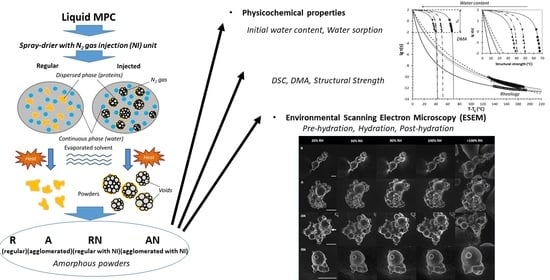
The Effect of High Protein Powder Structure on Hydration, Glass Transition, Water Sorption, and Thermomechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Manufacture of Milk Protein Concentrate Powders
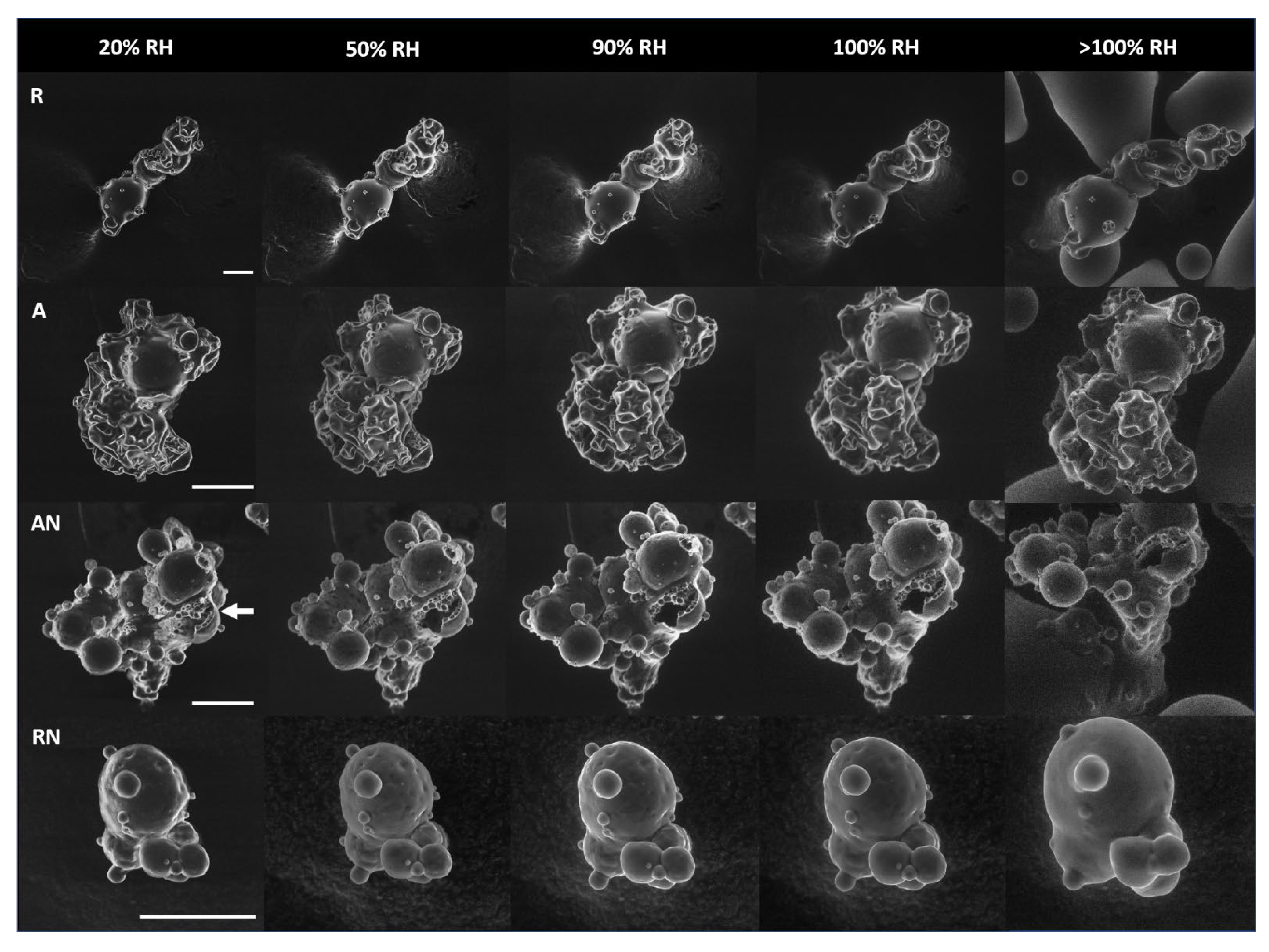
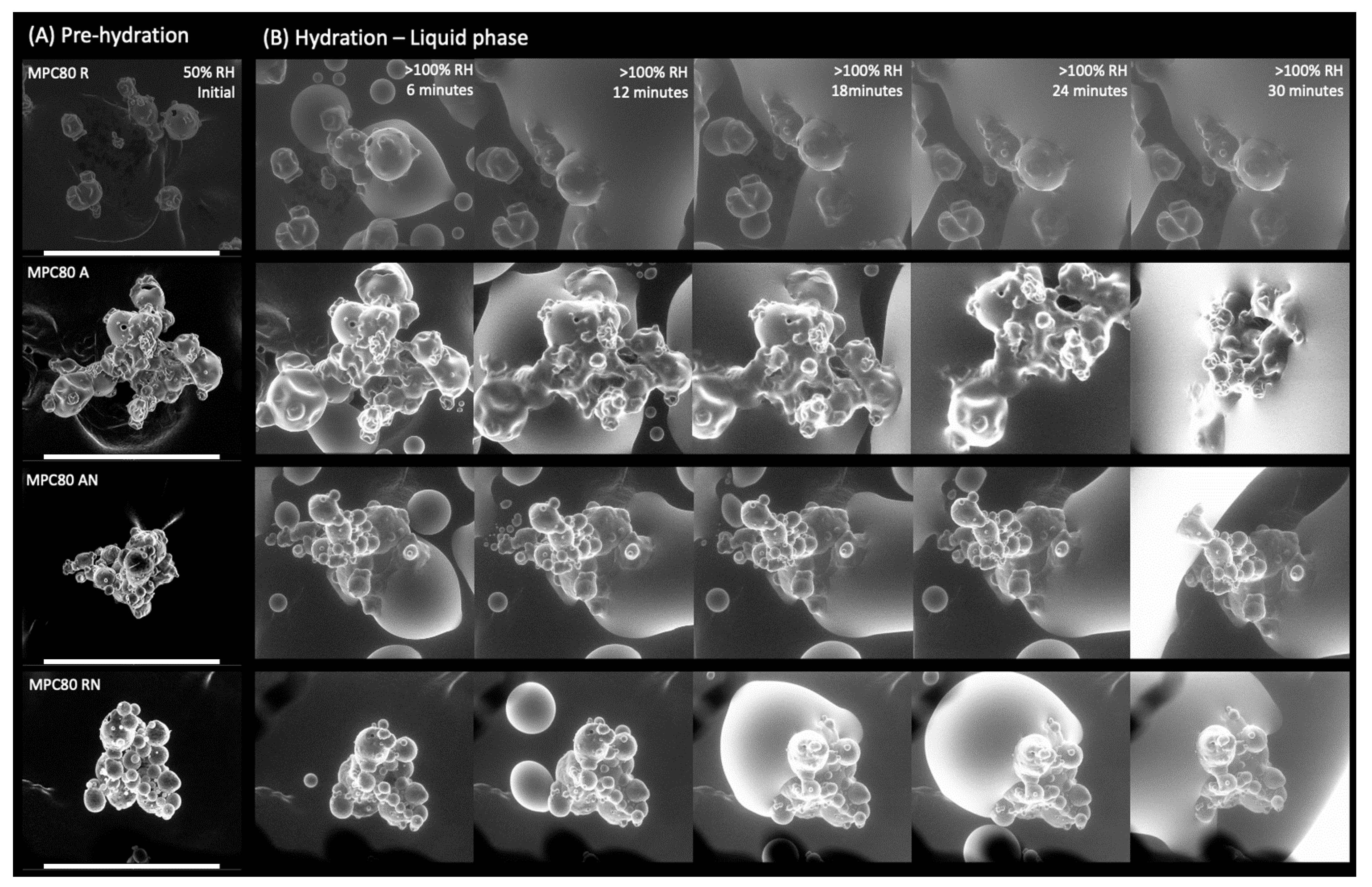
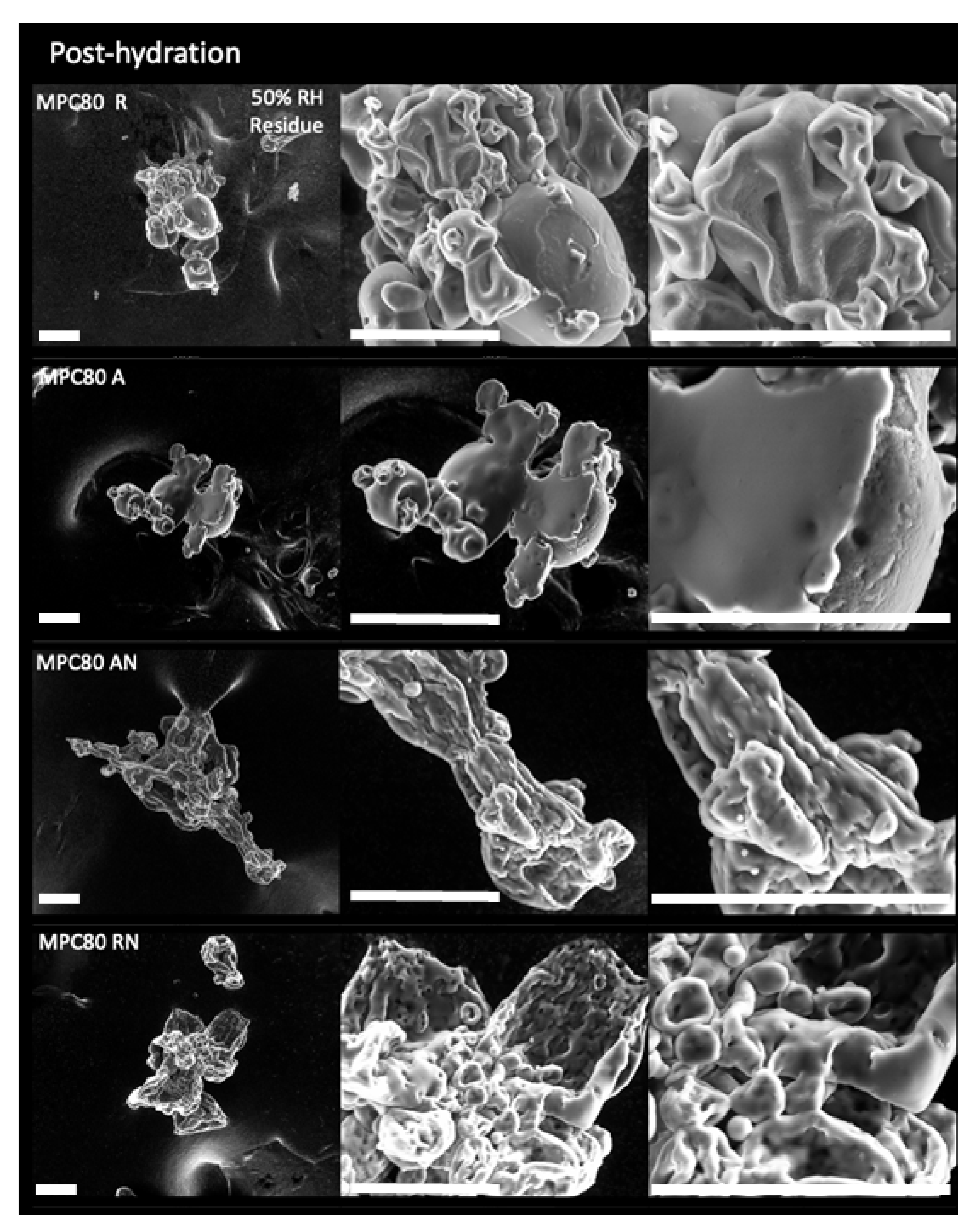
2.2. Environmental Scanning Electron Microscopy
2.3. Initial Water Content (IWC) Determination
2.4. Differential Scanning Calorimetry (DSC)
2.5. Dynamical Mechanical Analyses (DMA)
2.6. Rheology of MPC Dispersions
2.7. Calculation of the Williams–Landel–Ferry (WLF) Model Constants and Structural Strength Parameter
2.8. Data Analysis
3. Results and Discussions
3.1. Environmental Scanning Electron Microscopy
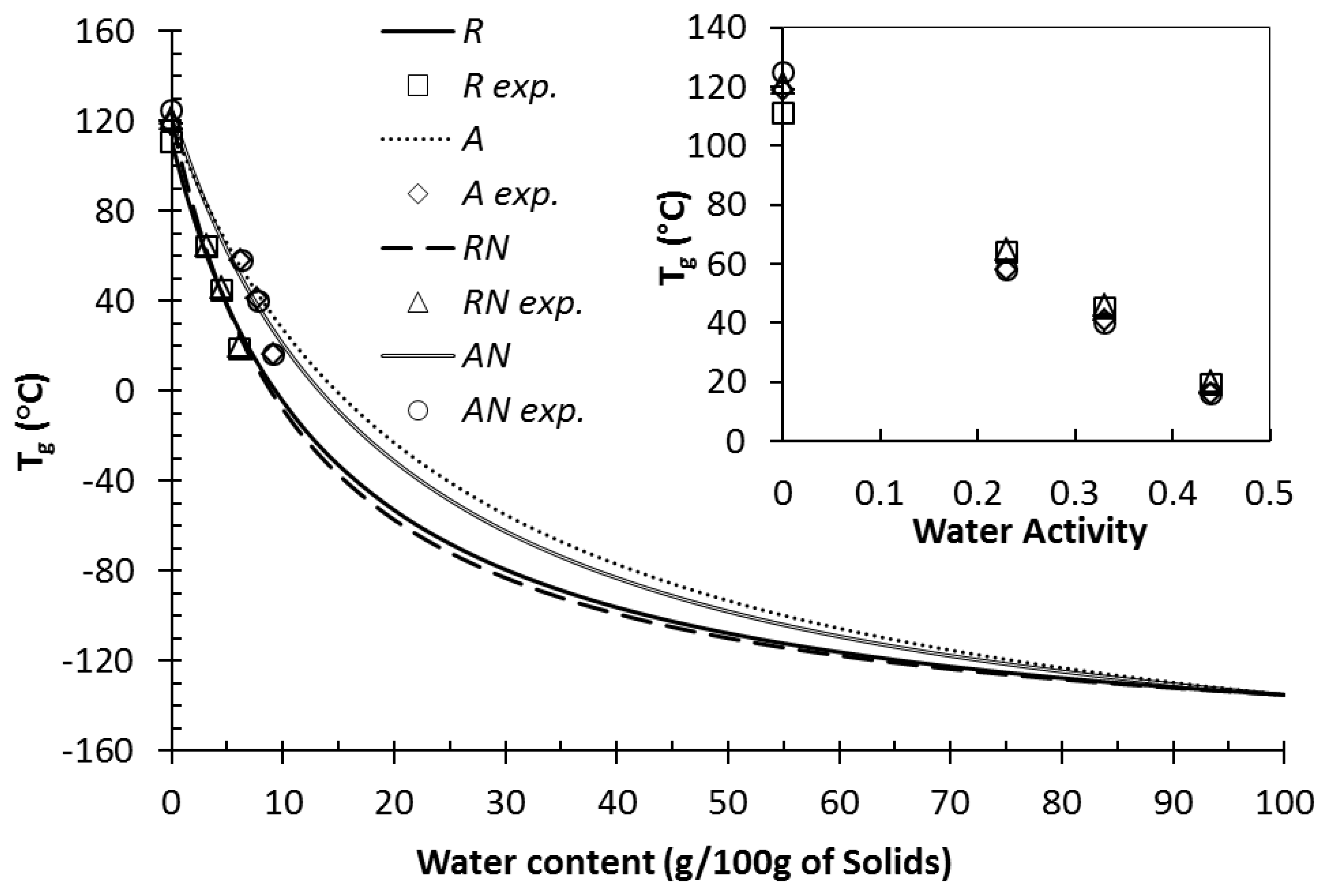
3.2. Water Content and Glass Transition Temperature of Anhydrous and Humidified Systems
3.3. Dynamic-Mechanical Properties
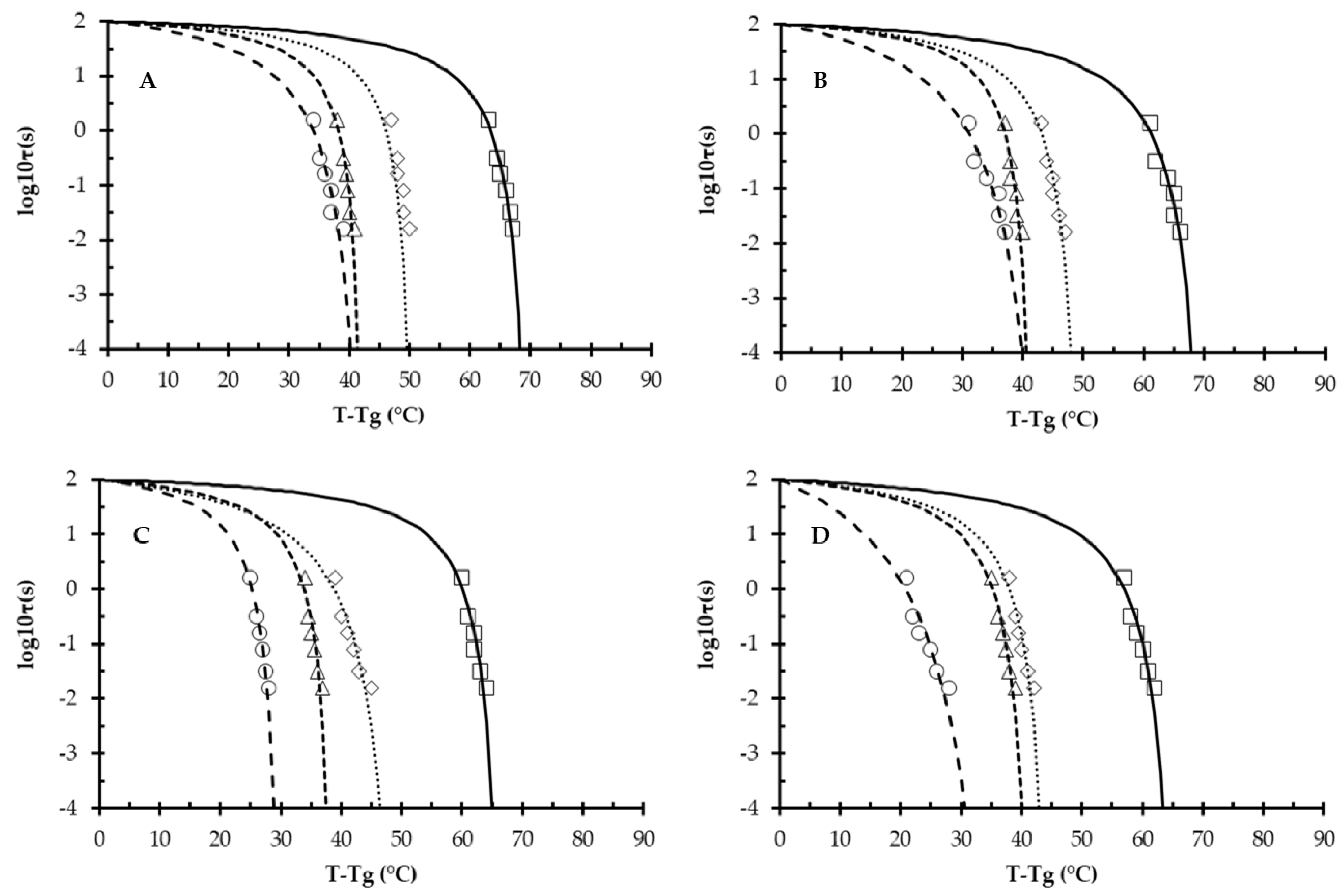
3.4. Williams–Landel–Ferry (WLF) Modeling and Structural Strength (S)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Baldwin, A.J.; Truong, G.N.T. Development of insolubility in dehydration of dairy milk powders. Food Bioprod. Process. 2007, 85, 202–208. [Google Scholar] [CrossRef]
- Jimenez-Flores, R.; Kosikowski, F.V. Properties of ultrafiltered skim milk retentate powders. J. Dairy Sci. 1986, 69, 329–339. [Google Scholar] [CrossRef]
- Schuck, P.; Piot, M.; Méjean, S.; Fauquant, J.; Brulé, G.; Maubois, J.L. Dehydration of an ultra-clean milk and micellar casein enriched milks. Lait 1994, 74, 47–63. [Google Scholar] [CrossRef]
- Dybing, S.T.; Bhaskar, G.V.; Dunlop, F.P.; Fayerman, A.M.; Whitton, M.J. Modified Milk Protein Concentrates and Their Use in Making Gels and Dairy Products. U.S. Patent 7,192,619, 20 March 2007. [Google Scholar]
- Anema, S.G.; Pinder, D.N.; Hunter, R.J.; Hemar, Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85). Food Hydrocoll. 2006, 20, 386–393. [Google Scholar] [CrossRef]
- Havea, P. Protein interactions in milk protein concentrate powders. Int. Dairy J. 2006, 16, 415–422. [Google Scholar] [CrossRef]
- Kelleher, C.M.; Tobin, J.T.; O’Mahony, J.A.; Kelly, A.L.; O’Callaghan, D.J.; McCarthy, N.A. A comparison of pilot-scale supersonic direct steam injection to conventional steam infusion and tubular heating systems for the heat treatment of protein-enriched skim milk-based beverages. Innov. Food Sci. Emerg. Technol. 2019, 52, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Pathania, S.; Ho, Q.T.; Hogan, S.A.; McCarthy, N.; Tobin, J.T. Applications of hydrodynamic cavitation for instant rehydration of high protein milk powders. J. Food Eng. 2018, 225, 18–25. [Google Scholar] [CrossRef]
- Hotchkiss, J.H.; Werner, B.G.; Lee, E.Y. Addition of carbon dioxide to dairy products to improve quality: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 158–168. [Google Scholar] [CrossRef]
- Marella, C.; Salunke, P.; Biswas, A.C.; Kommineni, A.; Metzger, L.E. Manufacture of modified milk protein concentrate utilizing injection of carbon dioxide. J. Dairy Sci. 2015, 98, 3577–3589. [Google Scholar] [CrossRef] [Green Version]
- Bouvier, J.M.; Collado, M.; Gardiner, D.; Scott, M.; Schuck, P. Physical and rehydration properties of milk protein concentrates: Comparison of spray-dried and extrusion-porosified powders. Dairy Sci. Technol. 2013, 93, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Kosasih, L.; Bhandari, B.; Prakash, S.; Bansal, N.; Gaiani, C. Effect of whole milk concentrate carbonation on functional, physicochemical and structural properties of the resultant spray dried powder during storage. J. Food Eng. 2016, 179, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Hanrahan, F.P.; Tamsma, A.; Fox, K.K.; Pallansch, M.J. Production and properties of spray-dried whole milk foam. J. Dairy Sci. 1962, 45, 27–31. [Google Scholar] [CrossRef]
- Bell, R.W.; Hanrahan, F.P.; Webb, B.H. Foam spray drying methods of making readily dispersible nonfat dry milk. J. Dairy Sci. 1963, 46, 1352–1356. [Google Scholar] [CrossRef]
- McSweeney, D.J.; Maidannyk, V.; O’Mahony, J.A.; McCarthy, N.A. Influence of nitrogen gas injection and agglomeration during spray drying on the physical and bulk handling properties of milk protein concentrate powders. J. Food Eng. 2021, 293, 110399. [Google Scholar] [CrossRef]
- Roos, Y.H. Water activity and glass transition. In Water Activity in Foods: Fundamentals and Applications; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 27–43. [Google Scholar]
- Roos, Y.H.; Fryer, P.J.; Knorr, D.; Schuchmann, H.P.; Schroën, K.; Schutyser, M.A.; Trystram, G.; Windhab, E.J. Food engineering at multiple scales: Case studies, challenges and the future—A European perspective. Food Eng. Rev. 2016, 8, 91–115. [Google Scholar] [CrossRef]
- Fan, F.; Roos, Y.H. Structural relaxations of amorphous lactose and lactose-whey protein mixtures. J. Food Eng. 2016, 173, 106–115. [Google Scholar] [CrossRef]
- Maidannyk, V. Strength Analysis for Understanding Structural Relaxations in Food Materials. Ph.D. Thesis, University College Cork, Cork, Ireland, 2017. [Google Scholar]
- Ubbink, J.; Dupas-Langlet, M. Rheology of carbohydrate blends close to the glass transition: Temperature and water content dependence of the viscosity in relation to fragility and strength. Food Res. Int. 2020, 138, 109801. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Maidannyk, V.A.; Roos, Y.H. Structural strength analysis of partially crystalline trehalose. LWT-Food Sci. Technol. 2018, 88, 9–17. [Google Scholar] [CrossRef]
- Maidannyk, V.A.; Lim, A.S.; Auty, M.A.E.; Roos, Y.H. Effects of lipids on the water sorption, glass transition and structural strength of carbohydrate-protein systems. Food Res. Int. 2019, 116, 1212–1222. [Google Scholar] [CrossRef] [Green Version]
- Lim, A.S.; Roos, Y.H. Amorphous wall materials properties and degradation of carotenoids in spray dried formulations. J. Food Eng. 2018, 223, 62–69. [Google Scholar] [CrossRef]
- Liu, W.; Roos, Y.H.; Fan, F. Physicochemical properties and relaxation time in strength analysis of amorphous poly (vinyl-pyrrolidone) and maltodextrin: Effects of water, molecular weight, and lactose addition. J. Food Eng. 2018, 226, 82–95. [Google Scholar] [CrossRef]
- Auty, M.A. Microscopy Techniques for Dairy Products—An Introduction. In Microstructure of Dairy Products; John Wiley & Sons: Hoboken, NJ, USA, 2018; p. 301. [Google Scholar]
- Cenini, V.L.; Gallagher, L.; McKerr, G.; McCarthy, N.A.; McSweeney, D.J.; Auty, M.A.E.; O’Hagan, B.M.G. A novel approach for dynamic in-situ surface characterisation of milk protein concentrate hydration and reconstitution using an environmental scanning electron microscope. Food Hydrocoll. 2020, 108, 105881. [Google Scholar] [CrossRef]
- Angell, C. Liquid fragility and the glass transition in water and aqueous solutions. Chem. Rev. 2002, 102, 2627–2650. [Google Scholar] [CrossRef]
- Silalai, N.; Roos, Y.H. Mechanical relaxation times as indicators of stickiness in skim milk–maltodextrin solids systems. J. Food Eng. 2011, 106, 306–317. [Google Scholar] [CrossRef]
- Potes, N.; Kerry, J.P.; Roos, Y.H. Additivity of water sorption, alpha-relaxations and crystallization inhibition in lactose–maltodextrin systems. Carbohydr. Polym. 2012, 89, 1050–1059. [Google Scholar] [CrossRef]
- Noel, T.R.; Parker, R.; Ring, S.G. Effect of molecular structure and water content on the dielectric relaxation behaviour of amorphous low molecular weight carbohydrates above and below their glass transition. Carbohydr. Res. 2000, 329, 839–845. [Google Scholar] [CrossRef]
- Roos, Y.H.; Drusch, S. Phase Transitions in Foods; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- McSweeney, D.J.; Maidannyk, V.; O’Mahony, J.A.; McCarthy, N.A. Rehydration properties of regular and agglomerated milk protein concentrate powders produced using nitrogen gas injection prior to spray drying. J. Food Eng. 2021, 305, 110597. [Google Scholar] [CrossRef]
- Ji, J.; Fitzpatrick, J.; Cronin, K.; Maguire, P.; Zhang, H.; Miao, S. Rehydration behaviours of high protein dairy powders: The influence of agglomeration on wettability, dispersibility and solubility. Food Hydrocoll. 2016, 58, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Rupp, L.S.; Molitor, M.S.; Lucey, J.A. Effect of processing methods and protein content of the concentrate on the properties of milk protein concentrate with 80% protein. J. Dairy Sci. 2018, 101, 7702–7713. [Google Scholar] [CrossRef]
- Menczel, J.D.; Judovits, L.; Prime, R.B.; Bair, H.E.; Reading, M.; Swier, S. Differential scanning calorimetry (DSC). Therm. Anal. Polym. Fundam. Appl. 2009, 7, 239. [Google Scholar]
- Haque, M.K.; Roos, Y.H. Differences in the physical state and thermal behavior of spray-dried and freeze-dried lactose and lactose/protein mixtures. Innov. Food Sci. Emerg. Technol. 2006, 7, 62–73. [Google Scholar] [CrossRef]
- Qiao RDeng, H.; Putz, K.W.; Brinson, L.C. Effect of particle agglomeration and interface on the glass transition temperature of polymer nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 740–748. [Google Scholar] [CrossRef]
- Kelly, G.M.; O’Mahony, J.A.; Kelly, A.L.; Huppertz, T.; Kennedy, D.; O’Callaghan, D.J. Influence of protein concentration on surface composition and physico-chemical properties of spray-dried milk protein concentrate powders. Int. Dairy J. 2015, 51, 34–40. [Google Scholar] [CrossRef]
- Cicerone, M.T.; Pikal, M.J.; Qian, K.K. Stabilization of proteins in solid form. Adv. Drug Deliv. Rev. 2015, 93, 14–24. [Google Scholar] [CrossRef] [Green Version]
- O’Donoghue, L.T.; Haque, M.; Hogan, S.A.; Laffir, F.R.; O’Mahony, J.A.; Murphy, E.G. Dynamic Mechanical Analysis as a Complementary Technique for Stickiness Determination in Model Whey Protein Powders. Foods 2020, 9, 1295. [Google Scholar] [CrossRef]
- Meinders, M.B.; van Vliet, T. Modeling water sorption dynamics of cellular solid food systems using free volume theory. Food Hydrocoll. 2009, 23, 2234–2242. [Google Scholar] [CrossRef]
- Peleg, M. On the use of the WLF model in polymers and foods. Crit. Rev. Food Sci. Nutr. 1992, 32, 59–66. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H.; Reid, D.S. Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Crit. Rev. Food Sci. Nutr. 1991, 30, 115–360. [Google Scholar] [CrossRef]
| RH % | Water Content (g/100 g of Dry Solids) | |||
|---|---|---|---|---|
| R | A | RN | AN | |
| IWC | 5.37 | 4.96 | 5.59 | 5.16 |
| 11% | 2.4 ± 0.58 | 4.8 ± 0.26 * | 2.7 ± 0.26 | 5.0 ± 0.37 * |
| 23% | 3.2 ± 0.01 | 6.2 ± 0.10 * | 3.2 ± 0.06 | 6.3 ± 0.12 * |
| 33% | 4.6 ± 0.02 | 7.7 ± 0.14 * | 4.5 ± 0.02 | 7.8 ± 0.15 * |
| 44% | 6.1 ± 0.05 | 9.1 ± 0.06 * | 6. 0 ± 0.01 | 9.2 ± 0.21 * |
| RH % | Glass Transition Temperature (Tg) of MPC Systems, °C | |||
|---|---|---|---|---|
| R | A | RN | AN | |
| 0 | 111 ± 5 | 119 ± 3 * | 121 ± 3 * | 125 ± 5 * |
| 23 | 64 ± 3 | 58 ± 3 * | 65 ± 2 | 58 ± 3 * |
| 33 | 45 ± 2 | 41 ± 5 * | 46 ± 1 | 40 ± 4 * |
| 44 | 19 ± 4 | 16 ± 3 * | 20 ± 4 | 16 ± 3 * |
| RH 0% | R | A | RN | AN | |
| f, Hz | logτ, s | Tα, °C | Tα, °C | Tα, °C | Tα, °C |
| 0.1 | 0.20 | 174 ± 7 | 180 ± 11 * | 181 ± 11 * | 182 ± 9 * |
| 0.5 | −0.49 | 176 ± 5 | 181 ± 7 * | 183 ± 9 * | 184 ± 11 * |
| 1.0 | −0.80 | 177 ± 6 | 183 ± 8 * | 184 ± 9 * | 185 ± 9 * |
| 5.0 | −1.50 | 178 ± 7 | 184 ± 5 * | 184 ± 7 * | 186 ± 9 * |
| 10.0 | −1.80 | 178 ± 6 | 185 ± 11 * | 185 ± 12 * | 187 ± 10 * |
| RH 23% | R | A | RN | AN | |
| f, Hz | logτ, s | Tα, °C | Tα, °C | Tα, °C | Tα, °C |
| 0.1 | 0.20 | 111 ± 5 | 101 ± 7 * | 104 ± 7 | 96 ± 4 * |
| 0.5 | −0.49 | 112 ± 4 | 102 ± 6 * | 105 ± 7 * | 97 ± 7 * |
| 1.0 | −0.80 | 112 ± 6 | 102 ± 5 * | 107 ± 6 | 98 ± 5 * |
| 5.0 | −1.50 | 113 ± 5 | 104 ± 4 * | 108 ± 6 | 99 ± 7 * |
| 10.0 | −1.80 | 114 ± 7 | 105 ± 5 * | 110 ± 7 | 100 ± 9 * |
| RH 33% | R | A | RN | AN | |
| f, Hz | logτ, s | Tα, °C | Tα, °C | Tα, °C | Tα, °C |
| 0.1 | 0.20 | 83 ± 5 | 78 ± 4 * | 80 ± 4 | 75 ± 5 * |
| 0.5 | −0.49 | 84 ± 4 | 79 ± 6 * | 81 ± 5 | 76 ± 4 * |
| 1.0 | −0.80 | 85 ± 5 | 79 ± 4 * | 82 ± 4 | 78 ± 5 * |
| 5.0 | −1.50 | 85 ± 6 | 80 ± 5 * | 82 ± 3 | 78 ± 6 * |
| 10.0 | −1.80 | 86 ± 5 | 81 ± 5 * | 83 ± 5 | 79 ± 5 * |
| RH 44% | R | A | RN | AN | |
| f, Hz | logτ, s | Tα, °C | Tα, °C | Tα, °C | Tα, °C |
| 0.1 | 0.20 | 53 ± 3 | 47 ± 5 * | 45 ± 3 * | 37 ± 4 * |
| 0.5 | −0.49 | 54 ± 5 | 48 ± 3 * | 46 ± 2 * | 38 ± 5 * |
| 1.0 | −0.80 | 55 ± 4 | 50 ± 4 | 47 ± 4 * | 39 ± 3 * |
| 5.0 | −1.50 | 56 ± 5 | 52 ± 4 | 47 ± 3 * | 42 ± 3 * |
| 10.0 | −1.80 | 58 ± 4 | 53 ± 5 | 48 ± 2 * | 44 ± 4 * |
| System | RH 0% | RH 23% | RH 33% | RH 44% | ||||
|---|---|---|---|---|---|---|---|---|
| -C1, s | -C2, °C | -C1, s | -C2, °C | -C1, s | -C2, °C | -C1, s | -C2, °C | |
| R | 0.2 ± 0.06 | 71.0 ± 3.2 | 0.2 ± 0.06 | 52.6 ± 2.7 * | 0.3 ± 0.09 | 43.5 ± 3.3 * | 0.6 ± 0.1 * | 44.1 ± 2.1 * |
| A | 0.3 ± 0.05 | 71.8 ± 3.7 | 0.4 ± 0.09 | 50.8 ± 2.5 * | 0.3 ± 0.11 | 42.8 ± 2.3 * | 1.0 ± 0.3 * | 46.5 ± 2.9 * |
| RN | 0.2 ± 0.05 | 67.6 ± 3.7 | 0.7 ± 0.08 * | 51.5 ± 2.2 * | 0.4 ± 0.09 | 39.9 ± 3.6 * | 0.5 ± 0.09 * | 31.5 ± 3.4 * |
| AN | 0.4 ± 0.09 | 67.2 ± 5.3 | 0.4 ± 0.08 | 45.9 ± 2.7 * | 0.4 ± 0.10 | 43.0 ± 2.4 * | 1.9 ± 0.2 * | 40.2 ± 2.4 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maidannyk, V.A.; McSweeney, D.J.; Montgomery, S.; Cenini, V.L.; O’Hagan, B.M.G.; Gallagher, L.; Miao, S.; McCarthy, N.A. The Effect of High Protein Powder Structure on Hydration, Glass Transition, Water Sorption, and Thermomechanical Properties. Foods 2022, 11, 292. https://doi.org/10.3390/foods11030292
Maidannyk VA, McSweeney DJ, Montgomery S, Cenini VL, O’Hagan BMG, Gallagher L, Miao S, McCarthy NA. The Effect of High Protein Powder Structure on Hydration, Glass Transition, Water Sorption, and Thermomechanical Properties. Foods. 2022; 11(3):292. https://doi.org/10.3390/foods11030292
Chicago/Turabian StyleMaidannyk, Valentyn A., David J. McSweeney, Sharon Montgomery, Valeria L. Cenini, Barry M. G. O’Hagan, Lucille Gallagher, Song Miao, and Noel A. McCarthy. 2022. "The Effect of High Protein Powder Structure on Hydration, Glass Transition, Water Sorption, and Thermomechanical Properties" Foods 11, no. 3: 292. https://doi.org/10.3390/foods11030292