactivation of Paraoxonase 1, an Antioxidant Enzyme. Lipids 2003, 38, 615–622. [Google Scholar] [CrossRef]
- Morris, G.; Puri, B.K.; Bortolasci, C.C.; Carvalho, A.; Berk, M.; Walder, K.; Moreira, E.G.; Maes, M. The Role of High-Density Lipoprotein Cholesterol, Apolipoprotein A and Paraoxonase-1 in the Pathophysiology of Neuroprogressive Disorders. Neurosci. Biobehav. Rev. 2021, 125, 244–263. [Google Scholar] [CrossRef]
- Mota, A.; Hemati-Dinarvand, M.; Akbar Taheraghdam, A.; Reza Nejabati, H.; Ahmadi, R.; Ghasemnejad, T.; Hasanpour, M.; Valilo, M. Association of Paraoxonse1 (PON1) Genotypes with the Activity of PON1 in Patients with Parkinson’s Disease. Acta Neurol. Taiwan 2019, 28, 66–74. [Google Scholar] [PubMed]



| Molecules | Related NDs | Formulation | Effects | Mechanism | Biological Model | References |
|---|---|---|---|---|---|---|
| PPARγ/α and TNF-α | Alzheimer, Parkinson and Huntington, CNS Hypoxia/Ischemia | Nanoemulsified PSO supplementation | Anti-inflammationIncreased fatty acid oxidation | Gene expression upregulation of PPARγ/α/β among others | Liver of high-fat diet-fed mice. | [12] |
| PuA | Improved glucose homeostasis and suppressed inflammation | Suppressed NF-κB activation and TNF-α expression via PuA Agonist effect of PPARγ | 3T3-L1 pre-adipocytes and obese/high-fat diet mice | [83,84] | ||
| Calpain | Alzheimer, Parkinson and Huntington’s diseases, Machado–Joseph disease, genetic Creutzfeldt–Jakob disease | PSO-nanoformulation(GranaGard) | Detention of the disease for 60–80 days and slower disease progression after. Decreased Aβ and p25 formation. | μ-calpain inhibition and nanoformulation antioxidant effect. | Mice | [43,88] |
| GLUT4 | Neurodegeneration | PSO | Decreased fasting blood sugar levels. | GLUT4 increased expression | Diabetic type II patients | [85] |
| HDL and PON1 | Alzheimer, Multiple Sclerosis, Parkinson, Huntington. | Microencapsulated pomegranate | Reduction in non-HDL sphingomyelinIncrease in HDL-cholesterol and HDL-phospholipidsIncrement in PON1 activity | Reduction in triglyceride content in HDL | Rabbits | [86] |
| Microencapsulated pomegranate | Decreased triglyceridesIncreased PON1 activity | Higher synthesis of PON1 protein. | Woman with Acute Coronary Syndrome | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Vázquez, C.M.; Martínez-Ávila, M.; Guajardo-Flores, D.; Antunes-Ricardo, M. Punicic Acid and Its Role in the Prevention of Neurological Disorders: A Review. Foods 2022, 11, 252. https://doi.org/10.3390/foods11030252
Guerra-Vázquez CM, Martínez-Ávila M, Guajardo-Flores D, Antunes-Ricardo M. Punicic Acid and Its Role in the Prevention of Neurological Disorders: A Review. Foods. 2022; 11(3):252. https://doi.org/10.3390/foods11030252
Chicago/Turabian StyleGuerra-Vázquez, Claudia M., Mariana Martínez-Ávila, Daniel Guajardo-Flores, and Marilena Antunes-Ricardo. 2022. "Punicic Acid and Its Role in the Prevention of Neurological Disorders: A Review" Foods 11, no. 3: 252. https://doi.org/10.3390/foods11030252
APA StyleGuerra-Vázquez, C. M., Martínez-Ávila, M., Guajardo-Flores, D., & Antunes-Ricardo, M. (2022). Punicic Acid and Its Role in the Prevention of Neurological Disorders: A Review. Foods, 11(3), 252. https://doi.org/10.3390/foods11030252