Eating Habits during the COVID-19 Pandemic and the Level of Antibodies IgG and FRAP—Experiences of Polish School Staff: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
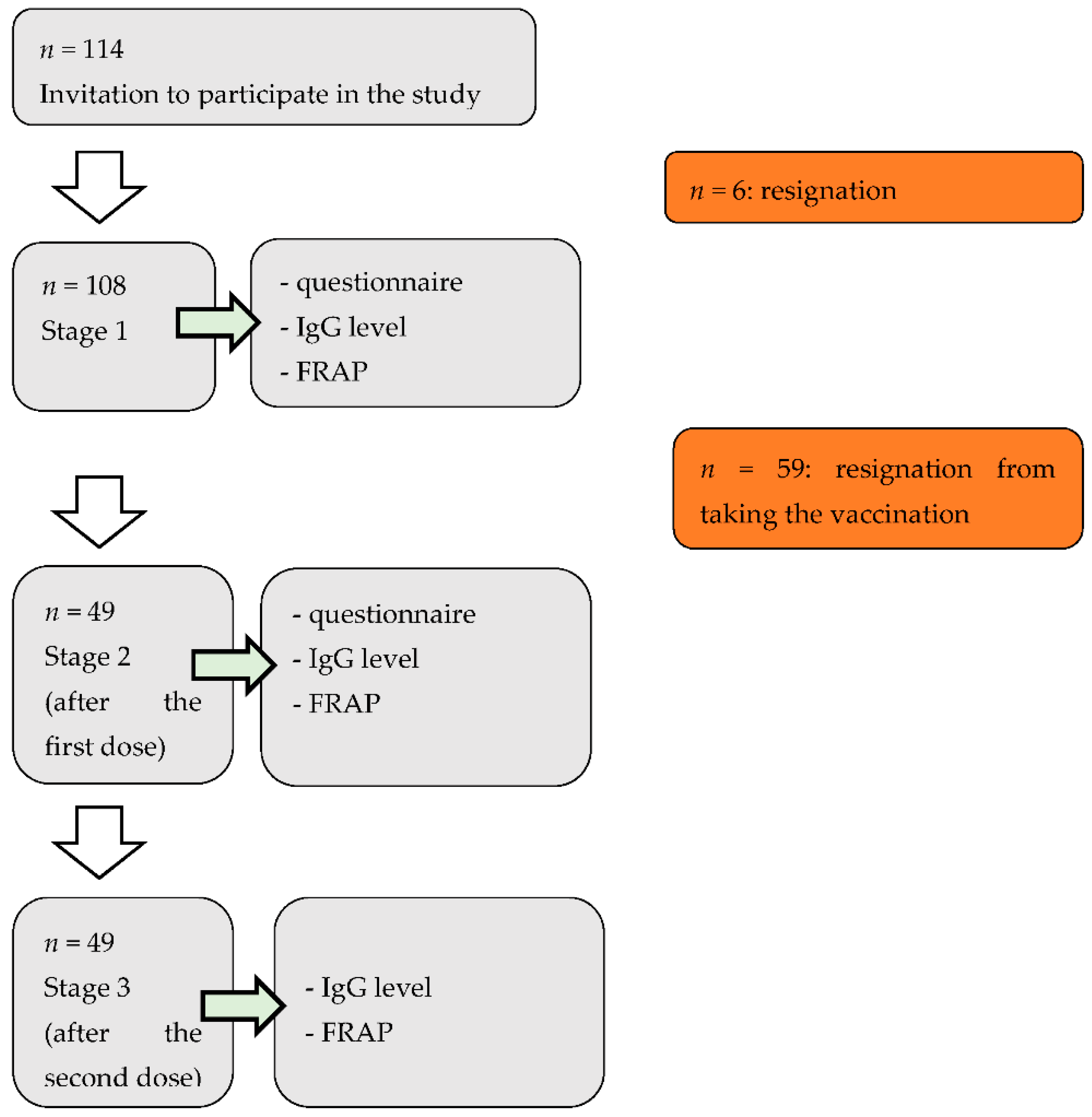
2.2. Study Design and Participants
2.3. Applied Questionaires
2.4. FRAP Assay
2.5. Antibodies Assay
2.6. Statystical Analysis
3. Results
3.1. Characteristics of the Group
3.2. COVID Infection-Symptoms, Health Background
3.3. Lifestyle
3.4. Consumption of Products
3.5. Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [Green Version]
- COVID-19—Public Health Emergency of International Concern (PHEIC) Global Research and Innovation Forum. Available online: https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum (accessed on 25 October 2021).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19). Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Laurent, S.; Onur, O.A.; Kleineberg, N.N.; Fink, G.R.; Schweitzer, F.; Warnke, C. A systematic review of neurological symptoms and complications of COVID-19. J. Neurol. 2021, 268, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Weerahandi, H.; Hochman, K.A.; Simon, E.; Blaum, C.; Chodosh, J.; Duan, E.; Garry, K.; Kahan, T.; Karmen-Tuohy, S.L.; Karpel, H.C. Post-Discharge Health Status and Symptoms in Patients with Severe COVID-19. J. Gen. Intern. Med. 2021, 36, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.S.; Rai, S.; Singh, N.K. Organizational resilience and social-economic sustainability: COVID-19 perspective. Environ. Dev. Sustain. 2021, 23, 12006–12023. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, S.; Allen, D.; Annan-Phan, S.; Bell, K.; Bolliger, I.; Chong, T.; Druckenmiller, H.; Huang, L.Y.; Hultgren, A.; Krasovich, E. The effect of large-scale anti-contagion policies on the COVID-19 pandemic. Nature 2020, 584, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Decision: Regulatory Approval of Pfizer/BioNTech Vaccine for COVID-19. Available online: https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19 (accessed on 25 October 2021).
- World Health Organization: 7 Vaccines Approved for Use by WHO. Available online: https://covid19.trackvaccines.org/agency/who/ (accessed on 25 October 2021).
- Bendau, A.; Plag, J.; Petzold, M.B.; Ströhle, A. COVID-19 vaccine hesitancy and related fears and anxiety. Int. Immunopharmacol. 2021, 97, 107724. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Zeyland, J.; Poniedziałek, B.; Małecka, I.; Wysocki, J. The Perception and Attitudes toward COVID-19 Vaccines: A Cross-Sectional Study in Poland. Vaccines 2021, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 25 October 2021).
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, E.; Cottrell, S.; Roberts, E.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, H.; Afshar, S.; Masoudi, M.R.; Gholizadeh, P.; Asgharzadeh, M.; Ganbarov, K.; Köse, S.; Yousefi, M.; Kafil, H.S. SARS-CoV-2 (Covid-19) vaccines structure, mechanisms and effectiveness: A review. Int. J. Biol. Macromol. 2021, 188, 740–750. [Google Scholar] [CrossRef]
- Jęśkowiak, I.; Wiatrak, B.; Grosman-Dziewiszek, P.; Szeląg, A. The Incidence and Severity of Post-Vaccination Reactions after Vaccination against COVID-19. Vaccines 2021, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Landry, C.A.; Paluszek, M.M.; Fergus, T.A.; McKay, D.; Asmundson, G.J.G. COVID stress syndrome: Concept, structure, and correlates. Depress. Anxiety 2020, 37, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nayar, K.R. COVID 19 and its mental health consequences. J. Ment. Health 2020, 30, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Valeria, S.; Davide, A.; Vincenzo, A. The Psychological and Social Impact of COVID-19: New Perspectives of Well-Being. Front. Psychol. 2020, 11, 25–50. [Google Scholar] [CrossRef]
- Sidor, A.; Rzymski, P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Scarmozzino, F.; Visioli, F. COVID-19 and the Subsequent Lockdown Modified Dietary Habits of Almost Half the Population in an Italian Sample. Foods 2020, 9, 675. [Google Scholar] [CrossRef]
- Castañeda-Babarro, A.; Arbillaga-Etxarri, A.; Gutiérrez-Santamaría, B.; Coca, A. Physical Activity Change during COVID-19 Confinement. Int. J. Environ. Res. Public Health 2020, 17, 6878. [Google Scholar] [CrossRef]
- Izzo, L.; Santonastaso, A.; Cotticelli, G.; Federico, A.; Pacifico, S.; Castaldo, L.; Colao, A.; Ritieni, A. An Italian Survey on Dietary Habits and Changes during the COVID-19 Lockdown. Nutrients 2021, 13, 1197. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 5574. [Google Scholar] [CrossRef]
- Gostner, J.M.; Becker, K.; Ueberall, F.; Fuchs, D. The good and bad of antioxidant foods: An immunological perspective. Food Chem. Toxicol. 2015, 80, 7279. [Google Scholar] [CrossRef] [PubMed]
- Harasym, J.; Oledzki, R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition 2014, 30, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Puchau, B.; Zulet, M.A.; González de Echávarri, A.; Hermsdorff, H.H.M.; Martínez, J.A. Dietary total antioxidant capacity is negatively associated with some metabolic syndrome features in healthy young adults. Nutrition 2010, 26, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Serafini, M.; Estruch, R.; Lamuela-Raventós, R.M.; Martínez-González, M.A.; Salas-Salvadó, J.; Fiol, M.; Lapetra, J.; Arós, F.; Covas, M.I.; et al. Mediterranean diet and non enzymatic antioxidant capacity in the PREDIMED study: Evidence for a mechanism of antioxidant tuning. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Zujko, M.; Witkowska, A. Antioxidant Potential and Polyphenol Content of Beverages, Chocolates, Nuts, and Seeds. Int. J. Food Prop. 2014, 17, 86–92. [Google Scholar] [CrossRef]
- Zujko, M.; Witkowska, A. Antioxidant Potential and Polyphenol Content of Selected Food. Int. J. Food Prop. 2011, 14, 300–308. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Bakaloudi, D.R.; Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Chourdakis, M. Impact of the first COVID-19 lockdown on body weight: A combined systematic review and a meta-analysis. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Maffoni, S.; Brazzo, S.; De Giuseppe, R.; Biino, G.; Vietti, I.; Pallavicini, C.; Cena, H. Lifestyle Changes and Body Mass Index during COVID-19 Pandemic Lockdown: An Italian Online-Survey. Nutrients 2021, 13, 1117. [Google Scholar] [CrossRef] [PubMed]
- Czenczek-Lewandowska, E.; Wyszyńska, J.; Leszczak, J.; Baran, J.; Weres, A.; Mazur, A.; Lewandowski, B. Health behaviours of young adults during the outbreak of the COVID-19 pandemic—A longitudinal study. BMC Public Health 2021, 21, 1038. [Google Scholar] [CrossRef] [PubMed]
- Al-Domi, H.; AL-Dalaeen, A.; AL-Rosan, S.; Batarseh, N.; Nawaiseh, H. Healthy nutritional behavior during COVID-19 lockdown: A cross-sectional study. Clin. Nutr. ESPEN 2021, 42, 132–137. [Google Scholar] [CrossRef]
- Elmacıoğlu, F.; Emiroğlu, E.; Ülker, M.; Özyılmaz Kırcali, B.; Oruç, S. Evaluation of nutritional behaviour related to COVID-19. Public Health Nutr. 2021, 24, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, H.; Sharps, M.; Cunliffe, L.; van den Tol, A. Eating in the lockdown during the COVID-19 pandemic; self-reported changes in eating behaviour, and associations with BMI, eating style, coping and health anxiety. Appetite 2021, 161, 105082. [Google Scholar] [CrossRef] [PubMed]
- Cecchetto, C.; Aiello, M.; Gentili, C.; Ionta, S.; Osimo, S.A. Increased emotional eating during COVID-19 associated with lockdown, psychological and social distress. Appetite 2021, 160, 105122. [Google Scholar] [CrossRef]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Zachary, Z.; Brianna, F.; Brianna, L.; Garrett, P.; Jade, W.; Alyssa, D.; Mikayla, K. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes. Res. Clin. Pract. 2020, 14, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Ponzo, V.; Rosato, R.; Scumaci, E.; Goitre, I.; Benso, A.; Belcastro, S.; Crespi, C.; De Michieli, F.; Ghigo, E.; et al. Changes in Weight and Nutritional Habits in Adults with Obesity during the “Lockdown” Period Caused by the COVID-19 Virus Emergency. Nutrients 2020, 12, 2016. [Google Scholar] [CrossRef] [PubMed]
- Flaudias, V.; Iceta, S.; Zerhouni, O.; Rodgers, R.F.; Billieux, J.; Llorca, P.M.; Boudesseul, J.; de Chazeron, I.; Romo, L.; Maurage, P.; et al. COVID-19 pandemic lockdown and problematic eating behaviors in a student population. J. Behav. Addict. 2020, 9, 826–835. [Google Scholar] [CrossRef]
- Huber, B.C.; Steffen, J.; Schlichtiger, J.; Brunner, S. Altered nutrition behavior during COVID-19 pandemic lockdown in young adults. Eur. J. Nutr. 2021, 60, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- Poelman, M.P.; Gillebaart, M.; Schlinkert, C.; Dijkstra, S.C.; Derksen, E.; Mensink, F.; Hermans, R.C.J.; Aardening, P.; de Ridder, D.; de Vet, E. Eating behavior and food purchases during the COVID-19 lockdown: A cross-sectional study among adults in the Netherlands. Appetite 2021, 157, 105002. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.; Desai, T.; Sullivan, K.; Muniz, D.; Wells, A. Changes to Physical Activity, Sitting Time, Eating Behaviours and Barriers to Exercise during the First COVID-19 ‘Lockdown’ in an English Cohort. Int. J. Environ. Res. Public Health 2021, 18, 10025. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.; Duffy, F.; Newman, E.; Bravo, C.P.; Ates, H.H.; Sharpe, H. Exploring changes in body image, eating and exercise during the COVID-19 lockdown: A UK survey. Appetite 2021, 159, 105062. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.N.; Gregório, M.J.; Santos, R.; Marques, A.; Rodrigues, B.; Godinho, C.; Silva, C.S.; Mendes, R.; Graça, P.; Arriaga, M.; et al. Towards an In-Depth Understanding of Physical Activity and Eating Behaviours during COVID-19 Social Confinement: A Combined Approach from a Portuguese National Survey. Nutrients 2021, 13, 2685. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Sigodo, K.O.; Al-Ghadban, F.; Al-Lahou, B.; Alnashmi, M.; Hermassi, S.; Chun, S. Effects of COVID-19 Lockdown on Physical Activity and Dietary Behaviors in Kuwait: A Cross-Sectional Study. Nutrients 2021, 13, 2252. [Google Scholar] [CrossRef]
- Ogundijo, D.A.; Tas, A.A.; Onarinde, B.A. Exploring the Impact of COVID-19 Pandemic on Eating and Purchasing Behaviours of People Living in England. Nutrients 2021, 13, 1499. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [Green Version]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A Novel Antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef]
- Błaszczyk-Bębenek, E.; Jagielski, P.; Bolesławska, I.; Jagielska, A.; Nitsch-Osuch, A.; Kawalec, P. Nutrition Behaviors in Polish Adults before and during COVID-19 Lockdown. Nutrients 2020, 12, 3084. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Uzhova, I.; Verardo, V.; Artacho, R.; García-Villanova, B.; Guerra-Hernández, E.J.; Kapsokefalou, M.; Malisova, O.; Vlassopoulos, A.; Katidi, A.; et al. Impact of COVID-19 confinement on eating behaviours across 16 European countries: The COVIDiet cross-national study. Food Qual. Prefer. 2021, 93, 104231. [Google Scholar] [CrossRef]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Kilian, C.; Ferreira-Borges, C.; Jernigan, D.; Monteiro, M.; Parry, C.D.H.; Sanchez, Z.M.; Manthey, J. Alcohol use in times of the COVID 19: Implications for monitoring and policy. Drug Alcohol Rev. 2020, 39, 301–304. [Google Scholar] [CrossRef] [PubMed]
- González-Monroy, C.; Gómez-Gómez, I.; Olarte-Sánchez, C.M.; Motrico, E. Eating Behaviour Changes during the COVID-19 Pandemic: A Systematic Review of Longitudinal Studies. Int. J. Environ. Res. Public Health 2021, 18, 11130. [Google Scholar] [CrossRef] [PubMed]
- Castellana, F.; De Nucci, S.; De Pergola, G.; Di Chito, M.; Lisco, G.; Triggiani, V.; Sardone, R.; Zupo, R. Trends in Coffee and Tea Consumption during the COVID-19 Pandemic. Foods 2021, 10, 2458. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, E.; Díaz-Jimenez, J.; Rosety, I.; Alférez, M.J.M.; Díaz, A.J.; Rosety, M.A.; Ordonez, F.J.; Rosety-Rodriguez, M. Perceived Stress and Increased Food Consumption during the ‘Third Wave’ of the COVID-19 Pandemic in Spain. Nutrients 2021, 13, 2380. [Google Scholar] [CrossRef] [PubMed]
- Buckland, N.J.; Swinnerton, L.F.; Ng, K.; Price, M.; Wilkinson, L.L.; Myers, A.; Dalton, M. Susceptibility to increased high energy dense sweet and savoury food intake in response to the COVID-19 lockdown: The role of craving control and acceptance coping strategies. Appetite 2021, 158, 105017. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflug. Arch.-Eur. J. Physiol. 2012, 463, 121–137. [Google Scholar] [CrossRef] [Green Version]
- Wright, L.; Steptoe, A.; Fancourt, D. Are adversities and worries during the COVID-19 pandemic related to sleep quality? Longitudinal analyses of 46,000 UK adults. PLoS ONE 2021, 16, e0248919. [Google Scholar] [CrossRef]
- Du, C.; Zan, M.C.H.; Cho, M.J.; Fenton, J.I.; Hsiao, P.Y.; Hsiao, R.; Keaver, L.; Lai, C.-C.; Lee, H.; Ludy, M.-J.; et al. The Effects of Sleep Quality and Resilience on Perceived Stress, Dietary Behaviors, and Alcohol Misuse: A Mediation-Moderation Analysis of Higher Education Students from Asia, Europe, and North America during the COVID-19 Pandemic. Nutrients 2021, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Szczeklik, K.; Krzyściak, W.; Cibor, D.; Domagała-Rodacka, R.; Pytko-Polończyk, J.; Mach, T.; Owczarek, D. Markers of lipid peroxidation and antioxidant status in the serum and saliva of patients with active Crohn disease. Pol. Arch. Intern. Med. 2018, 128, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Peres Rubio, C.; de la Fuente, J.; Villar, M.; Merino, O.; Mosqueda, J.; Cerón, J.J. Changes in serum biomarkers of oxidative stress in cattle vaccinated with tick recombinant antigens: A pilot study. Vaccines 2020, 9, 3965. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Myśliwiec, H.; Choromańska, K.; Żendzian-Piotrowska, M.; Dadan, J.; Zalewska, A.; Maciejczyk, M. Impact of Weight Loss on the Total Antioxidant/Oxidant Potential in Patients with Morbid Obesity—A Longitudinal Study. Antioxidants 2020, 9, 376. [Google Scholar] [CrossRef]
- Verso, L.L.; Dumont, K.; Lessard, M.; Lauzon, K.; Provost, C.; Gagnon, C.A.; Chorfi, Y.; Guay, F. The administration of diets contaminated with low to intermediate doses of deoxynivalenol and supplemented with antioxidants and binding agents slightly affects the growth, antioxidant status, and vaccine response in weanling pigs. Sci. J. Anim. Sci. 2021, 99, skab238. [Google Scholar] [CrossRef]
| Parameter | n | Av. ± SD | Med. | Min.–Max | Q1–Q3 |
|---|---|---|---|---|---|
| Gender (n, W/M) | 89/19 | - | - | - | - |
| Age (years) | 108 | 46.3 ± 10.5 | 48.5 | 24.0–70.0 | 39.5–54.0 |
| Height (m) | 108 | 168.0 ± 6.6 | 168.0 | 153.0–190.0 | 163.0–170.5 |
| Body weight (kg) | 108 | 74.0 ± 13.5 | 70.0 | 55.0–115.0 | 63.0–83.0 |
| BMI (kg/m2) | 108 | 26.3 ± 4.3 | 25.6 | 19.3–41.7 | 23.0–29.1 |
| Work experience (years) | 108 | 16.6 ± 12.1 | 17.5 | 0.5–51.0 | 4.5–20.5 |
| Parameter | Total (n = 108) | Women (n = 89) | Men (n = 19) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Change in weight during a pandemic | |||
| No change | 51 (47.2) | 41 (46.1) | 10 (52.5) |
| It was increased in the range of 3–5 kg | 42 (39.0) | 36 (40.4) | 6 (31.6) |
| It was increased in the range above 10 kg | 5 (4.6) | 4 (4.5) | 1 (5.3) |
| It was reduced in the range of 3–5 kg | 1 (0.9) | 0 (0.0) | 1 (5.3) |
| It was reduced in the range of 6–10 kg | 8 (7.4) | 7 (7.9) | 1 (5.3) |
| It was reduced in the range above 10 kg | 1 (0.9) | 1 (1.1) | 0 (0.0) |
| Type of work performed at school (multiple choice question) | |||
| Teacher in grades 0–3 | 40 (37.0) | 38 (42.7) | 2 (10.5) |
| Teacher in grades 4–8 | 33 (30.6) | 23 (25.8) | 10 (52.6) |
| School administration | 10 (9.3) | 8 (9.0) | 2 (10.5) |
| School service | 29 (26.9) | 24 (27.0) | 5 (26.4) |
| How do you currently work? | |||
| Stationary | 55 (51.4) | 48 (53.9) | 7 (36.8) |
| Remotely | 21 (18.7) | 13 (14.6) | 8 (42.1) |
| Stationary and remotely | 32 (29.9) | 28 (31.5) | 4 (21.1) |
| How do you rate remote learning during a pandemic? | |||
| Comparable to traditional teaching | 16 (14.6) | 14 (15.7) | 2 (10.5) |
| Worse than traditional education | 92 (85.4) | 75 (84.3) | 17 (89.5) |
| Parameter | Total (n = 108) | Women (n = 89) | Men (n = 19) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Have you been tested positive for COVID-19? | |||
| Yes | 23 (21.3) | 23 (25.8) | 0 (0.0) |
| No | 85 (78.7) | 66 (74.2) | 19 (100.0) |
| Have you had a COVID-19 antibody test performed? | |||
| Yes | 8 (7.4) | 8 (9.0) | 0 (0.0) |
| No | 100 (92.6) | 81 (91.0) | 19 (100.0) |
| If you have had COVID-19, please mark the symptoms accompanying the disease (multiple choice question) | |||
| Fever of 38 °C and above | 11 (10.2) | 10 (11.2) | 1 (5.3) |
| Cough | 10 (9.3) | 9 (10.1) | 1 (5.3) |
| Diarrhea | 3 (2.8) | 3 (3.4) | 0 (0.0) |
| Nausea | 4 (3.7) | 4 (4.5) | 0 (0.0) |
| Vomiting | 1 (0.9) | 1 (1.1) | 0 (0.0) |
| Smell and taste disorders | 16 (14.8) | 15 (16.9) | 0 (0.0) |
| Conjunctivitis | 1 (0.9) | 1 (1.1) | 0 (0.0) |
| Difficulty breathing, difficulty drawing air | 7 (6.5) | 7 (7.9) | 0 (0.0) |
| Muscle aches, fatigue | 15 (13.9) | 14 (15.7) | 1 (5.3) |
| Other symptoms | 7 (6.5) | 7 (7.9) | 0 (0.0) |
| Have any of your household members had a positive COVID-19 test? | |||
| Yes | 14 (13.0) | 8 (9.0) | 6 (31.6) |
| No | 94 (87.0) | 81 (91.0) | 13 (68.4) |
| Were you in quarantine because of COVID-19? | |||
| Yes | 36 (33.3) | 29 (32.6) | 7 (36.8) |
| No | 72 (66.7) | 60 (67.4) | 12 (63.2) |
| For what reason were you in quarantine? (38 answers) | |||
| Own disease | 6 (5.6) | 6 (6.7) | 0 (0.0) |
| Household disease | 15 (13.9) | 11 (12.4) | 4 (21.1) |
| Co-worker disease | 10 (9.3) | 7 (7.9) | 3 (15.8) |
| Return from abroad | 2 (1.9) | 2 (2.2) | 0 (0.0) |
| Another | 5 (4.6) | 5 (5.6) | 0 (0.0) |
| Do you suffer from chronic diseases? | |||
| Yes | 37 (34.3) | 34 (38.2) | 3 (15.8) |
| No | 71 (65.7) | 55 (61.8) | 16 (84.2) |
| Have you been vaccinated against COVID-19? | |||
| No | 109 (100.0) | 89 (100.0) | 19 (100.0) |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Would you report your willingness to be vaccinated against COVID-19 if it was possible? | |||
| Yes | 61 (56.5) | 47 (52.8) | 14 (73.7) |
| No | 47 (43.5) | 42 (47.2) | 5 (26.3) |
| If not, why not? (30 answers) | |||
| I don’t believe vaccination is effective | 3 (2.8) | 2 (2.2) | 1 (5.3) |
| I do not like the type of vaccine offered to the education staff | 20 (18.5) | 17 (19.1) | 3 (15.8) |
| Other | 7 (6.5) | 7 (7.9) | 0 (0.0) |
| If you have had COVID-19, do you think that your current health has returned to its pre-disease state? (23 answers) | |||
| Yes | 6 (5.6) | 6 (6.7) | 0 (0.0) |
| No | 17 (15.7) | 14 (15.7) | 3 (15.8) |
| If you have suffered from COVID-19, what complications do you experience after the illness? (35 answers) | |||
| General | 7 (6.5) | 7 (7.9) | 0 (0.0) |
| From the respiratory system | 5 (4.6) | 5 (5.6) | 0 (0.0) |
| From the cardiovascular system | 6 (5.6) | 6 (6.7) | 0 (0.0) |
| Neurological and psychiatric | 8 (7.4) | 8 (9.0) | 0 (0.0) |
| From the gastrointestinal tract | 1 (0.9) | 1 (1.1) | 0 (0.0) |
| From the motor organ | 3 (2.8) | 3 (3.4) | 0 (0.0) |
| From the sensory organs and the throat | 5 (4.6) | 4 (4.5) | 1 (5.3) |
| Parameter | Total (n = 108) | Women (n = 89) | Men (n = 19) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Do you feel stress related to the pandemic? | |||
| Yes | 84 (77.8) | 71 (79.8) | 13 (68.4) |
| No | 24 (22.2) | 18 (20.2) | 6 (31.6) |
| What is the stress experienced during a pandemic related to? (multiple choice question) | |||
| Concern for own and family’s health | 88 (81.5) | 73 (82.0) | 15 (78.9) |
| Limited social life | 42 (38.9) | 36 (40.4) | 6 (31.6) |
| Care for job stability and earnings | 31 (28.7) | 26 (29.2) | 5 (26.3) |
| Online learning and limited access to computer hardware | 18 (16.7) | 17 (19.1) | 1 (5.3) |
| Concern for the level of the teaching of their students | 46 (42.6) | 40 (44.9) | 6 (31.6) |
| Other | 2 (1.9) | 2 (2.2) | 0 (0.0) |
| How have your hygiene habits changed during the pandemic? (multiple choice question) | |||
| They have not changed | 15 (13.9) | 13 (14.6) | 2 (10.5) |
| I wash my hands more often | 72 (66.7) | 59 (66.3) | 13 (68.4) |
| I disinfect my hands more often | 79 (73.1) | 66 (74.2) | 13 (68.4) |
| I wear the mask in public places | 93 (86.1) | 76 (85.4) | 15 (78.9) |
| Other | 3 (2.8) | 2 (2.2) | 0 (0.0) |
| Do you smoke cigarettes? | |||
| Yes, regularly | 8 (7.4) | 3 (3.4) | 5 (26.3) |
| Yes, occasionally | 4 (3.7) | 3 (3.4) | 1 (5.3) |
| No | 95 (88.0) | 83 (93.2) | 12 (63.1) |
| I have smoked, but quit during the pandemic | 1 (0.9) | 0 (0.0) | 1 (5.3) |
| How do you evaluate the change in eating habits during the pandemic? | |||
| Positive change | 22 (20.4) | 20 (22.5) | 2 (10.5) |
| Negative change | 20 (18.5) | 13 (14.6) | 6 (31.6) |
| No change | 66 (61.1) | 55 (62.9) | 11 (57.9) |
| Physical Activity | Before the Pandemic * | During a Pandemic |
|---|---|---|
| n (%) | n (%) | |
| Lack of physical activity | 23 (21.3) | 38 (35.2) |
| 1–2 times a week, minimum 30 min | 43 (39.9) | 42 (38.9) |
| 3–5 times a week, minimum 30 min | 25 (23.1) | 17 (15.7) |
| More than 5 times a week, minimum 30 min | 17 (15.7) | 11 (10.2) |
| Product | Increase in Consumption | Decrease in Consumption |
|---|---|---|
| n (%) | n (%) | |
| Fruits, vegetables, salads | 22 (20.4) ** | 3 (2.8) |
| Honey and bee products | 17 (15.7) *** | 1 (0.9) |
| Nuts | 12 (11.1) * | 3 (2.8) |
| Milk and dairy products | 12 (11.1) | 5 (4.6) |
| Meat and meat products | 12 (11.1) | 17 (15.7) |
| Fish and processed fish | 18 (16.7) ** | 3 (2.8) |
| Eggs | 11 (10.2) * | 2 (1.9) |
| Bread | 7 (6.5) | 14 (13.0) |
| Groats, rice, cereals | 21 (19.4) ** | 7 (6.5) |
| Flour preparations (pies, pancakes, rolls, cookies) | 18 (16.7) | 13 (12.0) |
| Sweets | 21 (19.4) | 15 (13.9) |
| Ready-made dishes for quick preparation at home | 7 (6.5) | 25 (23.1) ** |
| Coffee | 16 (14.8) | 13 (12.0) |
| Tea | 21 (19.4) ** | 6 (5.6) |
| Juices | 10 (9.3) | 12 (11.1) |
| Water | 23 (21.3) *** | 4 (3.7) |
| Alcohol | 3 (2.8) | 12 (11.1) * |
| Parameter | Before the Pandemic | During a Pandemic |
|---|---|---|
| n (%) | n (%) | |
| Number of meals during the day | ||
| 1–2 meals | 11 (10.2) | 10 (9.2) |
| 3–5 meals | 96 (88.9) | 84 (77.8) |
| over 5 meals | 1 (0.9) | 14 (13.0) |
| Time spent in front of the computer | ||
| less than 2 h a day | 59 (54.7) *** | 18 (16.7) |
| 2–3 h a day | 31 (28.7) | 15 (13.9) |
| 4–5 h a day | 10 (9.2) | 21 (19.4) |
| 6–8 h a day | 3 (2.8) | 34 (31.5) |
| more than 8 h a day | 5 (4.6) | 20 (18.5) |
| Hours of sleep per day | ||
| 6 h or less | 31 (28.7) | 33 (30.6) |
| 7–9 h | 76 (70.4) | 71 (65.7) |
| 10 or more hours | 1 (0.9) | 4 (3.7) |
| Parameter | Before Vaccination (n = 108) | Before Vaccination (n = 49) | After First Vaccination (n = 49) | After Second Vaccination (n = 49) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| IG | ||||
| Under 18 U/mL | 58 (53.7) | 25 (51.0) | 5 (10.2) | 0 (0.0) |
| 18–22 U/mL | 8 (7.4) | 4 (8.2) | 0 (0.0) | 0 (0.0) |
| Above 22 U/mL | 42 (38.9) | 20 (40.8) | 44 (89.8) | 49 (100.0) |
| Index of Positivity | ||||
| Under 0.9 | 58 (53.7) | 25 (51.0) | 5 (10.2) | 0 (0.0) |
| 0.9–1.1 | 8 (7.4) | 4 (8.2) | 0 (0.0) | 0 (0.0) |
| Above 1.1 | 42 (38.9) | 20 (40.8) | 44 (89.8) | 49 (100.0) |
| Parameter | Av. ± SD | Med. (Q1–Q3) | p |
|---|---|---|---|
| FRAP—before vaccination (A) | 1453.3 ± 292.2 | 1428.0 (1271.0–1599.0) | p A/B < 0.0001 |
| FRAP—after 1 dose (B) | 1539.7 ± 285.0 | 1484.0 (1346.0–1721.0) | p A/C < 0.0001 |
| FRAP—after 2 doses (C) | 1613.1 ± 294.4 | 1581.0 (1423.0–1787.0) | p B/C < 0.0001 |
| Group | Parameter 1 | Parameter 2 | R, p |
|---|---|---|---|
| IgG level below 400 (n = 24) | FRAP before vaccination | FRAP after first dose | 0.92, 0.0001 |
| FRAP before vaccination | FRAP after second dose | 0.93, 0.0001 | |
| FRAP after first dose | FRAP after second dose | 0.97, 0.0001 | |
| IgG before vaccination | IgG after first dose | 0.56, 0.0042 | |
| IgG before vaccination | IgG after second dose | 0.55, 0.0053 | |
| FRAP after second dose | Growth | 0.41, 0.0482 | |
| IgG level 400 (n = 25) | FRAP before vaccination | FRAP after first dose | 0.76, 0.0001 |
| FRAP before vaccination | FRAP after second dose | 0.78, 0.0001 | |
| FRAP before vaccination | BMI | 0.61, 0.0013 | |
| FRAP after first dose | FRAP after second dose | 0.98, 0.0001 | |
| FRAP after first dose | BMI | 0.68, 0.0002 | |
| FRAP after second dose | BMI | 0.70, 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puścion-Jakubik, A.; Olechno, E.; Socha, K.; Zujko, M.E. Eating Habits during the COVID-19 Pandemic and the Level of Antibodies IgG and FRAP—Experiences of Polish School Staff: A Pilot Study. Foods 2022, 11, 408. https://doi.org/10.3390/foods11030408
Puścion-Jakubik A, Olechno E, Socha K, Zujko ME. Eating Habits during the COVID-19 Pandemic and the Level of Antibodies IgG and FRAP—Experiences of Polish School Staff: A Pilot Study. Foods. 2022; 11(3):408. https://doi.org/10.3390/foods11030408
Chicago/Turabian StylePuścion-Jakubik, Anna, Ewa Olechno, Katarzyna Socha, and Małgorzata Elżbieta Zujko. 2022. "Eating Habits during the COVID-19 Pandemic and the Level of Antibodies IgG and FRAP—Experiences of Polish School Staff: A Pilot Study" Foods 11, no. 3: 408. https://doi.org/10.3390/foods11030408
APA StylePuścion-Jakubik, A., Olechno, E., Socha, K., & Zujko, M. E. (2022). Eating Habits during the COVID-19 Pandemic and the Level of Antibodies IgG and FRAP—Experiences of Polish School Staff: A Pilot Study. Foods, 11(3), 408. https://doi.org/10.3390/foods11030408









