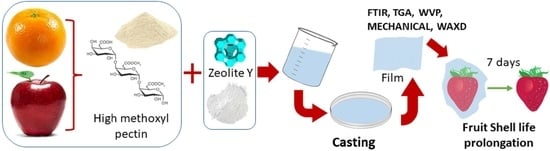
Novel Biocomposite Films Based on High Methoxyl Pectin Reinforced with Zeolite Y for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Films
2.3. Biocomposites’ Characterization
2.3.1. FTIR-ATR Analysis
2.3.2. WAXD Analysis
2.3.3. Water Vapor Permeability (WVP)
2.3.4. Mechanical Properties
2.3.5. Thermogravimetric Analysis
2.3.6. Statistical Analysis
3. Results
3.1. FTIR-ATR Analysis
3.2. Wide-Angle X-ray Diffraction (WAXD)
3.3. Water Vapor Permeability (WVP)
3.4. Mechanical Analysis
3.5. Thermogravimetric Analysis (TGA)
3.6. Applicative Potential
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tiseo, I. Global Plastic Production 1950–2020. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 21 December 2021).
- Marsh, K.; Bugusu, B. Food Packaging: Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Nesic, A.R.; Seslija, S.I. 19–The Influence of Nanofillers on Physical–Chemical Properties of Polysaccharide-Based Film Intended for Food Packaging. In Food Packaging; Academic Press: Cambridge, MA, USA, 2017; pp. 637–697. ISBN 9780128043028. [Google Scholar]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Sharma, B.R.; Naresh, L.; Dhuldhoya, N.C.; Merchant, S.U.; Merchant, U.C. An Overview on Pectins. Times Food Process. J. 2006, 23, 44–51. [Google Scholar]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Noreen, A.; Nazli, Z.-H.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Tabasum, S.; Zuber, M.; Zia, K.M. Pectins functionalized biomaterials; a new viable approach for biomedical applications: A review. Int. J. Biol. Macromol. 2017, 101, 254–272. [Google Scholar] [CrossRef]
- Nesic, A.R. Pectin Films for Application in Food Packaging: Review. In Pectin: Chemical Properties, Uses and Health Benefits; Bush, P., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 225–253. ISBN 978-1-63321-439-2. [Google Scholar]
- Saha, N.R.; Sarkar, G.; Roy, I.; Rana, D.; Bhattacharyya, A.; Adhikari, A.; Mukhopadhyay, A.; Chattopadhyay, D. Studies on methylcellulose/pectin/montmorillonite nanocomposite films and their application possibilities. Carbohydr. Polym. 2016, 136, 1218–1227. [Google Scholar] [CrossRef]
- Nesic, A.R.; Velickovic, S.J.; Antonovic, D.G. Novel composite films based on amidated pectin for cationic dye adsorption. Colloids Surf. B Biointerfaces 2014, 116, 620–626. [Google Scholar] [CrossRef]
- Mangiacapra, P.; Gorrasi, G.; Sorrentino, A.; Vittoria, V. Biodegradable nanocomposites obtained by ball milling of pectin and montmorillonites. Carbohydr. Polym. 2006, 64, 516–523. [Google Scholar] [CrossRef]
- da Costa, M.P.M.; de Mello Ferreira, I.L.; de Macedo Cruz, M.T. New polyelectrolyte complex from pectin/chitosan and montmorillonite clay. Carbohydr. Polym. 2016, 146, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Sustainable nanocomposites based on halloysite nanotubes and pectin/polyethylene glycol blend. Polym. Degrad. Stab. 2013, 98, 2529–2536. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.; Rawat, K.; Bohidar, H.B. Characterization of microstructure, viscoelasticity, heterogeneity and ergodicity in pectin?laponite?CTAB?calcium nanocomposite hydrogels. Carbohydr. Polym. 2016, 136, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Petrini, P.; Barcellona, G.; Roversi, T.; Piazza, L.; Visai, L.; Tanzi, M.C. Reactive hydroxyapatite fillers for pectin biocomposites. Mater. Sci. Eng. C 2014, 45, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julbe, A.; Drobek, M.; Zeolite, Y. Type. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 2060–2061. [Google Scholar] [CrossRef]
- Nesic, A.; Onjia, A.; Davidovic, S.; Dimitrijevic, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of pectin-sodium alginate based films for potential healthcare application: Study of chemico-physical interactions between the components of films and assessment of their antimicrobial activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef]
- Nesic, A.R.; Kokunesoski, M.J.; Ilic, S.M.; Gordic, M.V.; Ostojic, S.B.; Micic, D.M.; Velickovic, S.J. Biocomposite membranes of highly methylated pectin and mesoporous silica SBA-15. Compos. Part B Eng. 2014, 64, 162–167. [Google Scholar] [CrossRef]
- Černá, M.; Barros, A.S.; Nunes, A.; Rocha, S.M.; Delgadillo, I.; Čopíková, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, Y.; Li, Y.; Chen, W.; Liu, B. Synthesis and characterization of mesoporous zeolite Y by using block copolymers as templates. Chem. Eng. J. 2016, 284, 405–411. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O. Preparation and characterization of biodegradable methyl cellulose/montmorillonite nanocomposite films. Appl. Clay Sci. 2010, 48, 414–424. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier Science: New York, NY, USA, 2007; ISBN 978-0-444-53064-6. [Google Scholar]
- Martelli, M.R.; Barros, T.T.; de Moura, M.R.; Mattoso, L.H.C.; Assis, O.B.G. Effect of Chitosan Nanoparticles and Pectin Content on Mechanical Properties and Water Vapor Permeability of Banana Puree Films. J. Food Sci. 2013, 78, N98–N104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.-X.; Wang, Z.-W.; Hu, C.-Y.; Wang, L. Properties of low methoxyl pectin-carboxymethyl cellulose based on montmorillonite nanocomposite films. Int. J. Food Sci. Technol. 2014, 49, 2592–2601. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Zhong, F. Characterization of tara gum edible films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Food Hydrocoll. 2015, 44, 309–319. [Google Scholar] [CrossRef]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.d.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017, 157, 167–175. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Zea-Redondo, L.; Moates, G.K.; Wellner, N.; Cross, K.; Waldron, K.W.; Azeredo, H.M.C. Pomegranate peel pectin films as affected by montmorillonite. Food Chem. 2016, 198, 107–112. [Google Scholar] [CrossRef]
- Lorevice, M.V.; Otoni, C.G.; de Moura, M.R.; Mattoso, L.H.C. Chitosan nanoparticles on the improvement of thermal, barrier, and mechanical properties of high-and low-methyl pectin films. Food Hydrocoll. 2016, 52, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.-Y.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Rahmat, A.R.; WARahman, W.A.; Tan, A.-C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Yáñez-Fernández, J.; Castro-Muñoz, R. Dextran/chitosan blend film fabrication for bio-packaging of mushrooms (Agaricus bisporus). J. Food Process. Preserv. 2021, 45, e15489. [Google Scholar] [CrossRef]
- Dea, I.C.M.; Madden, J.K. Acetylated pectic polysaccharides of sugar beet. Food Hydrocoll. 1986, 1, 71–88. [Google Scholar] [CrossRef]
- Rolin, C. Chapter 10–Pectin. In Industrial Gums; Academic Press: Cambridge, MA, USA, 1993; pp. 257–293. ISBN 9780080926544. [Google Scholar]
- Voragen, F.; Schols, H.; Visser, R. (Eds.) Advances in Pectin and Pectinase Research; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 978-90-481-6229-1. [Google Scholar]
- Šupová, M.; Martynková, G.S.; Barabaszová, K. Effect of Nanofillers Dispersion in Polymer Matrices: A Review. Sci. Adv. Mater. 2011, 3, 1–25. [Google Scholar] [CrossRef]
- Kanagaraj, S.; Varanda, F.R.; Zhil’tsova, T.V.; Oliveira, M.S.A.; Simões, J.A.O. Mechanical properties of high density polyethylene/carbon nanotube composites. Compos. Sci. Technol. 2007, 67, 3071–3077. [Google Scholar] [CrossRef]
- Petersson, L.; Oksman, K. Biopolymer based nanocomposites: Comparing layered silicates and microcrystalline cellulose as nanoreinforcement. Compos. Sci. Technol. 2006, 66, 2187–2196. [Google Scholar] [CrossRef]
- Abdollahi, M.; Alboofetileh, M.; Rezaei, M.; Behrooz, R. Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers. Food Hydrocoll. 2013, 32, 416–424. [Google Scholar] [CrossRef]
- Miller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of nanoclay incorporation method on mechanical and water vapor barrier properties of starch-based films. Ind. Crops Prod. 2011, 33, 605–610. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Y.; Luo, W.; Fang, Y. Effects of clay-modifying agents on the morphology and properties of poly (methyl methacrylate)/clay nanocomposites synthesized via γ-ray irradiation polymerization. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3218–3226. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| SiO2/Al2O3 (mol/mol) | 30.3 |
| Mean particle size (µm) | 1.2 |
| Specific surface area (m2/g) | 710 |
| Specific volume (cm3/g) | 0.20 |
| Sample | Abbreviation |
|---|---|
| Citrus pectin + PG | PC |
| Citrus pectin + PG + ZY 0.05 wt% | PC/ZY0.05 |
| Citrus pectin + PG + ZY 0.1 wt% | PC/ZY0.1 |
| Citrus pectin + PG + ZY 0.2 wt% | PC/ZY0.2 |
| Apple pectin + PG | PA |
| Apple pectin + PG + ZY 0.05 wt% | PA/ZY0.05 |
| Apple pectin + PG + ZY 0.1 wt% | PA/ZY0.1 |
| Apple pectin + PG + ZY 0.2 wt% | PA/ZY0.2 |
| Sample | TS, MPa | ε, % | E, MPa | WVP × 10−10, g/m s Pa |
|---|---|---|---|---|
| PC | 41 ± 1 e | 15 ± 0.5 c | 1340 ± 50 c | 4.47 ± 0.2 d |
| PC/ZY0.05 | 45 ± 1 d | 12 ± 0.5 b | 1365 ± 40 c | 2.98 ± 0.0.2 c |
| PC/ZY0.1 | 57 ± 2 b | 12 ± 0.5 b | 1426 ± 30 b | 2.18 ± 0.1 a |
| PC/ZY0.2 | 68 ± 3 a | 12 ± 0.5 b | 1664 ± 40 a | 2.05± 0.1 a |
| PA | 38 ± 2 e | 12 ± 1 b | 1125 ± 70 d | 4.45 ± 0.3 d |
| PA/ZY0.05 | 43 ± 2 de | 10 ± 1 a | 1221 ± 60 d | 3.94 ± 0.2 d |
| PA/ZY0.1 | 47 ± 2 cd | 10 ± 1 a | 1355 ± 70 c | 3.12 ± 0.1 c |
| PA/ZY0.2 | 50 ± 2 c | 9 ± 1 a | 1453 ± 70 b | 2.70 ± 0.1 b |
| Sample | WL100, % | WL180, % | WL350, % | Tonset, °C | Tdegr, °C |
|---|---|---|---|---|---|
| PC | 6 | 8 | 64 | 191 | 223 |
| PC/ZY0.05 | 6 | 8 | 61 | 195 | 223 |
| PC/ZY0.1 | 5 | 7 | 57 | 195 | 223 |
| PC/ZY0.2 | 5 | 7 | 56 | 195 | 223 |
| PA | 5 | 6 | 65 | 191 | 219 |
| PA/ZY0.05 | 4 | 6 | 63 | 193 | 220 |
| PA/ZY0.1 | 4 | 6 | 62 | 193 | 220 |
| PA/ZY0.2 | 5 | 8 | 59 | 192 | 221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesic, A.; Meseldzija, S.; Cabrera-Barjas, G.; Onjia, A. Novel Biocomposite Films Based on High Methoxyl Pectin Reinforced with Zeolite Y for Food Packaging Applications. Foods 2022, 11, 360. https://doi.org/10.3390/foods11030360
Nesic A, Meseldzija S, Cabrera-Barjas G, Onjia A. Novel Biocomposite Films Based on High Methoxyl Pectin Reinforced with Zeolite Y for Food Packaging Applications. Foods. 2022; 11(3):360. https://doi.org/10.3390/foods11030360
Chicago/Turabian StyleNesic, Aleksandra, Sladjana Meseldzija, Gustavo Cabrera-Barjas, and Antonije Onjia. 2022. "Novel Biocomposite Films Based on High Methoxyl Pectin Reinforced with Zeolite Y for Food Packaging Applications" Foods 11, no. 3: 360. https://doi.org/10.3390/foods11030360
APA StyleNesic, A., Meseldzija, S., Cabrera-Barjas, G., & Onjia, A. (2022). Novel Biocomposite Films Based on High Methoxyl Pectin Reinforced with Zeolite Y for Food Packaging Applications. Foods, 11(3), 360. https://doi.org/10.3390/foods11030360