Kinetic Study of Encapsulated β-Carotene Degradation in Aqueous Environments: A Review
Abstract
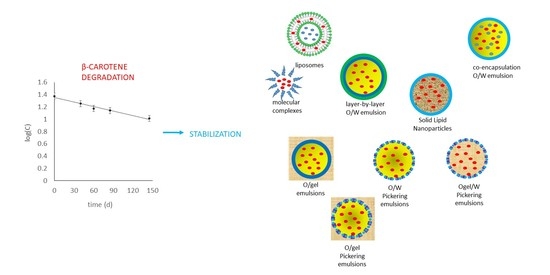
:1. Introduction
2. Methods
2.1. Literature Study
2.2. Modelling
3. Results
3.1. Rate of β-Carotene Degradation in Carrot Juice and Puree
3.2. Rate of Encapsulated β-Carotene Degradation in Water-Soluble Molecular Complexes
3.3. Rate of β-Carotene Degradation in Liposomes Dispersed in Water
3.4. Rate of Encapsulated β-Carotene Degradation in Oil-in-Water (O/W) and Oil-in-Gel (O/gel) Emulsions
3.5. Rate of Encapsulated β-Carotene Degradation in Solid Lipid (SL) Nanoparticles and in Nanostructured Lipid (NL) Carriers Dispersed in Water
3.6. Rate of Encapsulated β-Carotene Degradation in Oil-in-Water, Oleogel-in-Water, and Oil-in-Gel Pickering Emulsions
| Matrix Ingredients | Matrix Structure | PS | ζ-Pot | T | k × 103 | t1/2 | Ref. |
|---|---|---|---|---|---|---|---|
| carrot | pasteurized juice (90 °C, 60 s) | n.d. | n.d. | 4 | 21 | 33 | [34] |
| pasteurized juice (6 μs at 200 Hz, 35 kV cm−1 for 1500 μs) | n.d. | n.d. | 4 | 18 | 39 | ||
| carrot | pasteurized juice (180 MPa, 1 pass and 60 °C) | 68 | −31 | 4 | 18 | 39 | [38] |
| β-carotene, chitooligosaccharides | molecular complexes | 0.079 | 16.5 | 24 | 24 | 28 | [43] |
| β-carotene, zein | molecular complexes | ~0.4 | −32 | 20 | 67 | 10 | [40] |
| β-carotene, zein, carboxymethylchitosan | molecular complexes | ~0.1 | −34 | 20 | 48 | 14 | |
| β-carotene, zein, carboxymethylchitosan, tea polyphenols | molecular complexes | ~0.08 | −40 | 20 | 18 | 39 | |
| β-carotene, yolk lecithin, cholesterol | liposomes | 0.247 | −25 | 25 | n.d. | 6 | [48] |
| β-carotene, yolk lecithin, cholesterol, ascorbic acid | liposomes | 0.253 | −26 | 25 | n.d. | >30 | |
| β-carotene, medium-chain triglyceride oil, gum arabic | O/W emulsion | n.d. | n.d. | 25 | 95.5 | 7.2 | [49] |
| β-carotene, medium-chain triglyceride oil, gum arabic, ascorbyl palmitate | O/W emulsion | 0.614 | −23.8 | 25 | 13.9 | 50 | |
| β-carotene, medium-chain triglyceride oil, gum arabic, α-tocopherol | O/W emulsion | 0.644 | −26.6 | 25 | 14.3 | 48 | |
| β-carotene, medium-chain triglyceride oil, whey protein | O/W emulsion | 0.300 | −15 | 55 | n.d. | 3 | [53] |
| β-carotene, medium-chain triglyceride oil, whey protein, flaxseed gum | LBL O/W emulsion | 0.300 | −15 | 55 | n.d. | 6 | |
| β-carotene, corn oil, ferulic acid-conjugated curdlan | O/W emulsion | 3.62 | −30 | 25 | n.d. | 7 | [55] |
| β-carotene, sunflower oil, tartary buckwheat bran protein | O/W emulsion | 0.959 | −8.47 | 55 | n.d. | ~7 | [56] |
| β-carotene, sunflower oil, non-covalent tartary buckwheat bran protein–rutin complex | O/W emulsion | 0.265 | −28.7 | 55 | n.d. | ~14 | |
| β-carotene, sunflower oil, covalent tartary buckwheat bran protein–rutin complex | O/W emulsion | 0.243 | −32.7 | 55 | n.d. | >>28 | |
| β-carotene, camellia oil, low-methoxy pectin | O/W emulsion | 20 | ~22 | 4 | n.d. | ~4 | [57] |
| β-carotene, camellia oil, covalent low-methoxy pectin/soy peptide complex | O/W emulsion | 11 | ~18 | 4 | n.d. | >>28 | |
| β-carotene, camellia oil, covalent low-methoxy pectin/corn peptide complex | O/W emulsion | 17 | ~20 | 4 | n.d. | ~4 | |
| β-carotene, camellia oil, covalent low-methoxy pectin/whey protein peptide complex | O/W emulsion | ~14 | ~ 18 | 4 | n.d. | >>28 | |
| β-carotene, canola oil, Tween 80, alginate | O/W emulsion | n.d. | n.d. | 25 | n.d. | 48 | [61] |
| β-carotene, canola oil, Tween 80, alginate, calcium | O/gel emulsion | n.d. | n.d. | 25 | n.d. | 99 | |
| β-carotene, tristearin, Quillaja saponin | SL nanoparticles | <0.200 | n.d. | 25 | n.d. | >50 | [72] |
| β-carotene, tristearin, Quillaja saponin, high melting point lecithin | SL nanoparticles | <0.200 | n.d. | 25 | n.d. | >50 | |
| β-carotene, tristearin, Quillaja saponin, low melting point lecithin | SL nanoparticles | <0.200 | n.d. | 25 | n.d. | ~30 | |
| β-carotene, olive oil, Tween 80 | O/W emulsion | ~0.400 | −28 | 25 | n.d. | ~10 | [74]. |
| β-carotene, corn oil, Tween 80 | O/W emulsion | ~0.120 | −25 | 25 | n.d. | ~10 | |
| β-carotene, cocoa butter, Tween 80 | SL nanoparticles | ~0.200 | −39 | 25 | n.d. | ~40 | |
| β-carotene, hydrogenated coconut oil, Tween 80 | SL nanoparticles | ~0.200 | −33 | 25 | n.d. | ~10 | |
| β-carotene, medium-chain tryglicerides, Tween 80 | O/W emulsion | 0.106 | ~−30 | 25 | 170 | 4 | [75] |
| β-carotene, medium-chain triglycerides, 2% glyceryl stearate Tween 80 | NL carriers | 0.096 | ~−30 | 25 | 71 | 10 | |
| β-carotene, corn oil, gluten | O/W Pck emulsion | 9.4 | +22 | 25 | n.d. | >>30 | [77] |
| β-carotene, corn oil, gluten, xanthan gum | O/W Pck emulsion | 23.9 | −31 | 25 | n.d. | >>30 | |
| β-carotene, sunflower oil, medium-chain triglycerides, cellulose nanocrystals | O/W Pck emulsion | 3.14 | ~−42 | 55 | n.d. | ~5 | [78] |
| β-carotene, sunflower oil, medium-chain triglycerides, zein nanoparticles | O/W Pck emulsion | 5.11 | ~ +17 | 55 | n.d. | >28 | |
| β-carotene, sunflower oil, medium-chain triglycerides, 1/4 (w/w) zein nanoparticles/cellulose nanocrystals | O/W Pck emulsion | ~3.7 | ~−47 | 55 | n.d. | >28 | |
| β-carotene, soybean oil, cellulose nanocrystals | O/W Pck emulsion | 8.34 | n.d. | 25 | n.d. | 30 | [81] |
| β-carotene, soybean oil, beeswax, cellulose nanocrystals | Ogel/W Pck emulsion | 20.16 | n.d. | 25 | n.d. | 100 | |
| β-carotene, corn oil, gliadin | O/gel Pck emulsion | 4.9 | n.d. | 55 | n.d. | >28 | [84] |
| β-carotene, rosemary oil, chaperonin GroEL | O/W Pck emulsion | ~0.40 | −30 | 25 | n.d. | >35 | [85]. |
| Structure | Strategies to Increase β-Carotene Stability |
|---|---|
molecular complexes  | Use of carrier biopolymers with antioxidant activity to inhibit radical-mediated oxidation |
liposomes  | Co-encapsulation of antioxidant compounds to inhibit radical-mediated oxidation |
O/W emulsion  | Functionalization of the emulsifier to increase its oxygen barrier properties |
LBL O/W emulsion  | Addition of multiple biopolymer layers to decrease oxygen diffusion rate at the O/W interface |
O/gel emulsions  | Use of a gelled continuous phase to decrease oxygen diffusion rate at the air/continuous phase interface and at the continuous phase/oil droplet interface |
SL nanoparticles  | Use of a solid lipid phase to decrease oxygen diffusion rate through the core |
O/W Pck emulsions  | Use of colloidal surfactants to decrease oxygen diffusion rate at the O/W interface |
Ogel/W Pck emulsions  | Use of colloidal surfactants and an Ogel as a core to decrease oxygen diffusion rate at the Ogel/W interface and through the core |
O/gel Pck emulsions  | Use of colloidal surfactants and a gelled continuous phase to decrease oxygen diffusion rate at the air/continuous phase interface and at the continuous phase/oil droplet interface |
| Matrix Ingredients | Matrix Structure | T Range | Ea | Ref. |
|---|---|---|---|---|
| β-carotene, zein | molecular complexes | 4–60 | 13 (calculated) | [40] |
| β-carotene, zein, carboxymethyl chitosan | molecular complexes | 4–60 | 7 (calculated) | |
| β-carotene, zein, carboxymethyl chitosan, tea polyphenols | molecular complexes | 4–60 | 17 (calculated) | |
| β-carotene, medium-chain triglyceride oil, gum arabic, α-tocopherol | O/W emulsion | 4–65 | 49.8 | [49] |
| β-carotene, medium-chain triglyceride oil, gum arabic, ascorbyl palmitate | O/W emulsion | 4–65 | 45.7 | |
| β-carotene, canola oil, Tween 80, alginate | O/W emulsion | 4–37 | 51.1 | [61] |
| β-carotene, canola oil, Tween 80, alginate, calcium | O/gel emulsion | 4–37 | 47.6 | |
| β-carotene, medium-chain tryglicerides, Tween 80 | O/W emulsion | 4–35 | 58 (calculated) | [75] |
| β-carotene, medium chain tryglicerides, glyceryl stearate, Tween 80 | SL nanoparticles | 4–35 | 20 (calculated) |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Awasthi, S.; Awasthi, A. Role of vitamin A in child health and nutrition. Clin. Epidemiol. Glob. Health 2020, 8, 1039–1042. [Google Scholar] [CrossRef]
- Sherwin, J.C.; Reacher, M.H.; Dean, W.H.; Ngondi, J. Epidemiology of vitamin A deficiency and xerophthalmia in at-risk populations Epidemiology of vitamin A deficiency and xerophthalmia in at-risk populations. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qian, N.; Yan, L.; Jiang, H. Role of serum vitamin A and E in pregnancy. Exp. Ther. Med. 2018, 16, 5185–5189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meléndez-Martínez, A.J.; Böhm, V.; Borge, G.I.A.; Cano, M.P.; Fikselová, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandić, A.I.; Mapelli-Brahm, P.; et al. Carotenoids: Considerations for their use in functional foods, nutraceuticals, nutricosmetics, supplements, botanicals, and novel foods in the context of sustainability, circular economy, and climate change. Ann. Rev. Food Sci. Tech. 2021, 12, 433–460. [Google Scholar] [CrossRef]
- Maiani, G.; Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53 (Suppl. S12), S194–S218. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.; Biesalski, H.K. β-Carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [Green Version]
- Melendez-Martinez, A.J. An overview of carotenoids, apocarotenoids and vitamin A in agro-food, nutrition, health and disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [Green Version]
- Springmann, M.; Wiebe, K.; Mason-D’Croz, D.; Sulser, T.B.; Rayner, M.; Scarborough, P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: A global modelling analysis with country-level detail. Lancet Planet. Health 2018, 2, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Lavelli, V. Circular food supply chains—Impact on value addition and safety. Trends Food Sci. Technol. 2021, 114, 323–332. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents and research needs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–51. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites: A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palouck, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; McClements, D.J. Improving the bioavailability of oil-soluble vitamins by optimizing food matrix effects: A review. Food Chem. 2021, 348, 129148. [Google Scholar] [CrossRef] [PubMed]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Applying pulsed electric fields to whole carrots enhances the bioaccessibility of carotenoid and phenolic compounds in derived products. Foods 2021, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Elik, A.; Yanık, D.K.; Gogüs¸, F. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. LWT-Food Sci. Technol. 2020, 123, 109100. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Civan, M.; Kumcuoglu, S. Green ultrasound-assisted extraction of carotenoid and capsaicinoid from the pulp of hot pepper paste based on the bio-refinery concept. LWT-Food Sci. Technol. 2019, 113, 108320. [Google Scholar] [CrossRef]
- Patel, A.S.; Kar, A.; Dash, S.; Dash, S.K. Supercritical fluid extraction of β-carotene from ripe bitter melon pericarp. Sci. Rep. 2019, 9, 19266. [Google Scholar] [CrossRef]
- Del Pilar Sánchez-Camargo, A.; Gutierrez, L.F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narvaez-Cuenca, C.E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Mustafa, A.; Trevino, L.M.; Turner, C. Pressurized hot ethanol extraction of carotenoids from carrot by-products. Molecules 2012, 17, 1809–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara-Abia, S.; Gomez-Maqueo, A.; Welti-Chanes, J.; Pilar Cano, M. High hydrostatic pressure-assisted extraction of carotenoids from papaya (Carica papaya L. cv. Maradol) tissues using soybean and sunflower oil as potential green solvents. Food Eng. Rev. 2021, 13, 660–675. [Google Scholar] [CrossRef]
- Jayesree, N.; Hang, P.K.; Priyangaa, A.; Krishnamurthy, N.P.; Ramanan, R.N.; Turki, M.S.A.; Galanakis, M.C.; Ooi, C.W. Valorisation of carrot peel waste by water-induced hydrocolloidal complexation for extraction of carrot and pectin. Chemosphere 2021, 272, 129919. [Google Scholar] [CrossRef]
- De Freitas Santos, P.D.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef]
- Rehman, A.Q.T.; Jafari, S.M.; Assadpour, E.Q.S.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Sajid, B.; Mushtaq, W.A. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control Release 2019, 298, 38–67. [Google Scholar] [CrossRef]
- Martin, D.; Amado, A.M.; Gonzálvez, A.G.M.; Marques, P.M.; de Carvalho, L.A.E.B.; González Ureña, A. FTIR Spectroscopy and DFT calculations to probe the kinetics of β-carotene thermal degradation. J. Phys. Chem. A 2019, 123, 5266–5273. [Google Scholar] [CrossRef]
- Knockaert, G.; Pulissery, S.K.; Lemmens, L.; Buggenhout, S.V.; Hendrickx, M.; Loey, A.V. Carrot beta-carotene degradation and isomerization kinetics during thermal processing in the presence of oil. J. Agric. Food Chem. 2012, 60, 10312–10319. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Colle, I.J.P.; Lemmens, L.; Knockaert, G.; Loey, V.A.; Henderickx, M. Carotene degradation and isomerization during thermal processing: A review on kinetic aspects. Crit. Rev. Food Sci. Nutr. 2016, 56, 1844–1855. [Google Scholar] [CrossRef]
- Mordi, R.C. Mechanism of β-carotene degradation. Biochem. J. 1993, 292, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Huang, J.H. Degradation and isomerization of chlorophyll a and β-carotene as affected by various heating and illumination treatments. Food Chem. 1998, 62, 299–307. [Google Scholar] [CrossRef]
- Earle, R.L.; Earle, M.D. Unit Operations in Food Processing (Web ed.); Pergamon Press: Oxford, UK, 2004; Available online: https://www.nzifst.org.nz/resources/unitoperations/index.htm (accessed on 14 January 2022).
- Quitao-Teixeira, L.J.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Mota-Ramosa, A.; Martın-Belloso, O. Comparative study on antioxidant properties of carrot juice stabilised by high-intensity pulsed electric fields or heat treatments. J. Sci. Food Agric. 2009, 89, 2636–2642. [Google Scholar] [CrossRef]
- Wang, H.; Fang, X.-M.; Sutar, P.P.; Meng, J.S.; Wang, J.; Yu, X.-L.; Xiao, H.-W. Effects of vacuum-steam pulsed blanching on drying kinetics, colour, phytochemical contents, antioxidant capacity of carrot and the mechanism of carrot quality changes revealed by texture, microstructure and ultrastructure. Food Chem. 2021, 338, 127799. [Google Scholar] [CrossRef]
- European Chilled Food Federation. Recommendations for the Production of Prepackaged Chilled Food. 2006. Available online: https://www.ecff.net/wp-content/uploads/2018/10/ECFF_Recommendations_2nd_ed_18_12_06.pdf (accessed on 14 January 2022).
- Stinco, C.M.; Szczepańska, J.; Marszałek, K.; Pinto, C.A.; Inácio, R.S.; Mapelli-Brahm, P.; Barba, F.J.; Lorenzo, J.M.; Saraiva, J.A.; Meléndez-Martínez, A.J. Effect of high-pressure processing on carotenoids profile, colour, microbial and enzymatic stability of cloudy carrot juice. Food Chem. 2019, 299, 125112. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Bi, J.; Cao, F.; Ding, Y.; Peng, Y. Effects of high pressure homogenization on physical stability and carotenoid degradation kinetics of carrot beverage during storage. J. Food Eng. 2019, 263, 63–69. [Google Scholar] [CrossRef]
- Ozturk, B. Nanoemulsions for food fortification with lipophilic vitamins: Production challenges, stability, and bioavailability. Eur. J. Lipid Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]
- Ba, C.; Fu, Y.; Niu, F.; Wang, M.; Jina, B.; Lia, Z.; Chena, G.; Zhang, H.; Lia, X. Effects of environmental stresses on physiochemical stability of β-carotene in zein-carboxymethyl chitosan-tea polyphenols ternary delivery system. Food Chem. 2020, 331, 125878. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bockuviene, A.; Sereikaite, J. Preparation and characterisation of novel water-soluble β-carotene-chitooligosaccharides complexes. Carbohydr. Polym. 2019, 225, 115226. [Google Scholar] [CrossRef]
- Bockuviene, A.; Sereikaite, J. New β-carotene chitooligosaccharides complexes for food fortification: Stability study. Foods 2020, 9, 765. [Google Scholar] [CrossRef]
- Hu, K.; McClements, D.J. Fabrication of surfactant-stabilized zein nanoparticles: A pH modulated antisolvent precipitation method. Food Res. Int. 2014, 64, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, Y.Y.; Chen, G.W.; Shi, Y.G.; Li, X.M.; Zhang, H.; Shen, Y.L. Fabrication and characterization of carboxymethyl chitosan and tea polyphenols coating on zein nanoparticles to encapsulate β-carotene by anti-solvent precipitation method. Food Hydrocoll. 2018, 77, 577–587. [Google Scholar] [CrossRef]
- Taylor, T.M.; Weiss, J.; Davidson, P.M.; Bruce, B.D. Liposomal nanocapsules in food science and agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.M.; Morris, R.M. Thermal analysis of phase transition behavior in liposomes. Thermochim. Acta 1995, 248, 289–301. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zoub, Y.-X.; Luo, Z.-G.; Tamere, T.M. Co-encapsulation of vitamin C and β-carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Z.; Yang, J.; Gao, Y. Effects of antioxidants on the stability of β-carotene in O/W emulsions stabilized by Gum Arabic. J. Food Sci. Technol. 2015, 52, 3300–3311. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.S.; Decker, E.A.; McClements, D.J. Production and characterization of oil-in-water emulsions containing droplets stabilized by multilayer membranes consisting of β-lactoglobulin, k-carrageenan and gelatin. Langmuir 2005, 21, 5752–5760. [Google Scholar] [CrossRef]
- Shaw, L.A.; McClements, D.J.; Decker, E.A. Spray-dried multilayered emulsions as a delivery method for x-3 fatty acids into food systems. J. Agric. Food Chem. 2007, 55, 3112–3119. [Google Scholar] [CrossRef]
- Tikekar, R.V.; Nitin, A.J.N. Real-time measurement of oxygen transport across an oil–water emulsion interface. J. Food Eng. 2011, 103, 14–20. [Google Scholar] [CrossRef]
- Xu, D.; Qi, Y.; Wang, X.; Li, X.; Wang, S.; Cao, Y.; Wang, C.; Sun, B.; Decker, E.; Panya, A. The influence of flaxseed gum on the microrheological properties and physicochemical stability of whey protein stabilized β-carotene emulsions. Food Funct. 2017, 8, 415–423. [Google Scholar] [CrossRef]
- Cai, W.D.; Zhu, J.; Wu, L.X.; Qiao, Z.R.; Li, L.; Yan, J.K. Preparation, characterization, rheological and antioxidant properties of ferulic acid-grafted curdlan conjugates. Food Chem. 2019, 300, 125221. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cai, W.; Wang, Z.; Yan, J. Emulsifying properties of a ferulic acid-grafted curdlan conjugate and its contribution to the chemical stability of β-carotene. Food Chem. 2021, 339, 128053. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Geng, S.; Chen, C.; Liang, G.; Liu, B. Preparation and characterization of β-carotene nanoemulsions stabilized by complexes of tartary buckwheat bran protein and rutin. J. Food Process Preserv. 2021, 45, e15961. [Google Scholar] [CrossRef]
- Luo, S.-Z.; Wu, X.-Z.; Pan, L.-H.; Zheng, Z.; Zhang, M. Pectin-peptide complexes ameliorated physicochemical stabilities and in vitro digestion abilities of β-carotene loaded emulsions. Food Chem. 2021, 340, 128209. [Google Scholar] [CrossRef]
- Sun, J.; Liu, T.; Mu, Y.; Jing, H.; Obadi, M.; Xu, B. Enhancing the stabilization of β-carotene emulsion using ovalbumin-dextran conjugates as emulsifier. Colloids Surf. A Physicochem. Eng. Asp. 2022, 626, 126806. [Google Scholar] [CrossRef]
- Pénicaud, C.; Guilbert, S.; Peyron, S.; Gontard, N.; Guillard, V. Oxygen transfer in foods using oxygen luminescence sensors: Influence of oxygen partial pressure and food nature and composition. Food Chem. 2010, 123, 1275–1281. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjåk-Bræk, G.; Stokke, B.T. Similarities and differences between alginic acid gels and ionically crosslinked alginate gels. Food Hydrocoll. 2006, 20, 170–175. [Google Scholar] [CrossRef]
- Soukoulis, C.; Cambier, S.; Hoffmann, L.; Bohn, T. Chemical stability and bioaccessibility of β-carotene encapsulated in sodium alginate o/w emulsions: Impact of Ca2+ mediated gelation. Food Hydrocoll. 2016, 57, 301–310. [Google Scholar] [CrossRef]
- Silva, K.C.G.; Feltre, G.; Hubinger, M.D.; Sato, A.C.K. Protection and targeted delivery of β-carotene by starch-alginate-gelatin emulsion-filled hydrogels. J. Food Eng. 2021, 290, 110205. [Google Scholar] [CrossRef]
- MacArtain, P.; Jacquier, J.C.; Dawson, K.A. Physical characteristics of calcium induced k-carrageenan networks. Carbohydr. Polym. 2003, 53, 395–400. [Google Scholar] [CrossRef]
- Mangione, M.R.; Giacomazza, D.; Bulone, D.; Martorana, V.; Cavallaro, G.; San Biagio, P.L. K+ and Na+ effects on the gelation properties of k-carrageenan. Biophys. Chem. 2005, 113, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Mangione, M.R.; Giacomazza, D.; Bulone, D.; Martorana, V.; San Biagio, P.L. Thermoreversible gelation of j-carrageenan: Relation between conformational transition and aggregation. Biophys. Chem. 2003, 104, 95–105. [Google Scholar] [CrossRef]
- Soukoulis, C.; Tsevdou, M.; Andre, C.M.; Cambier, S.; Yonekura, L.; Taoukis, P.S.; Hoffmann, L. Modulation of chemical stability and in vitro bioaccessibility of beta-carotene loaded in kappa-carrageenan oil-in-gel emulsions. Food Chem. 2017, 220, 208–218. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef]
- Weiss, J.; Decker, E.A.; McClements, D.J.; Kristbergsson, K.; Helgason, T.; Awad, T. Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophys. 2008, 3, 146–154. [Google Scholar] [CrossRef]
- Cornacchia, L.; Roos, Y.H. State of dispersed lipid carrier and interface composition as determinants of beta-carotene stability in oil-in-water emulsions. J. Food Sci. 2011, 76, C1211–C1218. [Google Scholar] [CrossRef]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; Decker, E.A.; McClements, D.J.; Weiss, J. Impact of surfactant properties on oxidative stability of β-carotene encapsulated within solid lipid nanoparticles. J. Agric. Food Chem. 2009, 57, 8033–8040. [Google Scholar] [CrossRef]
- Salminen, H.; Gömmel, C.; Leuenberger, B.H.; Weiss, J. Influence of encapsulated functional lipids on crystal structure and chemical stability in solid lipid nanoparticles: Towards bioactive-based design of delivery systems. Food Chem. 2016, 190, 928–937. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Verkempinck, S.; Rijal, S.K.; Loey, A.V.; Grauwet, T.; Hendrickx, M. Lipid nanoparticles with fats or oils containing β-carotene: Storage stability and in vitro digestibility kinetics. Food Chem. 2019, 278, 396–405. [Google Scholar] [CrossRef]
- Hejri, A.; Khosravi, A.; Gharanjig, K.; Hejazi, M. Optimisation of the formulation of β-carotene loaded nanostructured lipid carriers prepared by solvent diffusion method. Food Chem. 2013, 141, 117–123. [Google Scholar] [CrossRef] [PubMed]
- De Abreu Martins, H.H.; Turmo-Ibarz, A.; Piccoli, R.H.; Martín-Belloso, O.; Salvia-Trujillo, L. Influence of lipid nanoparticle physical state on β-carotene stability kinetics under different environmental conditions. Food Funct. 2021, 12, 840–851. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-carotene in wheat gluten nanoparticle-xanthan gum stabilized Pickering emulsions: Enhancement of carotenoid stability and bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Z.; Guo, A.; Mackie, A.; Zhang, L.; Liao, W.; Mao, L.; Yuan, F.; Gao, Y. Zein colloidal particles and cellulose nanocrystals synergistic stabilization of Pickering emulsions for delivery β-carotene. J. Agric. Food Chem. 2021, 69, 12278–12294. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.M.; Barbut, S.; Marangoni, A.G. Edible oleogels for the oral delivery of lipid soluble molecules: Composition and structural design considerations. Trends Food Sci. Technol. 2016, 57, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.N.; Mao, L.; Lu, Y.; Yuan, F.; Gao, Y.X. Effect of monoglyceride content on the solubility and chemical stability of carotene in organogels. LWT-Food Sci. Technol. 2019, 106, 83–91. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, Z.; Wu, T. Encapsulation of β-carotene in oleogel-in-water Pickering emulsion with improved stability and bioaccessibility. Int. J. Biol. Macromol. 2020, 164, 1432–1442. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Arangoa, M.A.; Campanero, M.A.; Renedo, M.J.; Ponchel, G.; Irache, J.M. Gliadin nanoparticles as carriers for the oral administration of lipophilic drugs. Relationships between bioadhesion and pharmacokinetics. Pharm. Res. 2001, 18, 1521–1527. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, Y.; Wu, Z.; Miao, J.; Gao, H.; Ma, L.; Zou, L.; Peng, S.; Liu, C.; Liu, W. Gliadin nanoparticles Pickering emulgels for β-carotene delivery: Effect of particle concentration on the stability and bioaccessibility. Molecules 2020, 25, 4188. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, C.; Sun, H.; Wang, X.; Huang, F. Oil-in-water Pickering emulsions using a protein nano-ring as high-grade emulsifiers. Colloids Surf. B Biointerfaces 2020, 187, 110646. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavelli, V.; Sereikaitė, J. Kinetic Study of Encapsulated β-Carotene Degradation in Aqueous Environments: A Review. Foods 2022, 11, 317. https://doi.org/10.3390/foods11030317
Lavelli V, Sereikaitė J. Kinetic Study of Encapsulated β-Carotene Degradation in Aqueous Environments: A Review. Foods. 2022; 11(3):317. https://doi.org/10.3390/foods11030317
Chicago/Turabian StyleLavelli, Vera, and Jolanta Sereikaitė. 2022. "Kinetic Study of Encapsulated β-Carotene Degradation in Aqueous Environments: A Review" Foods 11, no. 3: 317. https://doi.org/10.3390/foods11030317