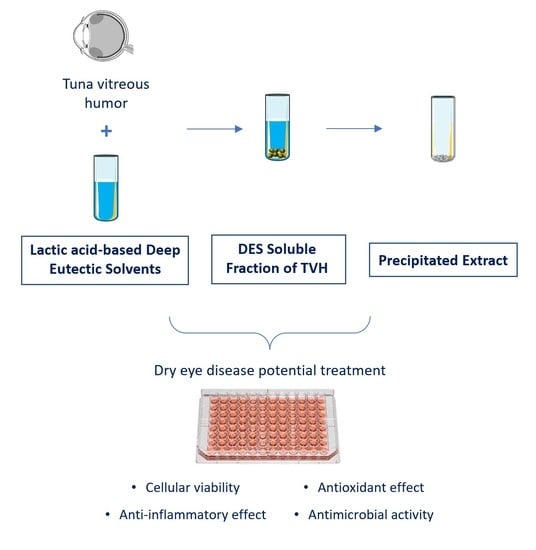
Potential Ophthalmological Application of Extracts Obtained from Tuna Vitreous Humor Using Lactic Acid-Based Deep Eutectic Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Raw Material Conditioning
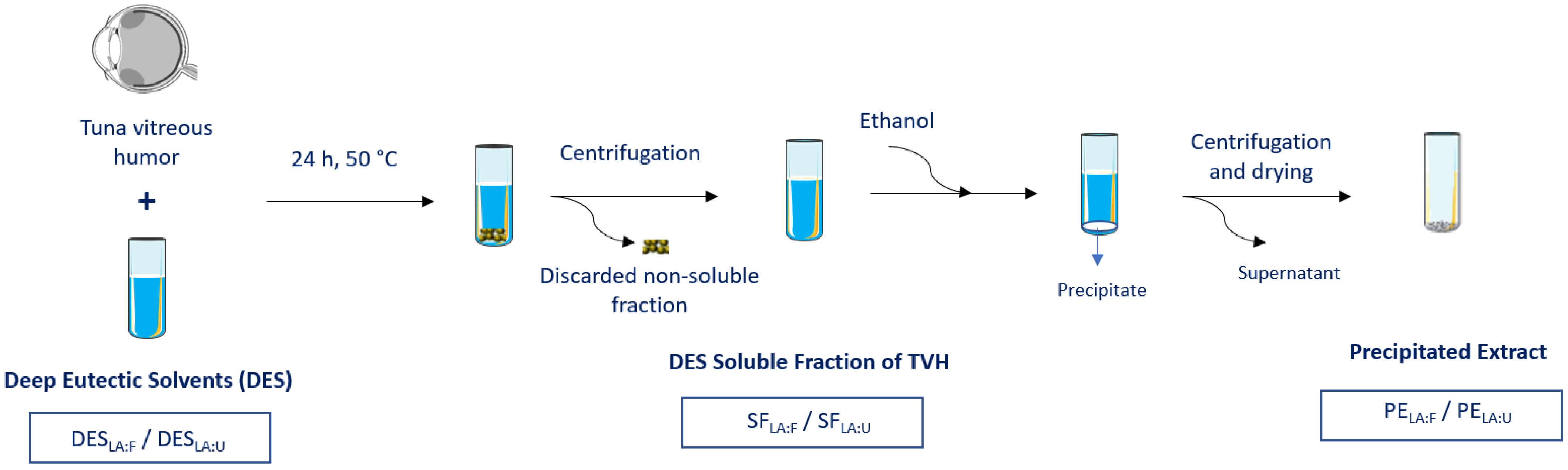
2.3. Extraction Process
2.4. HA Quantification
2.5. In Vitro Studies
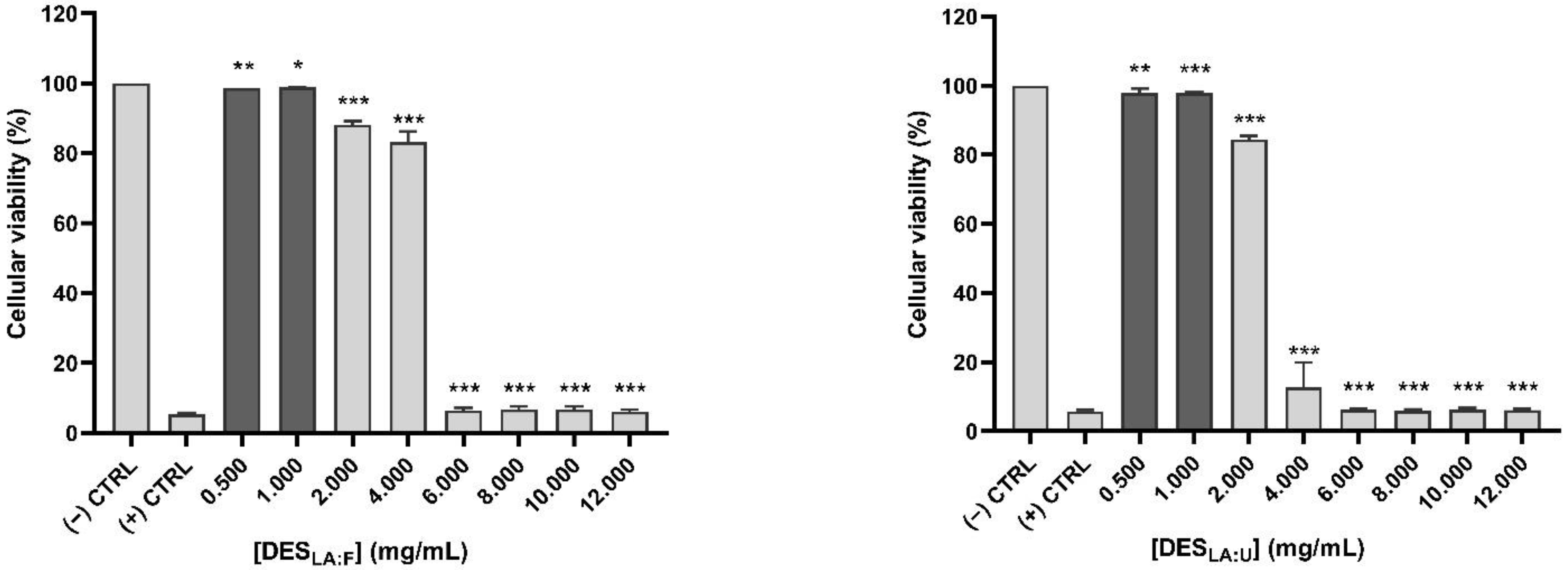
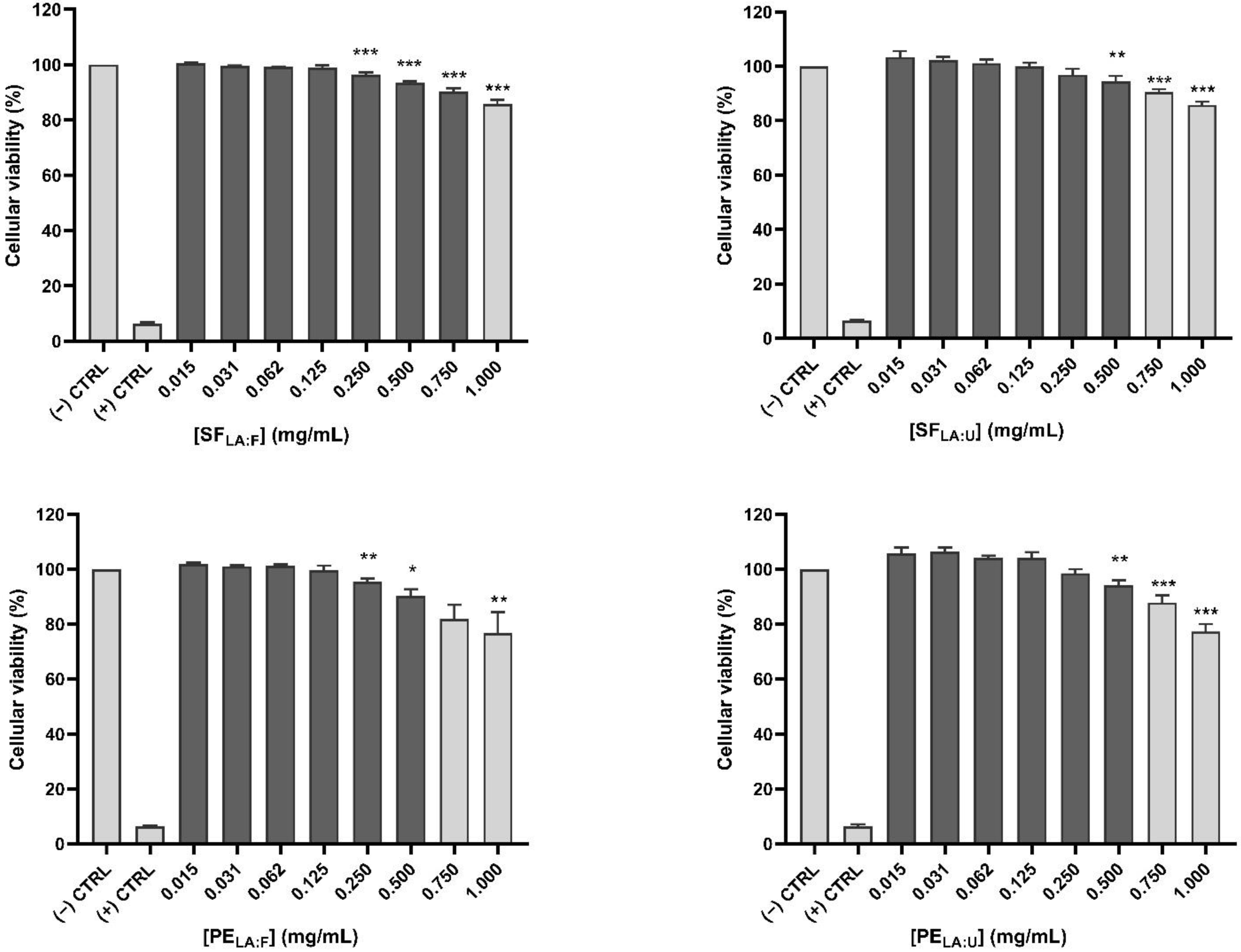
2.5.1. Cellular Viability
2.5.2. Antioxidant Effect
2.5.3. Evaluation of the Potential Inflammatory Response
2.5.4. Statistical Analysis
2.5.5. Antimicrobial Activity
3. Results and Discussion
3.1. Extraction Process
3.2. Cell Viability
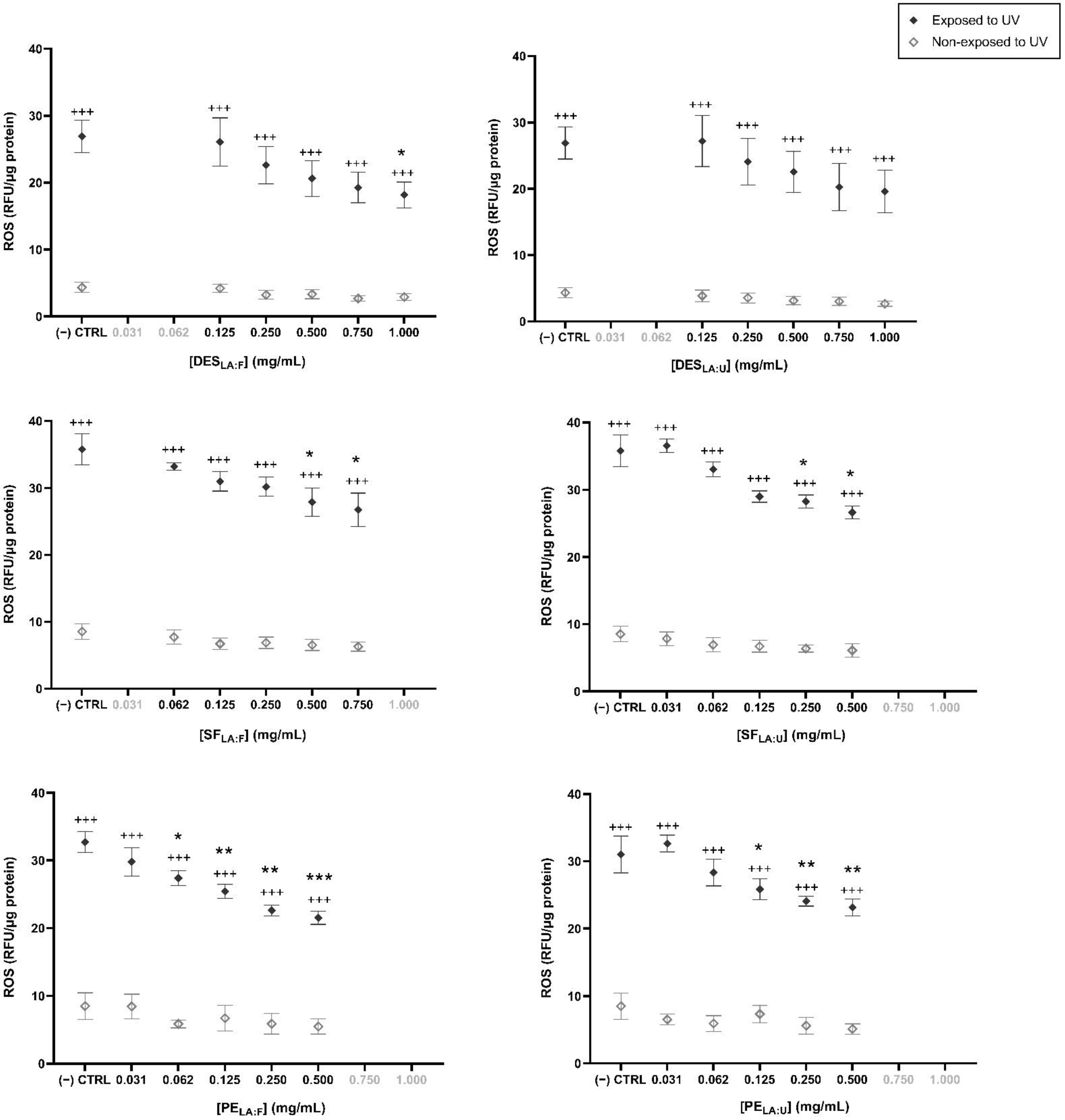
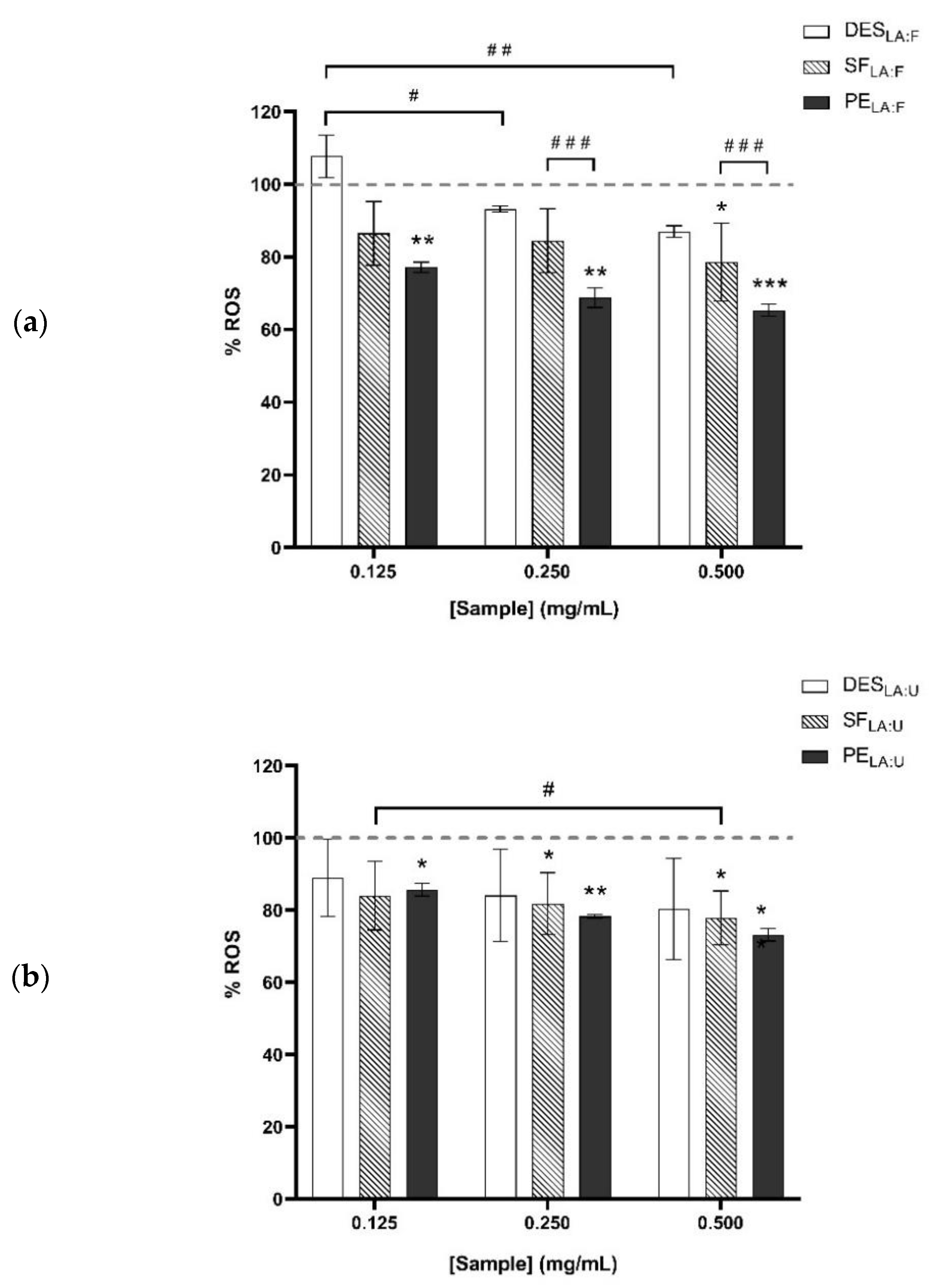
3.3. Antioxidant Effect
3.4. Evaluation of a Potential Inflammatory Response
3.5. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amberg, N.; Fogarassy, C. Green Consumer Behavior in the Cosmetics Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, C. Marine Natural Products in Medicinal Chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization. World Fisheries and Aquaculture. 2018. Available online: www.fao.org/publications (accessed on 27 August 2019).
- Govindharaj, M.; Roopavath, U.K.; Rath, S.N. Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J. Clean. Prod. 2019, 230, 412–419. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Mitigation of Food Wastage. Societal Costs and Benefits. 2013. Available online: https://www.fao.org/3/i3989e/i3989e.pdf (accessed on 15 December 2021).
- Abdallah, M.; Fernández, N.; Matias, A.A.; Bronze, M.D.R. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Shahbaz, K.; Bagh, F.S.G.; Mjalli, F.S.; AlNashef, I.M.; Hashim, M.A. Prediction of refractive index and density of deep eutectic solvents using atomic contributions. Fluid Phase Equilib. 2013, 354, 304–311. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of Improved Deep Eutectic Solvents Using Hole Theory. ChemPhysChem 2006, 7, 803–806. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pre-treatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef] [Green Version]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Rehmann, L.; Marzocchella, A. Deep Eutectic Solvents pre-treatment of agro-industrial food waste. Biotechnol. Biofuels 2018, 11, 37. [Google Scholar] [CrossRef]
- Chimphango, A.F.A.; Mugwagwa, L.R.; Swart, M. Extraction of Multiple Value-Added Compounds from Agricultural Biomass Waste: A Review. In Valorization of Biomass to Value-Added Commodities. Green Energy and Technology; Daramola, M., Ayeni, A., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Odeleye, T.; White, W.L.; Lu, J. Extraction techniques and potential health benefits of bioactive compounds from marine molluscs: A review. Food Funct. 2019, 10, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical application of glycosaminoglycans: A review. J. Tissue Eng. Regen. Med. 2018, 12, e23–e41. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Therapeutic Applications of Glycosaminoglycans. Curr. Med. Chem. 2006, 13, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Fondi, K.; Wozniak, P.A.; Schmidl, D.; Bata, A.M.; Witkowska, K.J.; Popa-Cherecheanu, A.; Schmetterer, L.; Garhöfer, G. Effect of Hyaluronic Acid/Trehalose in Two Different Formulations on Signs and Symptoms in Patients with Moderate to Severe Dry Eye Disease. J. Ophthalmol. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.J.; Lee, W.Y.; Kim, Y.J.; Hong, Y.P. A meta-analysis of the efficacy of hyaluronic acid eye drops for the treatment of dry eye syndrome. Int. J. Environ. Res. Public Health 2021, 18, 2383. [Google Scholar] [CrossRef]
- Limberg, M.B.; McCaa, C.; Kissling, G.E.; Kaufman, H.E. Topical application of hyaluronic acid and chondroitin sulfate in the treatment of dry eyes. Am. J. Ophthalmol. 1987, 103, 194–197. [Google Scholar] [CrossRef]
- Yokoi, N.; Komuro, A.; Nishida, K.; Kinoshita, S. Effectiveness of hyaluronan on corneal epithelial barrier function in dry eye. Br. J. Ophthalmol. 1997, 81, 533–536. [Google Scholar] [CrossRef] [Green Version]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- Labbé, A.; Wang, Y.X.; Jie, Y.; Baudouin, C.; Jonas, J.B.; Xu, L. Dry eye disease, dry eye symptoms and depression: The Beijing Eye Study. Br. J. Ophthalmol. 2013, 97, 1399–1403. [Google Scholar] [CrossRef]
- Brewitt, H.; Sistani, F. Dry Eye Disease: The Scale of the Problem. Surv. Ophthalmol. 2001, 45, S199–S202. [Google Scholar] [CrossRef]
- McCann, L.C.; Tomlinson, A.; Pearce, E.I.; Papa, V. Effectiveness of artificial tears in the management of evaporative dry eye. Cornea 2012, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4919. [Google Scholar] [CrossRef]
- Maccari, F.; Galeotti, F.; Volpi, N. Isolation and structural characterization of chondroitin sulfate from bony fishes. Carbohydr. Polym. 2015, 129, 143–147. [Google Scholar] [CrossRef]
- Volpi, N. Hyaluronic acid and chondroitin sulfate unsaturated disaccharides analysis by high-performance liquid chromatography and fluorimetric detection with dansylhydrazine. Anal. Biochem. 2000, 277, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Waitz, J.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Tenth; CLSI document No. M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1990. [Google Scholar]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Ang, B.C.H.; Sng, J.J.; Wang, P.X.H.; Htoon, H.M.; Tong, L.H.T. Sodium Hyaluronate in the Treatment of Dry Eye Syndrome: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, C.G.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents for the “green”extraction of vanillin from vanilla pods. Flavour Fragr. J. 2018, 33, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Şahin, S.; Kurtulbaş, E.; Bilgin, M. Special designed deep eutectic solvents for the recovery of high added-value products from olive leaf: A sustainable environment for bioactive materials. Prep. Biochem. Biotechnol. 2020, 51, 422–429. [Google Scholar] [CrossRef]
- Rondon-Berrios, H.; Tandukar, S.; Mor, M.K.; Ray, E.; Bender, F.H.; Kleyman, T.R.; Weisbord, S.D. Urea for the Treatment of Hyponatremia. Clin. J. Am. Soc. Nephrol. 2018, 13, 1627–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttunen, J.K. Fructose in medicine. Postgrad. Med. J. 1971, 47, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boomsma, B.; Bikker, E.; Lansdaal, E.; Stuut, P. L-Lactic Acid-A Safe Antimicrobial for Home-and Personal Care Formulations. SOFW J. 2015, 141, 2–5. [Google Scholar]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Yoshimura, K.; Inoue, Y.; Koizumi, A.; Suzuki, M.; Itakura, S. Effect of Lactic Acid Content into on during Miconazole Eye Drops to be Used for Infection Preventive. J. Drug Res. Dev. 2021, 7, 1–7. [Google Scholar] [CrossRef]
- Barclay, T.; Ginic-Markovic, M.; Cooper, P.; Petrovsky, N. The chemistry and sources of fructose and their effect on its utility and health implications. J. Excip. Food Chem. 2012, 3, 67–82. [Google Scholar]
- Schütte, E.; Reim, M. Fructose Metabolism of the Cornea. Ophthalmic Res. 1976, 8, 434–437. [Google Scholar] [CrossRef]
- Kinoshita, J.H. Physiological chemistry of the eye. Arch. Ophthalmol. 1962, 68, 554–570. [Google Scholar] [CrossRef]
- Galin, M.A.; Aizawa, F.; McLean, J.M. Urea as an Osmotic Ocular Hypotensive Agent in Glaucoma. AMA. Arch. Ophthalmol. 1959, 62, 347–352. [Google Scholar] [CrossRef]
- Gandhi, G.M.; Anasuya, S.R.; Kawathekar, P.; Bhaskarmall; Krishnamurthy, K.R. Urea in the management of advanced malignancies (Preliminary report). J. Surg. Oncol. 1977, 9, 139–146. [Google Scholar] [CrossRef]
- Fredriksson, T.; Gip, L. Urea creams in the treatment of dry skin and hand dermatitis. Int. J. Dermatol. 1975, 14, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Keisham, N.K.; Sharma, A. Correlation between Urea Levels in Lacrimal Fluid and Patho-Physiology of Dry Eye Syndromes. IOSR J. Dent. Med. Sci. 2020, 19, 29–34. [Google Scholar] [CrossRef]
- Jäger, K.; Kielstein, H.; Dunse, M.; Nass, N.; Paulsen, F.; Sel, S. Enzymes of urea synthesis are expressed at the ocular surface, and decreased urea in the tear fluid is associated with dry-eye syndrome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Catunda, R.-Q.; Vieira, J.-R.-C.; De Oliveira, E.-B.; Da Silva, E.-C.; Brasil, V.-L.-M.; Perez, D.-E.-D.C. Citotoxicity evaluation of three dental adhesives on vero cells in vitro. J. Clin. Exp. Dent. 2017, 9, e61. [Google Scholar] [CrossRef] [PubMed]
- Ahrari, F.; Tavakkol Afshari, J.; Poosti, M.; Brook, A. Cytotoxicity of orthodontic bonding adhesive resins on human oral fibroblasts. Eur. J. Orthod. 2010, 32, 688–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macri, A.; Scanarotti, C.; Bassi, A.M.; Giuffrida, S.; Sangalli, G.; Traverso, C.E.; Iester, M. Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative-free hyaluronic acid 0.15 % and vitamin B12 eye drops. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Nagata, T.; Kudo, H.; Müller-Lierheim, W.G.K.; Van Setten, G.-B.; Dogru, M.; Tsubota, K. The Effects of High Molecular Weight Hyaluronic Acid Eye Drop Application in Environmental Dry Eye Stress Model Mice. Int. J. Mol. Sci. 2020, 21, 3516. [Google Scholar] [CrossRef]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef] [Green Version]
- Roda, M.; Corazza, I.; Reggiani, M.L.B.; Pellegrini, M.; Taroni, L.; Giannaccare, G.; Versura, P. Dry Eye Disease and Tear Cytokine Levels—A Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3111. [Google Scholar] [CrossRef]
- Graham, J.E.; Moore, J.E.; Jiru, X.; Moore, J.E.; Goodall, E.A.; Dooley, J.; Hayes, V.E.A.; Dartt, D.A.; Downes, C.S.; Moore, T. Ocular Pathogen or Commensal: A PCR-Based Study of Surface Bacterial Flora in Normal and Dry Eyes. Investig. Opthalmol. Vis. Sci. 2007, 48, 5616–5623. [Google Scholar] [CrossRef]
- Pasricha, A.; Bhalla, P.; Sharma, K.B. Evaluation of Lactic Acid As an Antibacterial Agent. Indian J. Dermatol. Venereol. Leprol. 1979, 45, 159–161. [Google Scholar] [PubMed]
- Bucior, I.; Abbott, J.; Song, Y.; Matthay, M.A.; Engel, J.N. Sugar administration is an effective adjunctive therapy in the treatment of Pseudomonas aeruginosa pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozde Durmus, N.; Taylor, E.N.; Inci, F.; Kummer, K.M.; Tarquinio, K.M.; Webster, T.J. Fructose-enhanced reduction of bacterial growth on nanorough surfaces. Int. J. Nanomed. 2012, 7, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Atapi, N.E. Compararative Study on the Antibacterial Effect of Urea on Escherichia Coli and Staphylococcus Aureus. Ph.D. Dissertation, Makerere University, Kampala, Uganda, 2019. [Google Scholar]
- Cheng, H.M.; González, R.G.; Barnett, P.A.; Aguayo, J.B.; Wolfe, J.; Chylack, L.T. Sorbitol/fructose metabolism in the lens. Exp. Eye Res. 1985, 40, 223–229. [Google Scholar] [CrossRef]
- Chang, W.-H.; Liu, P.-Y.; Lin, M.-H.; Lu, C.-J.; Chou, H.-Y.; Nian, C.-Y.; Jiang, Y.-T.; Hsu, Y.-H. Applications of Hyaluronic Acid in Ophthalmology and Contact Lenses. Molecules 2021, 26, 2485. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
| Target Bacteria | Sample a | MIC (µL of Testing Sample/mL) | Sample Composition at MIC Value | MBC (µL of TESTING Sample/mL) | Sample Composition at MBC Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| [DES] (mg/mL) | [HA] (ng/mL) | [CHL] (µg/mL) | [DES] (mg/mL) | [HA] (ng/mL) | [CHL] (µg/mL) | ||||
| S. aureus | DESLA:F | 125 | 4 | — | — | 250 | 8 | — | — |
| DESLA:U | 250 | 8 | — | — | 500 | 16 | — | — | |
| SFLA:F | 250 | 8 | 2.30 | — | 250 | 8 | 2.30 | — | |
| SFLA:U | 250 | 4 | 0.67 | — | 500 | 16 | 2.70 | — | |
| PELA:F | >500 | — | >148,000 | — | >500 | — | >148,000 | — | |
| PELA:U | >500 | — | >76,000 | — | >500 | — | >76,000 | — | |
| HA-ED | >500 | — | >0.75 | — | >500 | — | >0.75 | — | |
| CHL-ED | 0.98 | — | — | 7.8 | 31.25 | — | — | 250 | |
| P. aeruginosa | DESLA:F | 125 | 4 | — | — | 125 | 4 | — | — |
| DESLA:U | 125 | 4 | — | — | 125 | 4 | — | — | |
| SFLA:F | 250 | 8 | 2.30 | — | 250 | 8 | 2.30 | — | |
| SFLA:U | 125 | 4 | 0.67 | — | 250 | 8 | 1.30 | — | |
| PELA:F | >500 | — | >148,000 | — | >500 | — | >148,000 | — | |
| PELA:U | >500 | — | >76,000 | — | >500 | — | >76,000 | — | |
| HA-ED | >500 | — | >0.75 | — | >500 | — | >0.75 | — | |
| CHL-ED | 15.63 | — | — | 125 | 15.63 | — | — | 125 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, M.M.; Leonardo, I.C.; Krstić, L.; Enríquez-de-Salamanca, A.; Diebold, Y.; González-García, M.J.; Gaspar, F.B.; Matias, A.A.; Bronze, M.R.; Fernández, N. Potential Ophthalmological Application of Extracts Obtained from Tuna Vitreous Humor Using Lactic Acid-Based Deep Eutectic Systems. Foods 2022, 11, 342. https://doi.org/10.3390/foods11030342
Abdallah MM, Leonardo IC, Krstić L, Enríquez-de-Salamanca A, Diebold Y, González-García MJ, Gaspar FB, Matias AA, Bronze MR, Fernández N. Potential Ophthalmological Application of Extracts Obtained from Tuna Vitreous Humor Using Lactic Acid-Based Deep Eutectic Systems. Foods. 2022; 11(3):342. https://doi.org/10.3390/foods11030342
Chicago/Turabian StyleAbdallah, Maha M., Inês C. Leonardo, Luna Krstić, Amalia Enríquez-de-Salamanca, Yolanda Diebold, María J. González-García, Frédéric B. Gaspar, Ana A. Matias, Maria Rosário Bronze, and Naiara Fernández. 2022. "Potential Ophthalmological Application of Extracts Obtained from Tuna Vitreous Humor Using Lactic Acid-Based Deep Eutectic Systems" Foods 11, no. 3: 342. https://doi.org/10.3390/foods11030342