Update on Biogenic Amines in Fermented and Non-Fermented Beverages
Abstract
:1. Introduction
2. Biogenic Amines in Fermented Alcoholic Beverages
2.1. Biogenic Amines’ Formation during the Winemaking Process
2.2. Biogenic Amine in Beer and Other Alcoholic Beverages
| Biogenic Amines (mg/L) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|
| Put | Cad | His | Spd | Spm | Tyr | Phe | Tryp | |
| 2.1–12.8 | 0.2–1.4 | nd-0.3 | 0.4–5.9 | tr-0.2 | Almeida et al., 2012 [44] | |||
| 1.6 | 1.0 | 0.9 | 2.1 | Matsheka et al., 2013 [45] | ||||
| 0.3–1.4 | 0.1–0.3 | nd-0.6 | 0.2–0.8 | 0.2–0.7 | nd-0.5 | nd-0.5 | 0.3–2.6 | Aflaki et al., 2014 [46] |
| 2.1–12.8 | 0.2–1.4 | nd-0.3 | 0.4–5.9 | Ordóñez et al., 2016 [47] | ||||
| nd-100.0 | nd-100.0 | nd-28.6 | nd-50.0 | nd-50.0 | nd->100.0 | nd-8.6 | nd-28.6 | Pradenas et al., 2016 [48] |
| 1.6–4.1 | 0.4–0.8 | nd | 0.1–58.3 | nd-0.4 | nd | Redruello et al., 2017 [49] | ||
| 3.6–8.9 | 0.0–1.3 | nd-5.7 | 0.0–4.0 | 0.9–6.5 | nd-0.3 | 0.1–0.5 | Poveda, 2019 [50] | |
| 22.4–72.2 | tr | nd | nd | tr-1.1 | tr-1.1 | nd | Angulo et al., 2020 [31] | |
| nd-11.4 | nd-12.7 | nd | nd | nd | nd | nd-6.3 | nd-4.1 | Bae et al., 2020 [51] |
| 1.5–8.2 | 0.2–1.4 | tr-0.8 | - | - | 0.4–6.0 | Bertuzzi et al., 2020 [52] | ||
| 3.2–7 | 0.6–1.1 | <LOQ-0.3 | <LOQ-1.4 | <LOQ->0.2 | Díaz-Liñán et al., 2021 [53] | |||
| 4.0–19 | 0.3–3.6 | 0.1–5.0 | 0.2–5.1 | <LOQ-0.8 | 0.4–31.7 | <LOQ-1.0 | <LOQ-76.6 | Nalazek-Rudnicka et al., 2021 [54] |
| 0.3 | 0.3 | 0.2 | 0.3 | 0.6 | 0.7 | 0.5 | 0.6 | Gil et al., 2022 [34] |
3. Biogenic Amine in Fermented Non-Alcoholic Beverages
4. Biogenic Amines in Non-Fermented Beverages
5. Harmful Effects of Biogenic Amines and Prevention Measures
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vasconcelos, H.; de Almeida, J.M.M.M.; Matias, A.; Saraiva, C.; Jorge, P.A.S.; Coelho, L.C.C. Detection of biogenic amines in several foods with different sample treatments: An overview. Trends Food Sci. Technol. 2021, 113, 86–96. [Google Scholar] [CrossRef]
- Ekici, K.; Omer, A.K. Biogenic amines formation and their importance in fermented foods. BIO Web Conf. 2020, 17, 00232. [Google Scholar] [CrossRef] [Green Version]
- Sahu, L.; Panda, S.K.; Paramithiotis, S.; Zdolec, N.; Ray, R.C. Biogenic amines in fermented foods: Overview. In Fermented Foods—Part I: Biochemistry and Biotechnology; Montet, D., Ramesh, C.R., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 318–332. [Google Scholar]
- Vinci, G.; Maddaloni, L. Biogenic amines in alcohol-free beverages. Beverages 2020, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Nalazek-Rudnicka, K.; Kubica, P.; Wasik, A. Discrepancies in determination of biogenic amines in beer samples by reversed phase and hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry. Microchem. J. 2020, 159, 105574. [Google Scholar] [CrossRef]
- Miranda, A.; Leça, J.M.; Pereira, V.; Marques, J.C. Analytical methodologies for the determination of biogenic amines in wines: An overview of the recent trends. J. Anal. Bioanal Sep. Tech. 2017, 2, 52–57. [Google Scholar]
- Restuccia, D.; Loizzo, M.R.; Spizzirri, U.G. Accumulation of biogenic amines in wine: Role of alcoholic and malolactic fermentation. Fermentation 2018, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Tristezza, M.; Vetrano, C.; Bleve, G.; Spano, G.; Capozzi, V.; Logrieco, A.; Mita, G.; Grieco, F. Biodiversity and safety aspects of yeast strains characterized from vineyards and spontaneous fermentations in the Apulia Region, Italy. Food Microbiol. 2013, 36, 335–342. [Google Scholar] [CrossRef]
- Andorrà, I.; Miró, G.; Espligares, N.; Mislata, A.M.; Puxeu, M.; Ferrer-Gallego, R. Wild yeast and lactic acid bacteria of wine. In Yeasts in Biotechnology; Peixoto Basso, T., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Čuš, F.; Česnik, H.B.; Bolta, Š.V. Pesticide residues, copper and biogenic amines in conventional and organic wines. Food Control 2022, 132, 108534. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Callejón, R.M.; Troncoso, A.M.; García–Parrilla, M.C. Evaluation of biogenic amines profile in opened wine bottles: Effect of storage conditions. J. Food Compos. Anal. 2017, 63, 139–147. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Simeonov, V.; Morrison, C.; Namieśnik, J. Impact of selected parameters of the fermentation process of wine and wine itself on the biogenic amines content: Evaluation by application of chemometric tools. Microchem. J. 2018, 142, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Costantini, A.; Vaudano, E.; Pulcini, L.; Carafa, T.; Garcia-Moruno, E. An overview of biogenic amines in wine. Beverages 2019, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.P.; Prats-Moya, M.S. Free amino acids and biogenic amines in Alicante Monastrell wines. Food Chem. 2012, 135, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Bach, B.; Le Quere, S.; Vuchot, P.; Grinbaum, M.; Barnavon, L. Validation of a method for the analysis of biogenic amines: Histamine instability during wine sample storage. Anal. Chim Acta 2012, 732, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Konakovsky, V.; Focke, M.; Hoffmann-Sommergruber, K.; Schmid, R.; Scheiner, O.; Moser, P.; Jarisch, R.; Hemmer, W. Levels of histamine and other biogenic amines in high-quality red wines. Food Addit. Contam. A 2011, 28, 408–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comuzzo, P.; Rauhut, D.; Werner, M.; Lagazio, C.; Zironi, R. A survey on wines from organic viticulture from different European countries. Food Control 2013, 34, 274–282. [Google Scholar] [CrossRef]
- Martuscelli, M.; Arfelli, G.; Manetta, A.C.; Suzzi, G. Biogenic amines content as a measure of the quality of wines of Abruzzo (Italy). Food Chem. 2013, 140, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.M.; Valente, I.M.; Rodrigues, J.A. Analysis of biogenic amines in wines by salting-out assisted liquid-liquid extraction and high-performance liquid chromatography with fluorimetric detection. Talanta 2014, 124, 146–251. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Congiu, F.; Serreli, G.; Mameli, S. Determination of dansylated amino acids and biogenic amines in Cannonau and Vermentino wines by HPLC-FLD. Food Chem. 2015, 175, 29–35. [Google Scholar] [CrossRef]
- Preti, R.; Antonelli, M.L.; Bernacchia, R.; Vinci, G. Fast determination of biogenic amines in beverages by a core-shell particle column. Food Chem. 2015, 187, 555–562. [Google Scholar] [CrossRef]
- Jastrzębska, A.; Piasta, A.; Kowalska, S.; Krzemiński, M.; Szłyk, E. A new derivatization reagent for determination of biogenic amines in wines. J. Food Compos. Anal. 2016, 48, 111–119. [Google Scholar] [CrossRef]
- Restuccia, D.; Sicari, V.; Pellicanò, T.M.; Spizzirri, U.G.; Loizzo, M.R. The impact of cultivar on polyphenol and biogenic amine profiles in Calabrian red grapes during winemaking. Food Res. Int. 2017, 102, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mitar, I.; Ljubenkov, I.; Rohtek, N.; Prkić, A.; Anđelić, I.; Vuletić, N. The content of biogenic amines in Croatian wines of different geographical origins. Molecules 2018, 23, 2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez-Ozaeta, I.; Amárita, F.; Lavilla, M.; Rainieri, S. Ecology of indigenous lactic acid bacteria from Rioja Alavesa re wines, focusing on biogenic amine production ability. LWT-Food Sci. Technol. 2019, 116, 108544. [Google Scholar] [CrossRef]
- Esposito, F.; Montuori, P.; Schettino, M.; Velotto, S.; Stasi, T.; Romano, R.; Cirillo, T. Levels of biogenic amines in red and white wine, dietary exposure, and histamine-mediated symptoms upon wine ingestion. Molecules 2019, 24, 3629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipe-Ribeiro, L.; Milheiro, J.; Ferreira, L.C.; Correia, E.; Cosme, F.; Nunes, F.M. Biogenic amines and polyamines in wines: Does Dekkera/Brettanomyces red wine spoilage increases the risk of intake by consumers? LWT-Food Sci. Technol. 2019, 115, 108488. [Google Scholar] [CrossRef]
- Milheiro, J.; Ferreira, L.C.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. A simple dispersive solid phase extraction clean-up/concentration method for selective and sensitive quantification of biogenic amines in wines using benzoyl chloride derivatization. Food Chem. 2019, 274, 110–117. [Google Scholar] [CrossRef]
- Palomino-Vasco, M.; Rodríguez-Cáceres, M.I.; Mora-Diez, N.; Pardo-Botelo, R.; Acedo-Valenzuela, M.I. Biogenic amines profile in red wines regarding aging and storage conditions. J. Food Compos. Anal. 2019, 83, 103295. [Google Scholar] [CrossRef]
- Žurga, P.; Vahčić, N.; Pasković, I.; Banović, M.; Staver, M.M. Occurrence of ochratoxin A and biogenic amines in Croatian commercial red wines. Foods 2019, 8, 348. [Google Scholar] [CrossRef] [Green Version]
- Angulo, M.F.; Flores, M.; Aranda, M.; Henríquez-Aedo, K. Fast and selective method for biogenic amines determination in wines and beers by ultra high-performances liquid chromatography. Food Chem. 2020, 309, 125689. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, J.M.; Simó, G.; Pérez-Magariño, S.; Cano-Mozo, E.; Fernández-Fernández, E.; Ruipérez, V.; Vila-Crespo, J. Evaluating the influence of simultaneous inoculation of SiO2-alginate encapsulated bacteria and yeasts on volatiles, amino acids, biogenic amines and sensory profile of red wine with lysozyme addition. Food Chem. 2020, 327, 126920. [Google Scholar] [CrossRef]
- Vinci, G.; Maddaloni, L.; Prencipe, S.A.; Ruggieri, R. Natural contaminants in wines: Determination of biogenic amines by chromatographic techniques. Int. J. Environ. Res. Public Health 2021, 18, 10159. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.L.; Amorim, C.G.; Montenegro, M.C.B.S.M.; Araújo, A.N. HPLC-potentiometric method for determination of biogenic amines in alcoholic beverages: A reliable approach for food quality control. Food Chem. 2022, 372, 131288. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Serreli, G.; Montoro, P.; D’Urso, G.; Congiu, F.; Kowalczyk, A. Biogenic amines and other polar compounds in long aged oxidized Vernaccia di Oristano white wines. Food Res. Int. 2018, 111, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Bordiga, M.; Locatelli, M.; Silva, C.; Câmara, J.S. Polyphenols, biogenic amines and amino acids patterns in Verdelho wines according to vintage. Microchem. J. 2020, 153, 104383. [Google Scholar] [CrossRef]
- Lorencová, E.; Salek, R.N.; Černíková, M.; Buňková, L.; Hýlková, A.; Buňka, F. Biogenic amines occurrence in beers produced in Czech microbreweries. Food Control 2020, 117, 107335. [Google Scholar] [CrossRef]
- Kalač, P.; Křížek, M. A review of biogenic amines and polyamines in beer. J. Inst. Brew. 2003, 109, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Lorencová, E.; Salek, R.N.; Buňková, L.; Szczybrochová, M.; Černíková, M.; Buňka, F. Assessment of biogenic amines profile in ciders from the Central Europe region as affected by storage time. Food Biosci. 2021, 41, 100957. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, Z.; Chen, G.; Liu, X.; You, J.; Zhang, C. Rapid analysis of biogenic amines from rice wine with isotope-coded derivatization followed by high performance liquid chromatography–tandem mass spectrometry. Food Chem. 2016, 192, 388–394. [Google Scholar] [CrossRef]
- Fong, F.L.Y.; El-Nezami, H.; Sze, E.T.P. Biogenic amines—Precursors of carcinogens in traditional Chinese fermented food. NFS J. 2021, 23, 52–57. [Google Scholar] [CrossRef]
- Silva, I.P.; Dias, L.G.; Oliveira da Silva, M.; Machado, C.S.; Branco Paula, V.M.; Evangelista-Barreto, N.S.; Lopes de Carvalho, C.A.; Estevinho, L.M. Detection of biogenic amines in mead of social bee. LWT-Food Sci. Technol. 2020, 121, 108969. [Google Scholar] [CrossRef]
- Cunha, S.C.; Lopes, R.; Fernandes, J.O. Biogenic amines in liqueurs: Influence of processing and composition. J. Food Compos. Anal. 2017, 56, 147–155. [Google Scholar] [CrossRef]
- Almeida, C.; Fernandes, J.O.; Cunha, S.C. A novel dispersive liquid-liquid microextraction (DLLME) gas chromatography-mass spectrometry (GC-MS) method for the determination of eighteen biogenic amines in beer. Food Control 2012, 25, 380–388. [Google Scholar] [CrossRef]
- Matsheka, M.I.; Magwamba, C.C.; Mpuchane, S.; Gashe, B.A. Biogenic amine producing bacteria associated with three different commercially fermented beverages in Botswana. Afr. J. Microbiol. Res. 2013, 7, 342–350. [Google Scholar]
- Aflaki, F.; Ghoulipour, V.; Saemian, N.; Sheibani, S. Biogenic amine contents in non-alcoholic beers: Screening and optimization of derivatization. Food Anal. Method 2014, 7, 713–720. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Troncoso, A.M.; García-Parrilla, M.D.C.; Callejón, R.M. Recent trends in the determination of biogenic amines in fermented beverages—A review. Anal. Chim. Acta 2016, 939, 10–25. [Google Scholar] [CrossRef]
- Pradenas, J.; Galarce-Bustos, O.; Henríquez-Aedo, K.; Mundaca-Uribe, R.; Aranda, M. Occurrence of biogenic amines in beers from Chilean market. Food Control 2016, 70, 138–144. [Google Scholar] [CrossRef]
- Redruello, B.; Ladero, V.; del Rio, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. A UHPLC method for the simultaneous analysis of biogenic amines, amino acids and ammonium ions in beer. Food Chem. 2017, 217, 117–124. [Google Scholar] [CrossRef]
- Poveda, J.M. Biogenic amines and free amino acids in craft beers from the Spanish market: A statistical approach. Food Control 2019, 96, 227–333. [Google Scholar] [CrossRef]
- Bae, C.M.; Shin, I.C.; Lee, W.; Lee, H.H.; Choi, Y.E.; Kim, Y.J.; Lee, G.H.; Jeong, K.J.; Choi, S.B. Monitoring of biogenic amines content in commercial fermented alcoholic beverages in Gangwon-do and risk assessment. J. Environ. Health Sci. 2020, 46, 324–334. [Google Scholar]
- Bertuzzi, T.; Mulazzi, A.; Rastelli, S.; Donadini, G.; Rossi, F.; Spigno, G. Targeted healthy compounds in small and large-scale brewed beers. Food Chem. 2020, 310, 125935. [Google Scholar] [CrossRef]
- Díaz-Liñán, M.C.; Cárdenas, S.; López-Lorente, A.I. Unmodified cellulose filter paper, a sustainable and affordable sorbent for the isolation of biogenic amines from beer samples. J. Chromatogr. A 2021, 1651, 462297. [Google Scholar] [CrossRef] [PubMed]
- Nalazek-Rudnicka, K.; Wojnowski, W.; Wasik, A. Occurrence and levels of biogenic amines in beers produced by different methods. Foods 2021, 10, 2902. [Google Scholar] [CrossRef] [PubMed]
- Song, N.E.; Cho, H.S.; Baik, S.H. Bacteria isolated from Korean black raspberry vinegar with low biogenic amine production in wine. Braz. J. Microbiol. 2016, 47, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef] [Green Version]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Picci, N. Brewing effect on levels of biogenic amines in different coffee samples as determined by LC-UV. Food Chem. 2015, 175, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Picci, N.; Restuccia, D. Extraction efficiency of different solvents and LC-UV determination of biogenic amines in tea leaves and infusions. J. Anal. Methods Chem. 2016, 2016, 8715287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbi, L.; Ciano, S.; Rapa, M.; Ruggieri, R. Biogenic Amines Determination in “Plant Milks”. Beverages 2019, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Preti, R.; Bernacchia, R.; Vinci, G. Chemometric evaluation of biogenic amines in commercial fruit juices. Eur. Food Res. Technol. 2016, 242, 2031–2039. [Google Scholar] [CrossRef]
- Basheer, C.; Wong, W.; Makahleh, A.; Tameem, A.A.; Salhin, A.; Saad, B.; Lee, H.K. Hydrazone-based ligands for micro-solid phase extraction-high performance liquid chromatographic determination of biogenic amines in orange juice. J. Chromatogr. A 2011, 1218, 4332–4339. [Google Scholar] [CrossRef]
- Zhong, J.; Ye, X.; Fang, Z.; Xie, G.; Liao, N.; Shu, J.; Liu, D. Determination of biogenic amines in semi-dry and semi-sweet Chinese rice wines from the Shaoxing region. Food Control 2012, 28, 151–156. [Google Scholar] [CrossRef]
- Özdestan, Ö. Evaluation of bioactive amine and mineral levels in Turkish coffee. Food Res. Int. 2014, 61, 167–175. [Google Scholar] [CrossRef]
- Lee, S.; Yoo, M.; Shin, D. The identification and quantification of biogenic amines in Korean turbid rice wine, Makgeolli by HPLC with mass spectrometry detection. LWT-Food Sci. Technol. 2015, 62, 350–356. [Google Scholar] [CrossRef]
- Sun, S.Y.; Chen, Z.X.; Jin, C.W. Combined influence of lactic acid bacteria starter and final pH on the induction of malolactic fermentation and quality of cherry wines. LWT-Food Sci. Technol. 2018, 89, 449–456. [Google Scholar] [CrossRef]
- Li, H.; Jiang, D.; Liu, W.; Yang, Y.; Zhang, Y.; Jin, C.; Sun, S. Comparison of fermentation behaviors and properties of raspberry wines by spontaneous and controlled alcoholic fermentations. Food Res. Int. 2020, 128, 108801. [Google Scholar] [CrossRef]
- Lázaro de la Torre, C.A.; Conte-Junior, C.A. Detection of biogenic amines: Quality and toxicity indicators in food of animal origin. In Food Control and Biosecurity; Handbook of Food, Bioengineering; Holban, A.M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 16, pp. 225–257. [Google Scholar]
- Ladero, V.; Calles-Enríquez, M.; Fernández, M.; Alvarez, M.A. Toxicological effects of dietary biogenic amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Benkerroum, N. Biogenic amines in dairy products: Origin, incidence, and control means. Compr. Rev. Food Sci. F 2016, 15, 801–826. [Google Scholar] [CrossRef] [Green Version]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.C. Histamine intolerance: The current state of the art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Paparella, A. An overview of histamine and other biogenic amines in fish and fish products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef]
- Borah, A.; Paul, R.; Mazumder, M.K.; Bhattacharjee, N. Contribution of beta-phenethylamine, a component of chocolate and wine, to dopaminergic neurodegeneration: Implications for the pathogenesis of Parkinson’s disease. Neurosci. Bull. 2013, 29, 655–660. [Google Scholar] [CrossRef] [Green Version]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Fernandez, M.; Martin, M.C.; Ruas-Madiedo, P.; Alvarez, M.A. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 2017, 218, 249–255. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Fernandez, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. The biogenic amine tryptamine, unlike β-phenylethylamine, shows in vitro cytotoxicity at concentrations that have been found in foods. Foods 2020, 331, 127303. [Google Scholar] [CrossRef] [PubMed]
- Jaguey-Hernández, Y.; Aguilar-Arteaga, K.; Ojeda-Ramirez, D.; Añorve-Morga, J.; González-Olivares, L.G.; Castañeda-Ovando, A. Biogenic amines levels in food processing: Efforts for their control in foodstuffs. Food Res. Int. 2021, 144, 110341. [Google Scholar] [CrossRef] [PubMed]
- Resolution OIV-CST. OIV Code of good vitivinicultural practices in order to minimize the presence of biogenic amines in vine-based products. In Proceedings of the General Director of the OIV Secretary of the General Assembly, Porto, Portugal, 24 June 2011; pp. 369–2011.

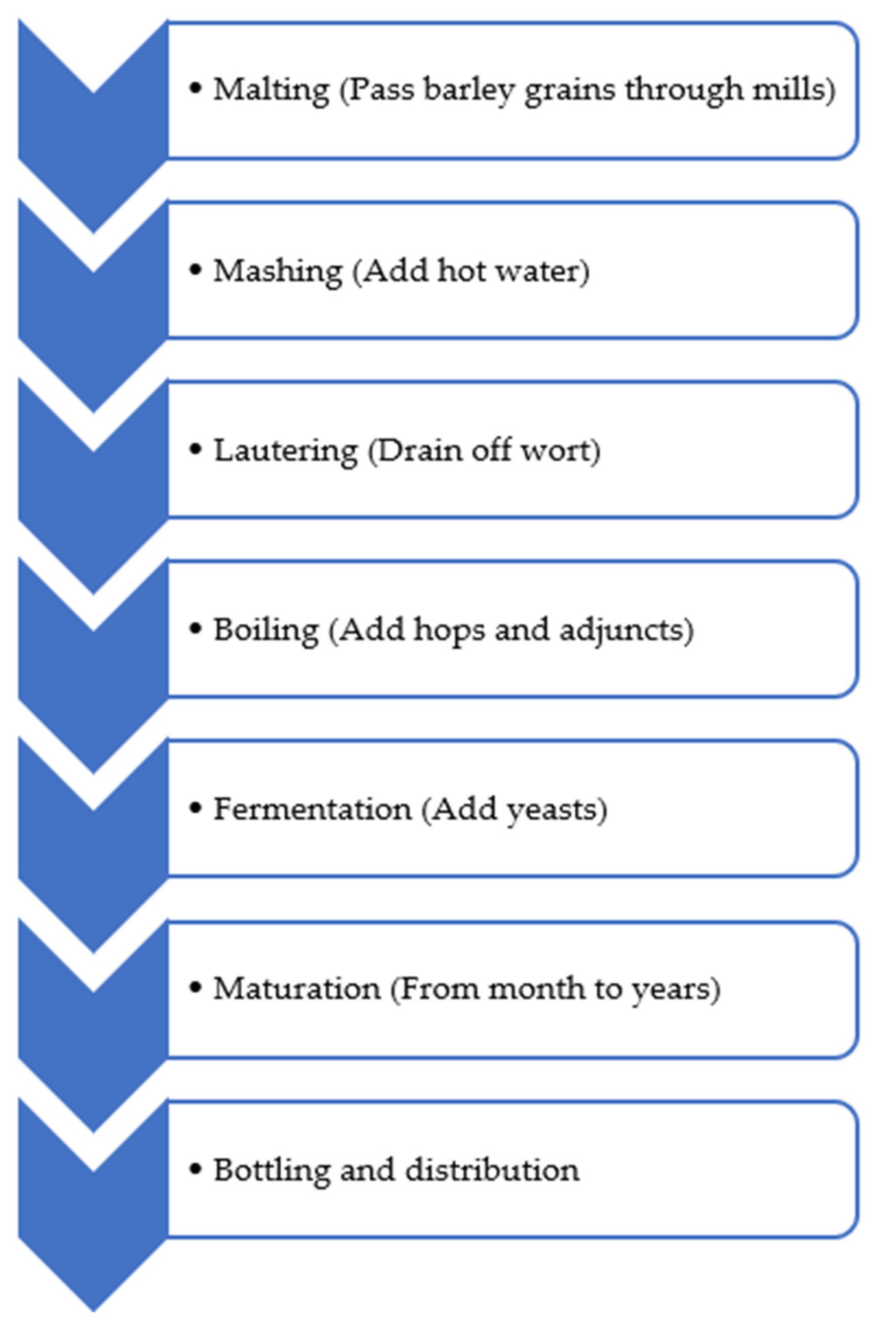
| Biogenic Amine | Formula | Chemical Structure | Molecular Weight (g/mol) | ||
|---|---|---|---|---|---|
| Aliphatic | Aromatic | Heterocyclic | |||
| Histamine | 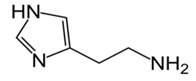 | ♦ | 111.15 | ||
| Tyramine |  | ♦ | 137.18 | ||
| Putrescine | 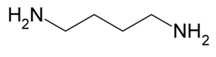 | ♦ | 88.15 | ||
| Cadaverine |  | ♦ | 102.18 | ||
| Spermidine | 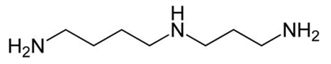 | ♦ | 145.25 | ||
| Spermine |  | ♦ | 202.34 | ||
| Wine | Biogenic Amines (mg/L) | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Met | Eth | Put | Cad | His | Spd | Spm | Tyr | Phe | Tryp | ||
| Red | nd | 1.7–8.0 | 7.6–35.7 | nd | nd-18.7 | 1.1–17.8 | nd-16.2 | Arrieta and Prats-Moya, 2012 [14] | |||
| 0.4–36.6 | 1.7–10.5 | 3.7–48.7 | 0.1–1.8 | <0.5–14.1 | <0.1–12.4 | <0.1–2.7 | Bach et al., 2012 [15] | ||||
| 2.9–122.0 | 0.5–26.9 | 1.1–10.7 | Konakowky et al., 2011 [16] | ||||||||
| 7.1–19.0 | 2.2–16.2 | 0.5–37.3 | Comuzzo et al., 2013 [17] | ||||||||
| 0.8–6.5 | 2.4–31.8 | 0–1.1 | 0–10.8 | nd | nd | 0–18.8 | nd | Martuscelli et al., 2013 [18] | |||
| 0.1 | 1.1 | 1.5 | 0.1 | 23.1 | nq | 0.2 | Ramos et al., 2014 [19] | ||||
| 0.2–1.7 | 4.1–11.3 | 10.2–32.8 | 0.6–2.4 | tr-8.1 | nd-1.3 | nd | 2.9–11.5 | tr-1.2 | tr-0.1 | Tuberoso et al., 2015 [20] | |
| nd-1.4 | 0.7–1.9 | 3.8–11.1 | 0.5–1.6 | nd-1.0 | nq-0.7 | nq-1.1 | 0.7–2.0 | 0.2–1.1 | nd | Preti et al., 2015 [21] | |
| 7.1–11.9 | 2.5–3.6 | nd-2.6 | nd-2.9 | Jastrzębska et al., 2016 [22] | |||||||
| 8.2–16.2 | 4.3–12.3 | 3.1–15.9 | 3.4–21.0 | 2.1–14.5 | nd-3.9 | Restuccia et al., 2017 [23] | |||||
| <0.1 | <0.2 | 0.8–7.5 | <0–2.0 | <0–2.3 | <0–0.5 | <0–0.3 | <0–2.0 | <0–1.2 | Mitar et al., 2018 [24] | ||
| <0.1–1.6 | <0.2–1.2 | <0–3.8 | <0–0.5 | <0–9.6 | <0–6.1 | <0–3.6 | <0–3.0 | <0–9.2 | |||
| 2.3–5.0 | 0.8–2.1 | nd-1.0 | nd-0.5 | Diez Ozaeta et al., 2019 [25] | |||||||
| 24.8–34.2 | 1.1 | nd | 1.3–2.5 | 1.8 | 1.8–4.3 | Esposito et al., 2019 [26] | |||||
| 10.0 | 1.7 | 2.4 | 3.4 | ||||||||
| 0.7–10.4 | 1.8–82.1 | 0–31.7 | 0–28.1 | 0–1.8 | 0–8.4 | Filipe-Ribeiro et al., 2019 [27] | |||||
| 0.9–10.4 | 1.8–82.1 | <LOQ-20.5 | <LOQ-28.1 | <LOQ-1.6 | <LOQ-6.9 | Milheiro et al., 2019 [28] | |||||
| 5.9–42.6 | nd-4.3 | nd-10.3 | nd-4.1 | nd-0.2 | nd | Palomino-Vasco et al., 2019 [29] | |||||
| 5.1–16.7 | nd-2.2 | 1.1–9.1 | nd-7.2 | nd-0.5 | nd | ||||||
| 2.0–14.1 | 0.1–3.0 | 0.1–7.1 | 0.1–8.4 | Žurga et al., 2019 [30] | |||||||
| 24.8–34.2 | tr-1.1 | tr | 1.3–2.5 | 1.8–4.3 | tr-3.5 | nd | Angulo et al., 2020 [31] | ||||
| 4.8–5.3 | 0.7 | 0.4–0.6 | 0.6–1.0 | 0.4–1.2 | 0.3 | Rodríguez-Nogales et al., 2020 [32] | |||||
| nd-10.5 | nd-3.4 | nd-7.6 | nd-1.3 | nd-1.6 | nd-6.6 | nd-3.8 | nd-2.5 | Vinci et al., 2021 [33] | |||
| 0.3 | 0.4 | 0.4 | 0.4 | 0.8 | 0.7 | 0.8 | 2.3 | 0.5 | 1.1 | Gil et al., 2022 [34] | |
| White | 1.1–8.6 | 0.8–12.8 | 0.3–1.2 | 0.3.4 | nd | nd | 0–6.8 | nd | Martuscelli et al., 2013 [18] | ||
| 0.2–0.4 | 0.5 | 0.2–0.3 | nq-0.1 | 2.8–8.9 | nq-0.1 | Ramos et al., 2014 [19] | |||||
| 0.4–2.2 | 1.2–6.6 | 1.5–10.6 | 0.5–2.5 | nd | tr | nd | tr | nd-1.8 | tr-0.1 | Tuberoso et al., 2015 [20] | |
| nd-2.9 | nd-1.7 | nd-1.8 | nd-1.5 | Jastrzębska et al., 2016 [22] | |||||||
| <0.1–1.4 | <0.2 | 1.0–2.1 | tr | <0.03–0.6 | <0.03 | <0–0.4 | <0–0.4 | <0–1.2 | Mitar et al., 2018 [24] | ||
| <0.1 | <0.2–0.6 | 0.3–1.5 | tr | tr | tr | tr | tr | <0–0.7 | |||
| nd-7.0 | 0.7–4.2 | 2.8–25.3 | 0.2–1.1 | tr-16.6 | nd-0.2 | nd | nd-6.0 | 0.2–2.4 | nd-0.4 | Tuberoso et al., 2018 [35] | |
| 1.9 | 1.5 | 0.8 | nd | nd | 0.4 | Esposito et al., 2019 [26] | |||||
| 0.2–3.0 | 0.1–1.2 | nd-3.8 | 0.1 | Perestrelo et al., 2020 [36] | |||||||
| nd-4.2 | nd-4.2 | nd-4.4 | nd-1.0 | nd-1.6 | nd-3.7 | nd-3.2 | nd-1.3 | Vinci et al., 2021 [33] | |||
| 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 1.0 | 1.0 | 0.7 | 0.6 | 0.8 | Gil et al., 2022 [34] | |
| Product | Biogenic Amines (mg/L) | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Met | Eth | Put | Cad | His | Spd | Spm | Tyr | Phe | Tryp | ||
| Rice wine | nd-58.9 | nd-29.9 | nd-24.9 | nd | nd | 1.2–37.6 | nd | nd | Zhong et al., 2012 [62] | ||
| Brewed coffee | 0.5–1.6 | 1.5–9.1 | nd | nd | nd | nd-19.7 | nd-5.0 | nd-20.2 | Özdestan, 2014 [63] | ||
| Ground coffee | 3.9–15.2 | 13.6–75.1 | nd | nd | nd | 22.5–99.6 | nd-22.8 | 3.0–37.9 | |||
| Espresso coffee | 0.6–2.3 | 0.2–1.8 | 0.2–1.6 | 0.5–1.2 | nd-2.0 | 0.3–1.9 | 0.2–1.2 | ni | Restuccia et al., 2015 [57] | ||
| Rice wine | 1.1–59.9 | 0–0.7 | 0.2–9.6 | 0–0.2 | 0.3–1.5 | 0.4–37.1 | 0.1–3.8 | nd-0.5 | Lee et al., 2015 [64] | ||
| Fruit nectars | nd | nd-2.5 | 1.1–3.3 | 2.0–17.2 | nd | 1.3–3.0 | 1.5–3.6 | nd | nd | Preti et al., 2015 [21] | |
| Rice wine | nd-32.3 | nd-63.5 | nd-72.1 | nd-41.4 | Ordóñez et al., 2016 [47] | ||||||
| Vinegar | nd-3.2 | nd-0.1 | nd-0.3 | nd-0.2 | |||||||
| Cyder | nd-12.3 | - | nd-6.9 | nd-5.0 | |||||||
| Orange juice | 0.1-2.2 | - | tr | tr | |||||||
| Apricot juice | nd | nd | 1.4-7.1 | 4.0-17.9 | nd | 2.0–2.5 | 1.2–2.5 | nd | nd | ni | Preti et al., 2016 [60] |
| Peach 50% juice | nd | nd | 1.4–3.2 | 2.0–10.1 | nd | 1.3–2.0 | 1.2–2.7 | nd | nd | ni | |
| Peach 70% juice | nd | nd | 2.3–3.6 | 4.1–6.1 | nd | 2.0–4.4 | 1.4–1.9 | nd | nd | ni | |
| Pear 50% juice | nd | nd | 1.1–2.7 | 1.9–8.3 | nd | 1.2–1.8 | 1.2–3.5 | nd | nd | ni | |
| Pear 70% juice | nd | 1.1–1.2 | 1.4–4.4 | 3.8–6.2 | nd | 1.9–2.7 | 1.2–1.4 | nd | nd | ni | |
| Apple concentrate juice | nd | nd-0.4 | 0.6–1.7 | 0.6–4.3 | nd | 0.2–0.7 | 0.2–1.0 | nd | nd | ni | |
| Pineapple concentrate juice | nd | 0.2–1.7 | 1.5–2.0 | nd-3.1 | nd | 2.6–5.4 | 1.5–3.2 | nd | nd | ni | |
| Grapefruit concentrate juice | nd-1.2 | 6.2–13.0 | 7.2–20.8 | 0.4–2.3 | nd | 1.0–2.2 | 0.3–0.5 | nd | nd | ni | |
| Orange concentrate juice | nd-2.7 | 24.0–38.6 | 34.7–61.0 | nd | nd | 2.0–3.7 | 0.4–1.4 | nd | nd | ni | |
| Black tea infusion | 8.4–10.2 | nd-14.0 | nd-20.0 | 6.5–10.8 | nd-0.3 | nd | nd-2.0 | nd | Spizzirri et al., 2016 [58] | ||
| Green tea | 10.3–14.6 | nd | nd | 6.3–10.4 | nd-11.5 | nd | nd | nd | |||
| Tea drinks | nd-6.9 | nd | nd | 4.3–6.7 | nd | nd | nd | nd | |||
| Fruit liqueurs | tr-0.2 | tr-1.0 | tr-2.5 | tr-0.1 | tr-0.2 | tr | tr | Cunha et al., 2017 [43] | |||
| Herbs liqueurs | tr-1.1 | tr | tr | tr-0.1 | tr-0.2 | tr | tr | ||||
| Coffee liqueurs | tr-1.1 | tr-0.1 | tr-0.4 | tr | 0.0–0.2 | tr | tr | ||||
| Honey liqueurs | 0.1 | tr | 0.1–0.7 | tr-0.2 | tr | tr-0.1 | tr | ||||
| Fruit wine | 0–0.1 | 0–0.3 | 0–9.9 | 0–0.9 | 0–1.5 | tr | 0–4.0 | tr | tr | Płotka-Wasylka et al., 2018 [12] | |
| Cherry wine | 0.7–1.6 | 1.0–1.2 | 4.4–7.3 | 2.8–4.1 | 0.6–1.0 | 0.8–1.4 | Sun et al., 2018 [65] | ||||
| Raspberry wine | nd-3.3 | nd-1.1 | 0.6–2.4 | nd-2.6 | 1.1–11.3 | 0.2–4.1 | Li et al., 2020 [66] | ||||
| Chinese rice wines | nd-58.9 | nd-98.7 | nd-78.5 | nd-27.1 | nd-33.6 | nd-100.8 | nd | Fong et al., 2021 [41] | |||
| Cider with low alcoholic content | nd-45.9 | nd-42.9 | nd-9.3 | 0.7–7.6 | 1.1–9.9 | nd-47.5 | nd-7.7 | nd-1.1 | Lorencová et al., 2021 [39] | ||
| Cider with high alcoholic content | nd-45.0 | nd-19.9 | nd-18.1 | 1.2–4.8 | 1.7–9.1 | nd-47.3 | nd-9.6 | nd | |||
| Biogenic Amine | Toxicological Reactions | References |
|---|---|---|
| Cadaverine and Putrescine | Bradycardia, hypotension, increased cardiac output, paresis of the extremities. Gastric or intestinal cancer illness. N-nitrosamine formation reacting with nitrites. Potentiation of histamine’s toxic effect. | Ladero et al., 2010 [68] Benkerroum, 2016 [69] |
| Histamine | Flushing, hives, rashes, swelling. Abdominal cramps, bloating, diarrhea, nausea, oral numbness, and thirst. Dizziness, faintness, headache, loss of sight, and tremor. Hypotonia, low blood pressure, myocardial disfunction, rapid and weak pulse, and shock. Dyspnea, rhinitis, respiratory distress, and sneezing. | Comas-Basté et al., 2020 [70] Visciano et al., 2020 [71] |
| Phenylethylamine | Attention deficit disorder, depression, hypertension, headache, migraine, epilepsy, schizophrenia, and Parkinson’s disease. | Borah et al., 2013 [72] Benkerroum, 2016 [69] |
| Spermine and spermidine | N-nitrosamine formation reacting with nitrites. Potentiate the toxicity of the other biogenic amines. | Vinci et al., 2020 [4] |
| Tyramine | Nausea and vomiting. Headache, migraine, neurological disorders, salivation, and tearing. Hypertension, peripheral vasoconstriction, and respiratory disorders. | del Rio et al., 2017 [73] |
| Tryptamine | High blood pressure, headache, vomiting, and perspiration. | del Rio et al., 2020 [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visciano, P.; Schirone, M. Update on Biogenic Amines in Fermented and Non-Fermented Beverages. Foods 2022, 11, 353. https://doi.org/10.3390/foods11030353
Visciano P, Schirone M. Update on Biogenic Amines in Fermented and Non-Fermented Beverages. Foods. 2022; 11(3):353. https://doi.org/10.3390/foods11030353
Chicago/Turabian StyleVisciano, Pierina, and Maria Schirone. 2022. "Update on Biogenic Amines in Fermented and Non-Fermented Beverages" Foods 11, no. 3: 353. https://doi.org/10.3390/foods11030353
APA StyleVisciano, P., & Schirone, M. (2022). Update on Biogenic Amines in Fermented and Non-Fermented Beverages. Foods, 11(3), 353. https://doi.org/10.3390/foods11030353






