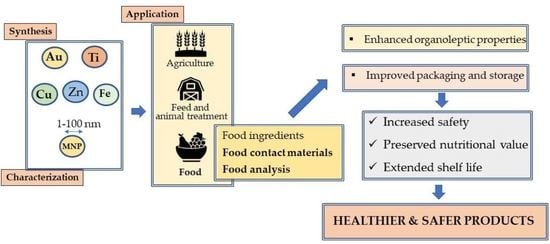
Metallic Nanoparticles in the Food Sector: A Mini-Review
Abstract
:1. Introduction
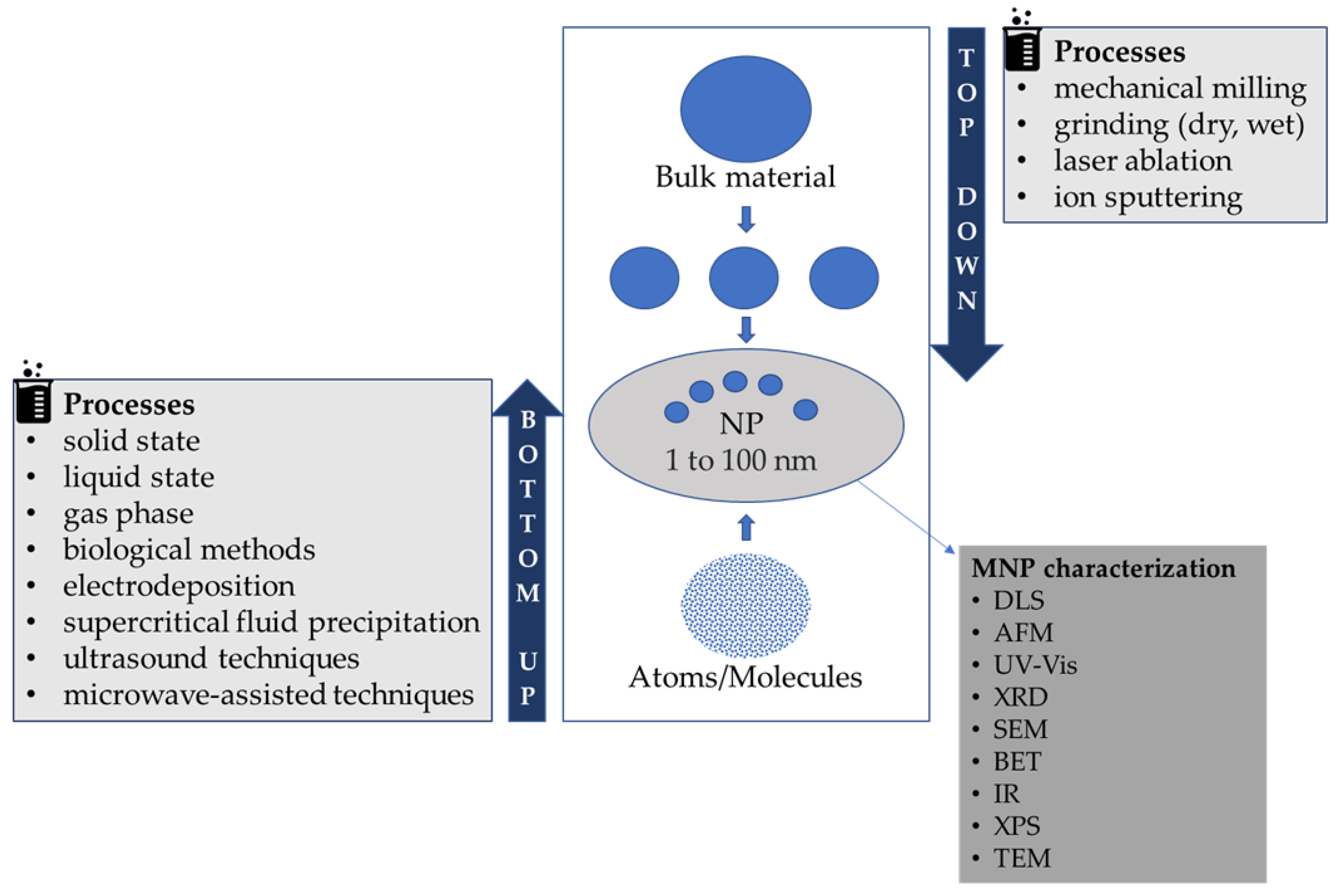
2. Synthesis and Stabilization of MNPs
3. Uses of MNPs in the Food Sector
- Develop antimicrobial agents with the potential to improve the shelf life of foods and prevent microbial growth. MNPs can destroy microbial cells through different mechanisms and have the potential to inhibit biofilm development [26]. Antimicrobial activity can result from MNP adsorption on the cell wall, destruction of the cell membrane by free radicals, induction of intracellular release of reactive oxygen species (ROS), interaction of metal ions with cell respiratory enzymes and interaction with DNA and proteins [27,28]. This antimicrobial activity depends on the MNP synthesis method, size, shape and type, and nature of the capping agents [23].
- Develop active, smart or biodegradable packaging with increased UV protection and antimicrobial activity, enhanced thermal, hydrophobicity (reduced water vapor permeability) and oxygen barrier properties; the ability to change product color; enhanced radical and oxygen scavenging activities; improved mechanical properties (tensile strength, film thickness and transparency, barrier properties), etc. [8]. Improved food packaging based on functional nanomaterials can be classified into four different categories: physically improved packaging (improved mechanical strength, temperature and moisture stability, gas barrier functions, flexibility and durability); biochemically improved packaging (improved biodegradability, edibility, biocompatibility, low-waste and eco-friendly features); improved packaging with active functions (effect on packaged foods with regard to taste, freshness and shelf life); improved packaging with smart functions (e.g., nanosensors to monitor food conditions such as oxygen levels, freshness and the presence of pathogens) [29].
3.1. Gold Nanoparticles (Au-NPs)
3.2. Silver Nanoparticles (Ag-NPs)
3.3. Copper Nanoparticles (Cu-NPs)
3.4. Zinc Nanoparticles (Zn-NPs)
3.5. Titanium Nanoparticles (Ti-NPs)
3.6. Other MNPs
4. Safety and Regulatory Issues
5. Final Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hadef, F. An Introduction to Nanomaterials. In Environmental Nanotechnology; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 1, pp. 1–58. [Google Scholar]
- Pérez, J.; Bax, L.; Escolano, C. Roadmap Report on Nanoparticles; W&W España s.l.: Barcelona, Spain, 2005. [Google Scholar]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J.-Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009, 157, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Heiligtag, F.J.; Niederberger, M. The fascinating world of nanoparticle research. Mater. Today 2013, 16, 262–271. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Mundekkad, D.; Ramalingam, C.; Shanker, R.; Kumar, A. Nanotechnology in agro-food: From field to plate. Food Res. Int. 2015, 69, 381–400. [Google Scholar] [CrossRef]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of nanomaterials in food packaging and its advanced future prospects. J. Inorg. Organomet. Polym. 2020, 30, 5180–5192. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Kolwas, K.; Derkachova, A. Impact of the interband transitions in gold and silver on the dynamics of propagating and localized surface plasmons. Nanomaterials 2020, 10, 1411. [Google Scholar] [CrossRef]
- Dobrucka, R.; Ankiel, M. Possible applications of metal nanoparticles in antimicrobial food packaging. J. Food Saf. 2019, 39, e12617. [Google Scholar] [CrossRef]
- dos Santos, C.A.; Ingle, A.P.; Rai, M. The emerging role of metallic nanoparticles in food. Appl. Microbiol. Biotechnol. 2020, 104, 2373–2383. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Alamilla-Beltrán, L.; Azuara-Nieto, E.; Hernández-Sánchez, H.; Téllez-Medina, D.I.; Martinez, C.J.; Gutierrez, G. High shear methods to produce nano-sized food related to dispersed systems. In Food Nanoscience and Nanotechnology; Hernández-Sánchez, H., Gutiérrez-López, G., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 145–161. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Metal-based nanoparticles, sensors, and their multifaceted application in food packaging. J. Nanobiotechnol. 2021, 19, 256. [Google Scholar] [CrossRef]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Buntinx, M.; Deferme, W.; Peeters, R. (Bio)polymer/ZnO nanocomposites for packaging applications: A review of gas barrier and mechanical properties. Nanomaterials 2019, 9, 1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Videira-Quintela, D.; Martin, O.; Montalvo, G. Recent advances in polymer-metallic composites for food packaging applications. Trends Food Sci. Technol. 2021, 109, 230–244. [Google Scholar] [CrossRef]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An overview of biodegradable packaging in food industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Muddapur, U. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 2014, 2014, 510246. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, M.; Priya, K.; Nancy, F.; Noorlidah, A.; Ahmed, A. Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind. Crops Prod. 2013, 41, 235–240. [Google Scholar] [CrossRef]
- Bachheti, R.K.; Fikadu, A.; Bachheti, A.; Husen, A. Biogenic fabrication of nanomaterials from flower-based chemical compounds, characterization and their various applications: A review. Saudi J. Biol. Sci. 2020, 27, 2551–2562. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef] [Green Version]
- Pomogailo, A.D.; Kestelman, V.N. Principles and mechanisms of nanoparticle stabilization by polymers. In Metallopolymer Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2005; pp. 65–113. [Google Scholar]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Ranjan, S.; Dasgupta, N.; Chakraborty, A.R.; Samuel, S.M.; Ramalingam, C.; Shanker, R.; Kumar, A. Nanoscience and nanotechnologies in food industries: Opportunities and research trends. J. Nanopart. Res. 2014, 16, 2464. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Moniz, F.B.; Brandhoff, M.P.; Gottardo, S.; Marvin, H.J.P.; Mech, A.; Pesudo, L.Q.; et al. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Moradi, M. Improvement of food packaging based on functional nanomaterial. In Nanotechnology: Applications in Energy, Drug and Food; Siddiquee, S., Melvin, G., Rahman, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 309–344. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Shen, Y.; Yang, M.; Li, X.; Han, X.; Jiang, X.; Zhao, B. SERS strategy based on the modified Au nanoparticles for highly sensitive detection of bisphenol A residues in milk. Talanta 2018, 179, 37–42. [Google Scholar] [CrossRef]
- Fan, X.; Xing, L.; Ge, P.; Cong, L.; Hou, Q.; Ge, Q.; Liu, R.; Zhang, W.; Zhou, G. Electrochemical sensor using gold nanoparticles and plasma pretreated graphene based on the complexes of calcium and Troponin C to detect Ca2+ in meat. Food Chem. 2020, 307, 125645. [Google Scholar] [CrossRef]
- Messaoud, N.B.; Ghica, M.E.; Dridi, C.; Ben Ali, M.; Brett, C.M. Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens. Actuators B Chem. 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Pissuwan, D.; Gazzana, C.; Mongkolsuk, S.; Cortie, M.B. Single and multiple detections of foodborne pathogens by gold nanoparticle assays. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1584. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Mohan, C.O.; Gunasekaran, S.; Ravishankar, C.N. Chitosan-capped gold nanoparticles for indicating temperature abuse in frozen stored products. NPJ Sci. Food 2019, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. A label-free ultrasensitive microfluidic surface Plasmon resonance biosensor for Aflatoxin B(1) detection using nanoparticles integrated gold chip. Food Chem. 2020, 307, 125530. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.G.; Buyukgoz, G.G.; Soforoglu, M.; Tamer, U.; Suludere, Z.; Boyaci, I.H. Alkaline phosphatase labeled SERS active sandwich immunoassay for detection of Escherichia coli. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 194, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhuang, Z.; Wang, Z.; Li, S.; Zhong, H.; Liu, Z.; Guo, Z.; Zhang, W. Black phosphorus-Au filter paper-based three-dimensional SERS substrate for rapid detection of foodborne bacteria. Appl. Surf. Sci. 2019, 497, 143825. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Zhong, H.-X.; Luo, H.; Zhang, R.-Y.; Huang, J.-R. Recombinase polymerase amplification combined with unmodified gold nanoparticles for Salmonella detection in milk. Food Anal. Methods 2018, 12, 190–197. [Google Scholar] [CrossRef]
- Du, J.; Singh, H.; Dong, W.-J.; Bai, Y.-H.; Yi, T.-H. Colorimetric detection of Listeria monocytogenes using one-pot biosynthesized flower-shaped gold nanoparticles. Sens. Actuators B Chem. 2018, 265, 285–292. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.-S.; Chon, J.-W.; Kim, D.-H.; Hyeon, J.-Y.; Seo, K.-H. New colorimetric aptasensor for rapid on-site detection of Campylobacter jejuni and Campylobacter coli in chicken carcass samples. Anal. Chim. Acta 2018, 1029, 78–85. [Google Scholar] [CrossRef]
- Li, F.; Yang, G.; Aguilar, Z.P.; Lai, W.; Xu, H. Asymmetric polymerase chain assay combined with propidium monoazide treatment and unmodified gold nanoparticles for colorimetric detection of viable emetic Bacillus cereus in milk. Sens. Actuators B Chem. 2017, 255 Pt 2, 1455–1461. [Google Scholar] [CrossRef]
- Oh, S.Y.; Heo, N.S.; Shukla, S.; Cho, H.J.; Vilian, A.E.; Kim, J.; Lee, S.Y.; Han, Y.-K.; Yoo, S.M.; Huh, Y.S. Development of gold nanoparticle-aptamer-based LSPR sensing chips for the rapid detection of Salmonella typhimurium in pork meat. Sci. Rep. 2017, 7, 10130. [Google Scholar] [CrossRef] [Green Version]
- Song, C. Development of a lateral flow colloidal gold immunoassay strip for the simultaneous detection of Shigella boydii and Escherichia coli O157:H7 in bread, milk and jelly samples. Food Control. 2016, 59, 345–351. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Song, S.; Xu, L.; Zhu, J.; Kuang, H. Gold nanoparticle-based paper sensor for multiple detection of 12 Listeria spp. by P60-mediated monoclonal antibody. Food Agric. Immunol. 2017, 28, 274–287. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.-W.; Pu, H.; Wei, Q. Ultrasensitive analysis of kanamycin residue in milk by SERS-based aptasensor. Talanta 2019, 197, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L.; Xu, L.; Song, S.; Kuang, H.; Cui, G.; Xu, C. Gold immunochromatographic sensor for the rapid detection of twenty-six sulfonamides in foods. Nano Res. 2017, 10, 2833–2844. [Google Scholar] [CrossRef]
- Vaisocherová-Lísalová, H.; Víšová, I.; Ermini, M.L.; Springer, T.; Song, X.C.; Mrazek, J.; Lamačová, J.; Lynn, N.S.; Šedivák, P.; Homola, J. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens. Bioelectron. 2016, 80, 84–90. [Google Scholar] [CrossRef]
- Oh, S.Y.; Lee, M.J.; Heo, N.S.; Kim, S.; Oh, J.S.; Lee, Y.; Jeon, E.J.; Moon, H.; Kim, H.S.; Park, T.J.; et al. Cuvette-type LSPR sensor for highly sensitive detection of melamine in infant formulas. Sensors 2019, 19, 3839. [Google Scholar] [CrossRef] [Green Version]
- Neethirajan, S.; Weng, X.; Tah, A.; Cordero, J.; Ragavan, K. Nano-biosensor platforms for detecting food allergens—New trends. Sens. Bio-Sens. Res. 2018, 18, 13–30. [Google Scholar] [CrossRef]
- El-Nour, K.M.A.; Salam, E.T.A.; Soliman, H.M.; Orabi, A.S. Gold nanoparticles as a direct and rapid sensor for sensitive analytical detection of biogenic amines. Nanoscale Res. Lett. 2017, 12, 231. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Lin, H.; Kannan, P.; Wang, C.; Zhong, Y.; Huang, Y.; Guo, Z. Enhanced antibacterial and food simulant activities of silver nanoparticles/polypropylene nanocomposite films. Langmuir 2018, 34, 14537–14545. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B.; Shankar, S.; Dennis, T.; Wacławek, S.; Černík, M.; Padil, V. Chitosan/gelatin/silver nanoparticles composites films for biodegradable food packaging applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Olmos, D.; Pontes-Quero, G.M.; Corral, A.; González-Gaitano, G.; Gonzãlez-Benito, J. Preparation and characterization of antimicrobial films based on LPDE/Ag nanoparticles with potential uses in food and health industries. Nanomaterials 2018, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Duarte, G.; Pérez-Cabrera, L.E.; Artés-Hernández, F.; Martínez-Hernández, G.B. Ag-chitosan nanocomposites in edible coatings affect the quality of fresh-cut melon. Postharvest Biol. Technol. 2019, 147, 174–184. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Mohammad-Razdari, A.; Yoosefian, S.H.; Izadi, Z. Effects of the combination of gamma irradiation and Ag nanoparticles polyethylene films on the quality of fresh bottom mushroom (Agaricus bisporus L.). J. Food Processing Preserv. 2018, 42, e13652. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, W.; Zhu, B.; Chen, H.; Chi, H.; Li, L.; Qin, Y.; Xue, J. The quality evaluation of postharvest strawberries stored in nano-Ag packages at refrigeration temperature. Polymers 2018, 10, 894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrami, A.; Mokarram, R.R.; Khiabani, M.S.; Ghanbarzadeh, B.; Salehi, R. Physico-mechanical and antimicrobial properties of tragacanth/hydroxypropyl methylcellulose/beeswax edible films reinforced with silver nanoparticles. Int. J. Biol. Macromol. 2019, 129, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.W.A.; Qaisar, M.; Jahangir, M.; Abbasi, K.S.; Khan, S.U.; Ali, N.; Liaquat, M. Influence of CMC- and guar gum-based silver nanoparticle coatings combined with low temperature on major aroma volatile components and the sensory quality of kinnow (Citrus reticulata). Int. J. Food Sci. Technol. 2016, 51, 2345–2352. [Google Scholar] [CrossRef]
- Mathew, S.; Snigdha, S.; Jyothis, M.; Radhakrishnan, E.K. Biodegradable and active nanocomposite pouches reinforced with silver nanoparticles for improved packaging of chicken sausages. Food Packag. Shelf Life 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Shankar, S.; Oun, A.A.; Rhim, J.W. Preparation of antimicrobial hybrid nano-materials using regenerated cellulose and metallic nanoparticles. Int. J. Biol. Macromol. 2018, 107 Pt A, 17–27. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose acetate/AgNPs-organoclay and/or thymol nano-biocomposite films with combined antimicrobial/antioxidant properties for active food packaging use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef] [Green Version]
- Kadam, D.; Momin, B.; Palamthodi, S.; Lele, S. Physicochemical and functional properties of chitosan-based nano-composite films incorporated with biogenic silver nanoparticles. Carbohydr. Polym. 2019, 211, 124–132. [Google Scholar] [CrossRef]
- Vishnuvarthanan, M.; Rajeswari, N. Preparation and characterization of carrageenan/silver nanoparticles/Laponite nanocomposite coating on oxygen plasma surface modified polypropylene for food packaging. J. Food Sci. Technol. 2019, 56, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, C.; Zhang, Y.; Yao, J.; Yang, W.; Hu, Q.; Wang, C.; Cao, C. Effect of stable antimicrobial nano-silver packaging on inhibiting mildew and in storage of rice. Food Chem. 2017, 215, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Deng, W.; Luo, J.; Deng, D. Multifunctional nano-cellulose composite films with grape seed extracts and immobilized silver nanoparticles. Carbohydr. Polym. 2019, 205, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sahareen, T. Investigation of cellulosic packets impregnated with silver nanoparticles for enhancing shelf-life of vegetables. LWT 2017, 86, 116–122. [Google Scholar] [CrossRef]
- Bittar, D.B.; Catelani, T.A.; Nigoghossian, K.; Barud, H.D.S.; Ribeiro, S.; Pezza, L.; Pezza, H.R. Optimized synthesis of silver nanoparticles by factorial design with application for the determination of melamine in milk. Anal. Lett. 2017, 50, 829–841. [Google Scholar] [CrossRef]
- Omole, R.; Torimiro, N.; Alayande, S.; Ajenifuja, E. Silver nanoparticles synthesized from Bacillus subtilis for detection of deterioration in the post-harvest spoilage of fruit. Sustain. Chem. Pharm. 2018, 10, 33–40. [Google Scholar] [CrossRef]
- Sachdev, D.; Kumar, V.; Maheshwari, P.H.; Pasricha, R.; Deepthi; Baghel, N. Silver based nanomaterial, as a selective colorimetric sensor for visual detection of post harvest spoilage in onion. Sens. Actuators B Chem. 2016, 228, 471–479. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull. Natl. Res. Cent. 2019, 43, 83. [Google Scholar] [CrossRef]
- Pandit, R.; Rai, M.; Santos, C.A. Enhanced antimicrobial activity of the food-protecting nisin peptide by bioconjugation with silver nanoparticles. Environ. Chem. Lett. 2017, 15, 443–452. [Google Scholar] [CrossRef]
- Madhavan, A.A.; Qotainy, R.; Nair, R. Synthesis of functionalized silver nanoparticles and its application as chemical sensor. In Proceedings of the 2019 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 26 March–10 April 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Ebrahimiasl, S.; ¸Rajabpour, A. Synthesis and characterization of novel bactericidal Cu/HPMC BNCs using chemical reduction method for food packaging. J. Food Sci. Technol. 2015, 52, 5982–5988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Jin, S.; Liu, X.; Chen, H.; He, J.; Li, J. Preparation and characterization of chitosan/soy protein isolate nanocomposite film reinforced by Cu nanoclusters. Polymers 2017, 9, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzi, F.; Zanfrognini, B.; Ruggeri, S.; Dossi, N.; Casagrande, G.M.; Piccin, E. Amperometric paper sensor based on Cu nanoparticles for the determination of carbohydrates. Sens. Actuators B Chem. 2017, 245, 352–358. [Google Scholar] [CrossRef]
- Tunesi, M.; Kalwar, N.H.; Abbas, M.W.; Karakuş, S.; Soomro, R.; Kilislioglu, A.; Abro, M.I.; Hallam, K.R. Functionalised CuO nanostructures for the detection of organophosphorus pesticides: A non-enzymatic inhibition approach coupled with nano-scale electrode engineering to improve electrode sensitivity. Sens. Actuators B Chem. 2018, 260, 480–489. [Google Scholar] [CrossRef]
- Al’Abri, A.M.; Halim, S.N.A.; Abu Bakar, N.K.; Saharin, S.M.; Sherino, B.; Nodeh, H.R.; Mohamad, S. Highly sensitive and selective determination of malathion in vegetable extracts by an electrochemical sensor based on Cu-metal organic framework. J. Environ. Sci. Health B 2019, 54, 930–941. [Google Scholar] [CrossRef]
- Castro Mayorga, J.L.; Rovira, M.J.F.; Mas, L.C.; Moragas, G.S.; Cabello, J.M.L. Antimicrobial nanocomposites and electrospun coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J. Appl. Polym Sci. 2018, 135, 45673. [Google Scholar] [CrossRef] [Green Version]
- Arfat, Y.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Physico-mechanical characterization and antimicrobial properties of fish protein isolate/fish skin gelatin-zinc oxide (ZnO) nanocomposite films. Food Bioprocess Technol. 2016, 9, 101–112. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lara-Lledo, M.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. LWT 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Alves, M.; Santos, C.; Ribeiro, I.; Rodrigues, C.; Coelhoso, I.; Fernando, A. Biodegradable chitosan films with ZnO nanoparticles synthesized using food industry by-products—production and characterization. Coatings 2021, 11, 646. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.-W. Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Divsalar, E.; Tajik, H.; Moradi, M.; Forough, M.; Lotfi, M.; Kuswandi, B. Characterization of cellulosic paper coated with chitosan-zinc oxide nanocomposite containing nisin and its application in packaging of UF cheese. Int. J. Biol. Macromol. 2018, 109, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef] [PubMed]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Li, W.; Li, M.; Deng, Q.; Wang, Y.; Li, C.; Chu, P.K. Ecofriendly and biodegradable soybean protein isolate films incorporated with ZnO nanoparticles for food packaging. ACS Appl. Bio Mater. 2019, 2, 2202–2207. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.M.; Muraleedharan, K. Flexible chitosan-nano ZnO antimicrobial pouches as a new material for extending the shelf life of raw meat. Int. J. Biol. Macromol. 2017, 97, 382–391. [Google Scholar] [CrossRef]
- Rezaei, F.; Shahbazi, Y. Shelf-life extension and quality attributes of sauced silver carp fillet: A comparison among direct addition, edible coating and biodegradable film. LWT 2018, 87, 122–133. [Google Scholar] [CrossRef]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.F.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated Type B gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Pirsa, S.; Shamusi, T. Intelligent and active packaging of chicken thigh meat by conducting nano structure cellulose-polypyrrole-ZnO film. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 798–809. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J.; Wu, C. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Lavinia, M.; Hibarturrahman, S.; Harinata, H.; Wardana, A.A. Antimicrobial activity and application of nanocomposite coating from chitosan and ZnO nanoparticle to inhibit microbial growth on fresh-cut papaya. Food Res. 2019, 4, 307–311. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO(2) composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y.; et al. Effects of different TiO2 nanoparticles concentrations on the physical and antibacterial activities of chitosan-based coating film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of antioxidant and antimicrobial coating based on whey protein nanofibrils with TiO2; nanotubes on the quality and shelf life of chilled meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, S.; Partovi, R.; Talebi, F.; Babaei, A. Chitosan/TiO2 nanoparticle/Cymbopogon citratus essential oil film as food packaging material: Physico-mechanical properties and its effects on microbial, chemical, and organoleptic quality of minced meat during refrigeration. J. Food Processing Preserv. 2020, 44, e14536. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Abdollahi, M. Effect of gelatin/agar bilayer film incorporated with TiO2 nanoparticles as a UV absorbent on fish oil photooxidation. Int. J. Food Sci. Technol. 2017, 52, 1862–1868. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal oxide-based nanocomposites in food packaging: Applications, migration, and regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Zhang, Y.; Shang, Y.; Zhang, X.; Wen, Y. Preparation of PAN@TiO2; nanofibers for fruit packaging materials with efficient photocatalytic degradation of ethylene. Materials 2019, 12, 896. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh-Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Ur Rehman, Z. Review of recent advances in polylactic acid/TiO2 composites. Materials 2019, 12, 3659. [Google Scholar] [CrossRef] [Green Version]
- Albelda, J.A.V.; Uzunoglu, A.; Santos, G.N.C.; Stanciu, L.A. Graphene-titanium dioxide nanocomposite based hypoxanthine sensor for assessment of meat freshness. Biosens. Bioelectron. 2017, 89 Pt 1, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Farhoodi, M.; Mohammadifar, M.A.; Mousavi, M.; Sotudeh-Gharebagh, R.; Emam-Djomeh, Z. Migration kinetics of ethylene glycol monomer from pet bottles into acidic food simulant: Effects of nanoparticle presence and matrix morphology. J. Food Processing Eng. 2017, 40, e12383. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Shang, Y.; Wen, Y. Electrospun nanofibers containing TiO2 for the photocatalytic degradation of ethylene and delaying postharvest ripening of bananas. Food Bioprocess Technol. 2019, 12, 281–287. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Lian, Z.; Zhang, Y.; Zhao, Y. Nano-TiO2 particles and high hydrostatic pressure treatment for improving functionality of polyvinyl alcohol and chitosan composite films and nano-TiO2 migration from film matrix in food simulants. Innov. Food Sci. Emerg. Technol. 2016, 33, 145–153. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.; Salama, H.H.; Assem, F.; El-Salam, M.H.A. Novel bionanocomposite materials used for packaging skimmed milk acid coagulated cheese (Karish). Int. J. Biol. Macromol. 2018, 115, 1002–1011. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, H.S.; El-Nagar, I.; El-Sayed, S.M. Preparation and characterization of novel bionanocomposites based on garlic extract for preserving fresh Nile tilapia fish fillets. RSC Adv. 2021, 11, 22571–22584. [Google Scholar] [CrossRef]
- He, L.; Wang, F.; Chen, Y.; Liu, Y. Rapid and sensitive colorimetric detection of ascorbic acid in food based on the intrinsic oxidase-like activity of MnO2 nanosheets. Luminescence 2018, 33, 145–152. [Google Scholar] [CrossRef] [Green Version]
- De Silva, R.T.; Mantilaka, P.; Ratnayake, S.; Amaratunga, G.; de Silva, K.N. Nano-MgO reinforced chitosan nanocomposites for high performance packaging applications with improved mechanical, thermal and barrier properties. Carbohydr. Polym. 2017, 157, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Foltynowicz, Z.; Bardenshtein, A.; Sängerlaub, S.; Antvorskov, H.; Kozak, W. Nanoscale, zero valent iron particles for application as oxygen scavenger in food packaging. Food Packag. Shelf Life 2017, 11, 74–83. [Google Scholar] [CrossRef]
- Cherpinski, A.; Gozutok, M.; Sasmazel, H.T.; Torres-Giner, S.; Lagaron, J.M. Electrospun oxygen scavenging films of poly(3-hydroxybutyrate) containing palladium nanoparticles for active packaging applications. Nanomaterials 2018, 8, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putri, V.J.; Warsiki, E.; Syamsu, K.; Iskandar, A. Application Nano Zeolite-Molybdate for Avocado Ripeness Indicator; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 347, p. 012063. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. New antioxidant multilayer packaging with nanoselenium to enhance the shelf-life of market food products. Nanomaterials 2018, 8, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hondred, J.A.; Nickmans, K.; Lub, J.; Debije, M.G.; Schenning, A.P. Printed graphene electrochemical biosensors fabricated by inkjet maskless lithography for rapid and sensitive detection of organophosphates. ACS Appl. Mater. Interfaces 2018, 10, 11125–11134. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.I.; Gülle, S.; Yilmaz, E.; Soylak, M. Trace determination of vitamin B12 in food samples by using Fe3O4 magnetic particles including multi-walled carbon nanotubes and nanodiamonds. Anal. Methods 2019, 11, 5108–5117. [Google Scholar] [CrossRef]
- Mirabi, A.; Dalirandeh, Z.; Rad, A.S. Preparation of modified magnetic nanoparticles as a sorbent for the preconcentration and determination of cadmium ions in food and environmental water samples prior to flame atomic absorption spectrometry. J. Magn. Magn. Mater. 2015, 381, 138–144. [Google Scholar] [CrossRef]
- Varaprasad, K.; Pariguana, M.; Raghavendra, G.M.; Jayaramudu, T.; Sadiku, R. Development of biodegradable metaloxide/polymer nanocomposite films based on poly-ε-caprolactone and terephthalic acid. Mater. Sci. Eng. C 2017, 70, 85–93. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Pakdel, P.M. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Preparation of multifunctional chitin nanowhiskers/ZnO-Ag NPs and their effect on the properties of carboxymethyl cellulose-based nanocomposite film. Carbohydr. Polym. 2017, 169, 467–479. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Ilyas, S.; Hezam, A.; Mathur, S.; Byrappa, K. Smart fortified PHBV-CS biopolymer with ZnO–Ag nanocomposites for enhanced shelf life of food packaging. ACS Appl. Mater. Interfaces 2019, 11, 48309–48320. [Google Scholar] [CrossRef] [PubMed]
- Ewelina, J.; Pavel, K.; Lesław, J.; Agnieszka, K.; Zuzana, B.; Vedran, M.; Małgorzata, M. Development of furcellaran-gelatin films with Se-AgNPs as an active packaging system for extension of mini kiwi shelf life. Food Packag. Shelf Life 2019, 21, 100339. [Google Scholar] [CrossRef]
- Panea, B.; Albertí, P.; Ripoll, G. Effect of high pressure, calcium chloride and ZnO-Ag nanoparticles on beef color and shear stress. Foods 2020, 9, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, M.C.; Tiimob, B.J.; Abdela, W.; Jeelani, S.; Rangari, V.K. Nano silica-carbon-silver ternary hybrid induced antimicrobial composite films for food packaging application. Food Packag. Shelf Life 2019, 19, 104–113. [Google Scholar] [CrossRef]
- Chi, H.; Song, S.; Luo, M.; Zhang, C.; Li, W.; Li, L.; Qin, Y. Effect of PLA nanocomposite films containing bergamot essential oil, TiO2 nanoparticles, and Ag nanoparticles on shelf life of mangoes. Sci. Hortic. 2019, 249, 192–198. [Google Scholar] [CrossRef]
- Beigmohammadi, F.; Peighambardoust, S.H.; Hesari, J.; Azadmard-Damirchi, S.; Khosrowshahi, N.K. Antibacterial properties of LDPE nanocomposite films in packaging of UF cheese. LWT 2016, 65, 106–111. [Google Scholar] [CrossRef]
- Wang, C.; Hu, L.; Zhao, K.; Deng, A.; Li, J. Multiple signal amplification electrochemiluminescent immunoassay for Sudan I using gold nanorods functionalized graphene oxide and palladium/aurum core-shell nanocrystallines as labels. Electrochim. Acta 2018, 278, 352–362. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active chicken meat packaging based on polylactide films and bimetallic Ag-Cu nanoparticles and essential oil. J. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Castro-Aguirre, E.; Auras, R. Mechanical, structural and thermal properties of Ag-Cu and ZnO reinforced polylactide nanocomposite films. Int. J. Biol. Macromol. 2016, 86, 885–892. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Jacob, H. Preparation and characterization of agar-based nanocomposite films reinforced with bimetallic (Ag-Cu) alloy nanoparticles. Carbohydr. Polym. 2017, 155, 382–390. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ejaz, M.; Jacob, H.; Ahmed, J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohydr. Polym. 2017, 157, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of antimicrobial packaging film made from poly(lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Li, L.; Zhang, H.; Yuan, M.; Qin, Y. Evaluation of PLA nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese. J. Food Processing Preserv. 2017, 42, e13362. [Google Scholar] [CrossRef]
- Paidari, S.; Ibrahim, S.A. Potential application of gold nanoparticles in food packaging: A mini review. Gold Bull. 2021, 54, 31–36. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Yang, S.Z.; Liu, Q.-A.; Liu, Y.-L.; Weng, G.-J.; Zhu, J.; Li, J.-J. Recent progress in the optical detection of pathogenic bacteria based on noble metal nanoparticles. Mikrochim. Acta 2021, 188, 258. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications—A review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshi, R.; Roy, S.; Ghosh, T.; Biswas, D.; Rhim, J.-W. Antimicrobial nanofillers reinforced biopolymer composite films for active food packaging applications—A review. Sustain. Mater. Technol. 2021, e00353. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of silver nanoparticles and their biomedical applications—A comprehensive review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Youssef, A.M.; El-Sayed, S.M.; Salama, H.H.; El-Sayed, H.S.; Dufresne, A. Evaluation of bionanocomposites as packaging material on properties of soft white cheese during storage period. Carbohydr. Polym. 2015, 132, 274–285. [Google Scholar] [CrossRef]
- Donglu, F.; Wenjian, Y.; Kimatu, B.M.; Mariga, A.M.; Liyan, Z.; Xinxin, A.; Qiuhui, H. Effect of nanocomposite-based packaging on storage stability of mushrooms (Flammulina velutipes). Innov. Food Sci. Emerg. Technol. 2016, 33, 489–497. [Google Scholar] [CrossRef]
- Khan, R.; Rehman, A.; Hayat, A.; Andreescu, S. Magnetic Particles-Based Analytical Platforms for Food Safety Monitoring. Magnetochemistry 2019, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Asensio-Ramos, M.; Rodríguez-Delgado, M.Á. Recent Applications of Magnetic Nanoparticles in Food Analysis. Processes 2020, 8, 1140. [Google Scholar] [CrossRef]
- Yu, X.; Zhong, T.; Zhang, Y.; Zhao, X.; Xiao, Y.; Wang, L.; Liu, X.; Zhang, X. Design, Preparation, and Application of Magnetic Nanoparticles for Food Safety Analysis: A Review of Recent Advances. J. Agric. Food Chem. 2022, 70, 46–62. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Scown, T.M.; Santos, E.; Johnston, B.D.; Gaiser, B.K.; Baalousha, M.; Mitov, S.; Lead, J.R.; Stone, V.; Fernandes, T.; Jepson, A.M.; et al. Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol. Sci. 2010, 115, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ilan, O.; Albrecht, R.M.; Fako, V.E.; Furgeson, D.Y. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In vitro methods for assessing nanoparticle toxicity. In Nanotoxicity. Methods in Molecular Biology; Zhang, Q., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1894. [Google Scholar] [CrossRef]
- Osman, I.F.; Baumgartner, A.; Cemeli, E.; Fletcher, J.N.; Anderson, D. Genotoxicity and cytotoxicity of zinc oxide and titanium dioxide in HEp-2 cells. Nanomedicine 2010, 5, 1193–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, B.; Br, T.; Czifra, G.; Tóth, B.I.; Kertész, Z.; Szikszai, Z.; Kiss, Z.; Juhász, I.; Zouboulis, C.C.; Hunyadi, J. Investigation of micronized titanium dioxide penetration in human skin xenografts and its effect on cellular functions of human skin-derived cells. Exp. Dermatol. 2008, 17, 659–667. [Google Scholar] [CrossRef]
- Bour, A.; Mouchet, F.; Cadarsi, S.; Silvestre, J.; Verneuil, L.; Baqué, D.; Chauvet, E.; Bonzom, J.-M.; Pagnout, C.; Clivot, H.; et al. Toxicity of CeO2 nanoparticles on a freshwater experimental trophic chain: A study in environmentally relevant conditions through the use of mesocosms. Nanotoxicology 2015, 10, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Rajput, V.; Minkina, T.; Fedorenko, A.; Sushkova, S.; Mandzhieva, S.; Lysenko, V.; Duplii, N.; Fedorenko, G.; Dvadnenko, K.; Ghazaryan, K. Toxicity of copper oxide nanoparticles on spring barley (Hordeum sativum distichum). Sci. Total Environ. 2018, 645, 1103–1113. [Google Scholar] [CrossRef]
- OECD. Recommendation of the Council on the Safety Testing and Assessment of Manufactured Nanomaterials; OECD/LEGAL/0400. Available online: https://legalinstruments.oecd.org/en/instruments/OECD-LEGAL-0400 (accessed on 8 January 2022).
- van der Meulen, B.; Bremmers, H.; Purnhagen, K.; Gupta, N.; Bouwmeester, H.; Geyer, L.L. Nano-specific regulation. In Governing Nano Foods: Principles-Based Responsive Regulation; van der Meulen, B., Bremmers, H., Purnhagen, K., Gupta, N., Bouwmeester, H., Geyer, L., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 43–45. [Google Scholar] [CrossRef]
- Pavlicek, A.; Part, F.; Rose, G.; Praetorius, A.; Miernicki, M.; Gazsó, A.; Huber-Humer, M. A European nano-registry as a reliable database for quantitative risk assessment of nanomaterials? A comparison of national approaches. NanoImpact 2021, 21, 100276. [Google Scholar] [CrossRef]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- European Commission. General Food Law. Available online: https://ec.europa.eu/food/horizontal-topics/general-food-law_en (accessed on 8 January 2022).
- Meghani, N.; Dave, S.; Kumar, A. Introduction to Nanofood. In Nano-Food Engineering. Food Engineering Series; Hebbar, U., Ranjan, S., Dasgupta, N., Mishra, R.K., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015. OJ L 327, 11.12.2015, p. 1–22. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32015R2283 (accessed on 8 January 2022).
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011. OJ L 304, 22.11.2011, p. 18–63. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1169 (accessed on 8 January 2022).
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008. OJ L 354, 31.12.2008, p. 16–33. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1333 (accessed on 8 January 2022).
- Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013. OJ L 181, 29.6.2013, p. 35–56. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32013R0609 (accessed on 8 January 2022).
- Commission Regulation (EU) No 10/2011 of 14 January 2011. OJ L 12, 15.1.2011, p. 1–89. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R0010 (accessed on 8 January 2022).
- Commission Regulation (EC) No 450/2009 of 29 May 2009. OJ L 135, 30.5.2009, p. 3–11. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R0450 (accessed on 8 January 2022).
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021, 19, e06768. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA J. 2021, 19, e06769. [Google Scholar] [CrossRef] [PubMed]
- FDA. U.S. Guidance for Industry: “Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology”. Fed. Regist. 2014, 79, 36534–36535. [Google Scholar]
- FDA. U.S. Guidance for Industry: “Assessing the Effects of Significant Manufacturing Process Changes, Including Emerging Technologies, on the Safety and Regulatory Status of Food Ingredients and Food Contact Substances, including Food Ingredients that Are Color Additives”. Fed. Regist. 2014, 79, 36533–36534. [Google Scholar]
- FDA’s Approach to Regulation of Nanotechnology Products. Available online: https://www.fda.gov/science-research/nanotechnology-programs-fda/fdas-approach-regulation-nanotechnology-products (accessed on 8 January 2022).

| MNP | Application | Film/Analyte/Food | Ref. |
|---|---|---|---|
| Au-NP | SERS sensor | Bisphenol/milk | [31] |
| Electrochemical sensor | Ca2+/meat | [32] | |
| Electrochemical biosensor | Bisphenol A/waters | [33] | |
| Rapid detection of single or multiple foodborne pathogens | Foodborne pathogens | [34] | |
| SPR with Au-NPs | − | [35] | |
| Temperature indicator | Chitosan-capped Au-NPs/frozen products | [36] | |
| SPR biosensor | Aflatoxin B1 | [37] | |
| SERS active sandwich immunoassay | E. coli | [38] | |
| Filter paper-based SERS substrates | Black phosphorus-Au/S. aureus, L. monocytogenes and E. coli | [39] | |
| Isothermal RPA detection | Salmonella detection/milk | [40] | |
| Colorimetric sensor | hlyA gene and genomic DNA of Listeria monocytogenes | [41] | |
| Aptasensor | C. jejuni and C. coli/chicken carcass | [42] | |
| Polymerase chain assay colorimetric sensor | Emetic Bacillus cereus/milk | [43] | |
| Portable plasmonic biosensor | Melamine/infant formula | [44] | |
| Colloidal Au immunochromatographic strip | Simultaneous detection of S. boydii + E. coli | [45] | |
| Paper sensor | Listeria spp./milk | [46] | |
| SERS-based aptasensor | Kanamycin residue/milk | [47] | |
| Immunochromatographic sensor | 26 sulfonamides/commercial honey | [48] | |
| Low-fouling SPR biosensor | E. coli and Salmonella spp./cucumber and hamburger | [49] | |
| Cuvette-type localized SPR optical biosensor | Melamine/infant formulas | [50] | |
| POC biosensors | Food allergens | [51] | |
| Optical sensor | Biogenic amines/poultry meat | [52] | |
| Ag-NP | Films with enhanced antibacterial and migration properties | PP-Ag nanocomposite | [53] |
| Biodegradable food packaging | Chitosan/gelatin/Ag-NP composites/carrot pieces | [54] | |
| Antimicrobial films | LDPE/Ag-NPs | [55] | |
| Edible coatings (thin layers of material on the product surface) | Ag-chitosan nanocomposites into chitosan coatings/fresh-cut melon | [56] | |
| Combined use of gamma irradiation and PE/Ag-NP films | PP/Ag-NPs/fresh bottom mushroom | [57] | |
| Multifunctional packaging | Chitosan coated PE films (lecithin-liposomes/laurel essential oil/Ag-NPs)/pork | [58] | |
| Active nanocomposite packaging film | PLA/Ag-NPs/strawberries | [59] | |
| Physico-mechanical and antimicrobial edible films | Tragacanth/HPMC/bees-wax/Ag-NPs | [60] | |
| Coating films | Guar gum-Ag coatings/coated kinnow (Citrus reticulata cv. Blanco) | [61] | |
| Antimicrobial food packaging | PVA/nanocellulose/Ag nanocomposite | [30] | |
| Packaging | Biodegradable PVA-montmorillonite K10 clay nanocomposite blend films with in situ generated ginger extract mediated Ag-NP pouches/chicken sausages | [62] | |
| Antimicrobial materials | Hybrid nanomaterials(cellulose/Ag-NPs) | [63] | |
| Active food packaging: antimicrobial/antioxidant | Cellulose acetate/AgNP-organoclay and/or thymol nanobiocomposite films | [64] | |
| Packaging | Chitosan based nanocomposite films incorporated with biogenic Ag-NPs | [65] | |
| Packaging | Carrageenan/Ag-NP/laponite nanocomposite coated on oxygen plasma surface–modified PP film | [66] | |
| Packaging | A. flavus/mildew and storage of rice | [67] | |
| Packaging | Nano-cellulose composite films/grape seed extracts/Ag-NPs (antimicrobial activity against E. coli and S. aureus + strong antioxidant activity) | [68] | |
| Packaging | Cellulosic packets impregnated with Ag-NPs/Aeromonas sp. isolated from rotten vegetables (tomatoes and cabbage) | [69] | |
| Colorimetric assay based on Ag-NPs | Melamine/milk | [70] | |
| Colorimetric sensor | Ag-NP solution synthesized using culture supernatant B. subtilis/volatile compounds released during the deterioration of Musa acuminata (banana) | [71] | |
| Colorimetric sensor | Ag-based nanomaterial/onion postharvest spoilage | [72] | |
| Packaging | Chitosan-Ag-NPs/minced meat | [73] | |
| Packaging | Agar film containing nanoAg conjugate (Cymbopogon citratus extract/nisin/Ag)/L. monocytogenes, S. aureus, P. fluorescens, A. niger and F. moniliforme | [74] | |
| Food spoilage detection and post-harvest spoilage | Cysteine and histidine incorporated Ag-NP lactic acid/fresh milk | [75] | |
| Cu-NP | Antibacterial surfaces | Antibacterial effect of Cu-polymer nanocomposites | [76] |
| Packaging | Biodegradable HPMC matrix/Cu-NPs/ S. aureus, S. epidermidis, B. cereus, E. coli, E. faecalis, Salmonella spp., P. aeruginosa/meat | [77] | |
| Packaging | Chitosan/soy protein isolate nanocomposite film | [78] | |
| Amperometric paper sensor | Carbohydrates/soft drinks | [79] | |
| Electrochemical biosensor | Organophosphorus pesticides (chlorpyrifos, fenthion and methylparathion)/cabbage and spinach extract | [80] | |
| Electrochemical sensor | Malathion/vegetable extracts | [81] | |
| Active biodegradable film | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites/CuO-NPs/ S. enterica, L. monocytogenes and murine norovirus | [82] | |
| Zn-NP | Active food packaging material | Fish protein isolate and fish skin gelatin/ZnO-NPs | [83] |
| Packaging | PLA/ZnO biocomposite films | [84] | |
| Antimicrobial packaging | PLA/ZnO nanocomposite coated paper | [85] | |
| Biodegradable film | Bionanocomposites of chitosan/ZnO-NPs/apple peels, fresh poultry meat | [86] | |
| Packaging | Gelatin/ZnO-NP nanocomposite film/ foodborne pathogenic bacteria | [87] | |
| Packaging | Nanocomposite film of chitosan-ZnO-cellulose/nisin/cheese | [88] | |
| Packaging | Hybrid nanocellulose-ZnO-NP-based composites | [89] | |
| Antimicrobial packaging | Chitosan/ZnO coated on PE film | [90] | |
| Biodegradable food packaging | Soybean protein/ZnO film | [91] | |
| Food packaging | Chitosan/ZnO antimicrobial pouches/raw meat | [92] | |
| Food packaging | Ziziphora clinopodioides essential oil/apple peel extract/ZnO-NPs/Listeria monocytogenes/sauced silver carp fillet | [93] | |
| Food packaging | ZnO nanorods/clove essential oil/Type B gelatin composite films/L. monocytogenes + Salmonella Typhimurium/shrimps | [94] | |
| Intelligent and active films | Cellulose modified with polypyrrole/ZnO/microbial load chicken thigh | [95] | |
| Multifunctional bionanocomposite films | Konjac glucomannan/chitosan/ZnO/mulberry anthocyanin extract/E. coli and S. aureus | [96] | |
| Coating | Chitosan/ZnO-NPs/microbial growth on fresh-cut papaya | [97] | |
| Ti-NP | Active and smart packaging | Chitosan-TiO2 composite film/antimicrobial activity against E. coli, S. aureus, C.albicans, A. niger/red grapes | [98] |
| Coating film | TiO2-NPs/chitosan | [99] | |
| Edible films and coatings | Whey protein nanofibrils/glycerol/TiO2/ L. monocytogenes, S. aureus, S. enteritidis, E. coli/meat | [100] | |
| Packaging | Chitosan film/Cymbopogon citratus essential oil/TiO2-NPs/minced meat | [101] | |
| UV absorbent film | Gelatin/agar bilayer film and nanocomposites/TiO2-NPs/fish oil | [102] | |
| Packaging | TiO2-NPs/PLA/several bacteria strains | [103] | |
| Active and smart packaging | PAN/TiO2 nanofibers/tomato fruit-ripening test | [104] | |
| Film | Whey protein/cellulose nanofiber/nanocomposite films/TiO2/rosemary essential oil/foodborne bacteria/lamb meat | [105] | |
| Food packaging | PLA/TiO2 composites | [106] | |
| Amperometric sensor | Graphene/TiO2 nanocomposite/hypoxanthine/meat freshness evaluation | [107] | |
| Packaging | PET/TiO2-NPs/ethylene glycol migration | [108] | |
| Active and smart packaging | PAN/nanofibers/postharvest ripening of bananas | [109] | |
| Active food packaging (ethylene scavenging + antimicrobial activity) | Chitosan/TiO2 nanocomposite film/S. aureus, E.coli, Salmonella Typhimurium, P. aeruginosa, Aspergillus and Penicillium | [110] | |
| Biodegradable food packaging | Starch/TiO2 bionanocomposite | [111] | |
| Biodegradable food packaging | Chitosan/PVA/Ti-NPs/olive oils | [112] | |
| Active packaging | Chitosan- TiO2 nanocomposite film/tomato storage shelf life | [113] | |
| Packaging | Chitosan/PVA/skimmed milk acid coagulated cheese (Karish) | [114] | |
| Edible coating | CMC/gum arabic/gelatin/garlic extract/TiO2-NPs/Nile tilapia fish fillets | [115] | |
| Other single metal MNPs | Optical biosensor | Single-layer MnO2 nanosheets/ascorbic acid/fresh oranges and orange juice | [116] |
| Packaging | Chitosan nanocomposite thin films/MgO | [117] | |
| Active and smart packaging | Nanoscale O2 scavengers (Fe particles) | [118] | |
| Active and smart packaging | O2 scavenging films made of PHB/Pd-NPs | [119] | |
| Active and smart packaging | Nano zeolite-Mo42−/avocado ripeness indicator | [120] | |
| Flexible antioxidant packaging (radical scavenging ability) | Embedded Se-NPs/multi-layer plastic/preservation of packages/hazelnuts, walnuts, potato chips | [121] | |
| Electrochemical biosensor | Graphene-based electrode/nano/microstructured Pt-NPs/phosphonate organophosphates | [122] | |
| Magnetic solid phase extraction HPLC-DAD | Fe3O4 magnetic NPs/multi-walled carbon nanotubes and nanodiamonds/vit.B12/milk-based infant formula, orange and peach juice, meat, salami, powder milk | [123] | |
| Magnetic solid phase extraction FAAS | Fe3O4 -sodium dodecyl sulfate-carbazone/ Cd/green tea. Lettuce, ginseng, rice, spice and carrot | [124] | |
| Mixed (bi-/ternary) MNPs | Nanocomposite films | ZnO/CuO-NPs/poly-ε-caprolactone/terephthalic acid | [125] |
| Packaging | Hybrid nanomaterials: Ag-NPs, CuO-NPs, ZnO-NPs/cellulose regenerated from cotton linter and microcrystalline cellulose | [63] | |
| Packaging | Starch-based nanocomposite films: single or combined Ag, ZnO and CuO-NPs/E. coli and S. aureus | [126] | |
| Packaging films | Chitin/ZnO/Ag-NPs/CMC/Gram(+) and Gram(−) bacteria | [127] | |
| Degradable biopolymer nanocomposite | ZnO/Ag nanocomposite/Thymus vulgaris leaf extract/PHB-co-3-hydroxyvalerate)-chitosan/poultries | [128] | |
| Films | Furcellaran/gelatin/Se-Ag-NPs/S. aureus, Multi Resistant S. aureus and E. coli/ kiwi (Actinidia arguta) storage | [129] | |
| Active packaging | Combination of high-pressure treatment, steak margination and MNPs LDPE/Ag and ZnO-NPs/beef color and shear stress | [130] | |
| Film | SiO2/carbon/Ag ternary hybrid polymeric composite/S. enteritidis | [131] | |
| Packaging films | PLA/bergamot essential oils (BEO) or PLA/BEO/nano-TiO2 or PLA/BEO/nano-TiO2 + nano-Ag/mangoes | [132] | |
| Packaging films | LDPE incorporating Ag/CuO/ZnO-NPs/ coliform/ultra-filtrated cheese | [133] | |
| Electrochemiluminescent immunoassay | Au nanorods functionalized graphene oxide and Pd/Au core-shell nanocrystallines/tomato and chili sauce and powder | [134] | |
| Active food packaging | PLA films/Ag/Cu-NPs/cinnamon essential oil/chicken meat | [135] | |
| Nanocomposite films | Ag/Cu and ZnO reinforced PLA | [136] | |
| Nanocomposite films | Ag/Cu agar-based/L. monocytogenes, Salmonella enterica serovar Typhimurium | [137] | |
| Active food packaging material | Ag/Cu guar gum nanocomposite films | [138] | |
| Packaging film | Ag/TiO2/PLA/E. coli, L. monocytogenes | [139] | |
| Packaging | PLA/TiO2 and PLA/TiO2/Ag composite films/cottage cheese samples | [140] |
| Law | Aim and Scope |
|---|---|
| Regulation (EC) No 178/2002 (General Food Law Regulation) | Laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety |
| Regulation (EU) 2015/2283 (Novel Foods) | Lays down rules for the placing of novel foods on the market within the Union |
| Regulation (EC) No 1333/2008 | Community lists of approved food additives, conditions of use of food additives in foods, and rules on the labelling of food additives |
| Regulation (EU) No 609/2013 | On food intended for infants and young children, food for special medical purposes, and total diet replacements for weight control |
| Regulation (EC) No 1334/2008 | On flavourings and certain food ingredients with flavouring properties |
| Regulation (EC) No 1332/2008 | On food enzymes |
| Directive 2002/46/EC | On the approximation of the laws of the Member States relating to food supplements |
| Regulation (EC) No 1925/2006 | On the addition of vitamins and minerals and of certain other substances to foods |
| Regulation (EC) No 1935/2004 | On materials and articles intended to come into contact with food |
| Commission Regulation (EU) No 10/2011 | On plastic materials and articles intended to come into contact with food |
| Commission Regulation (EC) No 450/2009 | On active and intelligent materials and articles intended to come into contact with food |
| Regulation (EU) No 1169/2011 | On the provision of food information to consumers |
| Regulation (EC) No 1924/2006 | On nutrition and health claims made on foods |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, C.; Almeida, A. Metallic Nanoparticles in the Food Sector: A Mini-Review. Foods 2022, 11, 402. https://doi.org/10.3390/foods11030402
Couto C, Almeida A. Metallic Nanoparticles in the Food Sector: A Mini-Review. Foods. 2022; 11(3):402. https://doi.org/10.3390/foods11030402
Chicago/Turabian StyleCouto, Cristina, and Agostinho Almeida. 2022. "Metallic Nanoparticles in the Food Sector: A Mini-Review" Foods 11, no. 3: 402. https://doi.org/10.3390/foods11030402
APA StyleCouto, C., & Almeida, A. (2022). Metallic Nanoparticles in the Food Sector: A Mini-Review. Foods, 11(3), 402. https://doi.org/10.3390/foods11030402