Waste Management in the Agri-Food Industry: The Conversion of Eggshells, Spent Coffee Grounds, and Brown Onion Skins into Carriers for Lipase Immobilization
Abstract
:1. Introduction
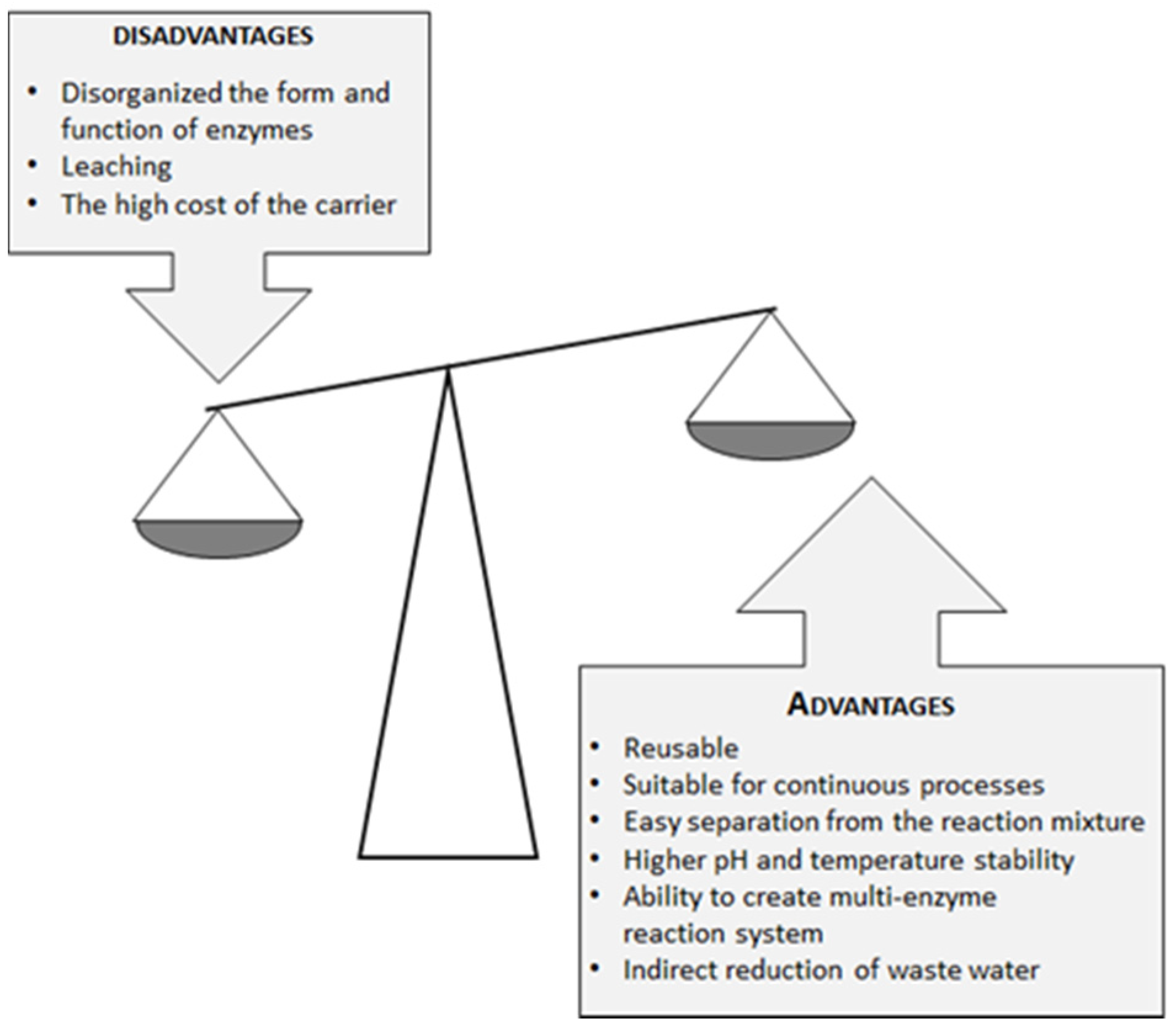
2. Lipases as Heterogeneous Biocatalysts
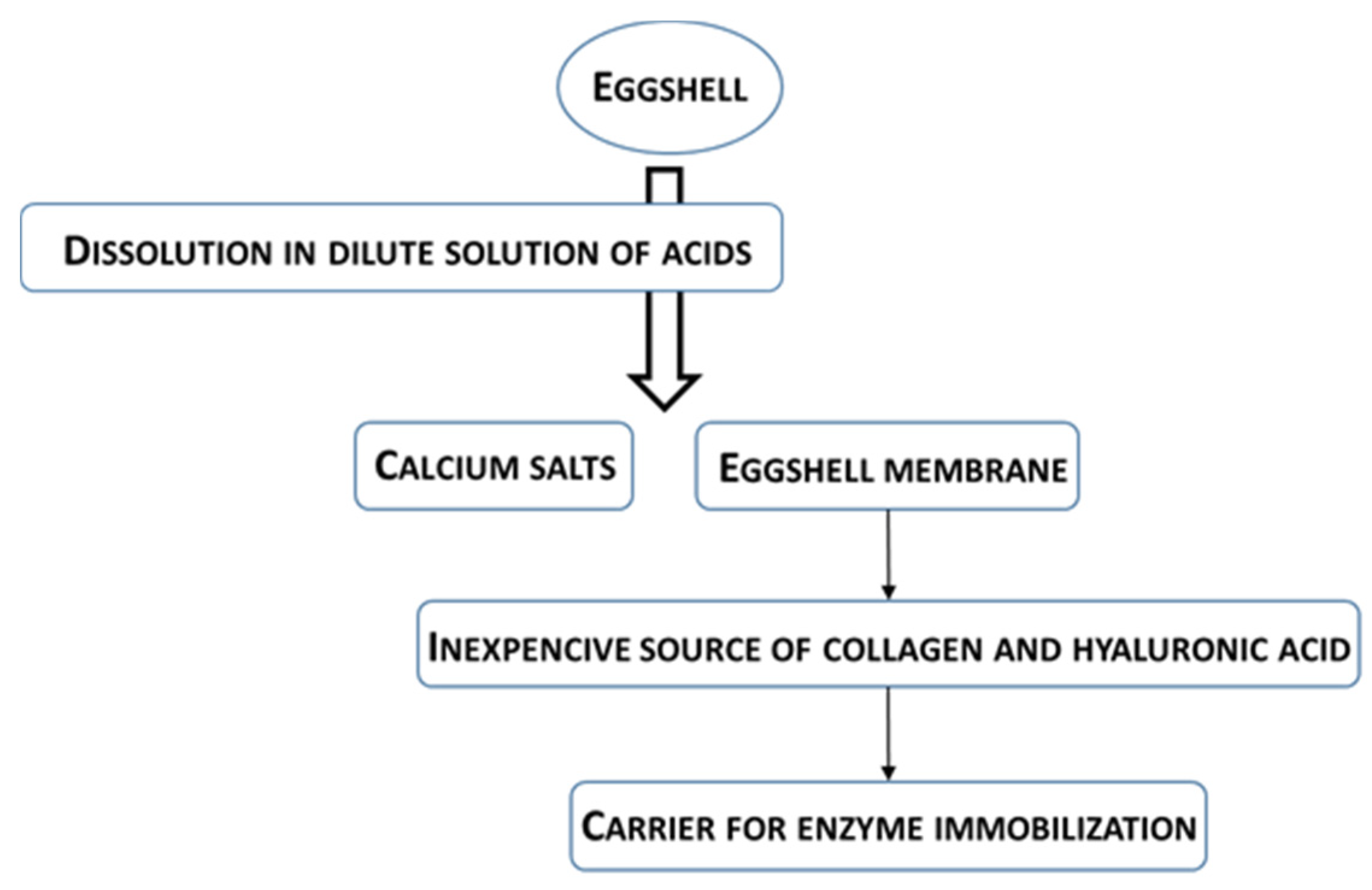
2.1. Carriers Based on Eggshell Membranes
2.2. Carriers Based on Spent Coffee Grounds and Brown Onion Skins
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 6, 238. [Google Scholar] [CrossRef] [Green Version]
- Raman, J.K.; Ting, V.F.W.; Pogaku, R. Life cycle assessment of biodiesel production using alkali, soluble and immobilized enzyme catalyst processes. Biomass Bioenergy 2011, 35, 4221–4229. [Google Scholar] [CrossRef]
- Agenda 2030. Available online: https://sdgs.un.org/2030agenda (accessed on 4 March 2021).
- Towards Sustainable Development. Available online: www.un-documents.net/ocf-02.htm (accessed on 4 March 2021).
- Stankevičienė, J.; Nikanorova, M. Eco-innovation as a pillar for sustainable development of circular economy. Bus. Theory Pract. 2020, 21, 531–544. [Google Scholar] [CrossRef]
- Circular Economy. Available online: http://www.fzoeu.hr/en/circular-economy/7659 (accessed on 4 March 2021).
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier-Coussy, H.; Jang, G.-W.; Verniquet, A.; Broeze, J.; et al. A research challenge vision regarding management of agricultural waste in a circular bio-based economy. Crit. Rev. Environ. Sci. Technol. 2018, 48, 614–654. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Garcia, G.; Stone, J.; Rahimifard, S. Opportunities for waste valorization in the food industry—A case study with four UK food manufacturers. J. Clean. Prod. 2018, 211, 1339–1356. [Google Scholar] [CrossRef]
- Budžaki, S.; Šalić, A.; Zelić, B.; Tišma, M. Enzyme-catalysed Biodiesel Production from Edible and Waste Cooking Oils. Chem. Biochem. Eng. Q. 2015, 29, 329–333. [Google Scholar] [CrossRef]
- Budžaki, S.; Miljić, G. Otpadno ulje i Nusproizvodi Prehrambene Industrije Kao Sirovine za Proizvodnju Biodizela. In Neke Mogućnosti Iskorištenja Nusproizvoda Prehrambene Industrije; Šubarić, D., Ed.; Josip Juraj Strossmayer University of Osijek, Faculty of Food Technology Osijek: Osijek, Croatia, 2017; pp. 165–177. [Google Scholar]
- Tran, N.N.; Tišma, M.; Budžaki, S.; McMurchie, E.J.; Gonzalez, O.M.M.; Hessel, V.; Ngothai, Y. Scale-up and economic analysis of biodiesel production from recycled grease trap waste. Appl. Energy 2018, 229, 142–150. [Google Scholar] [CrossRef]
- Tran, N.N.; Tišma, M.; Budžaki, S.; McMurchie, T.; Ngothai, Y.; Morales-Gonzalez, O.M.; Hessel, V. Production of Biodiesel from Recycled Grease Trap Waste: A Review. Ind. Eng. Chem. Res. 2021, 60, 16547–16560. [Google Scholar] [CrossRef]
- Budžaki, S.; Sundaram, S.; Tišma, M.; Hessel, V. Cost analysis of oil cake-to-biodiesel production in packed-bed micro-flow reactors with immobilized lipases. J. Biosci. Bioeng. 2019, 128, 98–102. [Google Scholar] [CrossRef]
- Budžaki, S.; Strelec, I.; Krnić, M.; Alilović, K.; Tišma, M.; Zelić, B. Proximate analysis of cold-press oil cakes after biological treatment with Trametes versicolor and Humicola grisea. Eng. Life Sci. 2018, 18, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Borges, J.; Alvim-Ferraz, C.; Almeida, M.F.; Dias, J.M.; Budžaki, S. Enzymatic Esterification of Pre-Treated and Untreated Acid Oil Soapstock. In WASTES—Solutions, Treatments and Opportunities II: Selected Papers from the 4th Edition of the International Conference on Wastes: Solutions, Treatments and Opportunities; Vilarinho, C., Castro, F., de Lurdes Lopes, M., Eds.; CRC Press (Taylor & Francis Group): London, UK, 2017; pp. 241–246. [Google Scholar]
- Budžaki, S.; Miljić, G.; Tišma, M.; Sundaram, S.; Hessel, V. Is there a future for enzymatic biodiesel industrial production in microreactors? Appl. Energy 2017, 201, 124–134. [Google Scholar] [CrossRef]
- Budžaki, S.; Miljić, G.; Tišma, M.; Sundaram, S.; Hessel, V. Cost analysis of enzymatic biodiesel production in small-scaled packed-bed reactors. Appl. Energy 2018, 210, 268–278. [Google Scholar] [CrossRef]
- Bilal, M.; Wang, Z.; Ciu, J.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Environmental impact of lignocellulosic waste and their effective exploitation as smart cariers—A drive twords greener and eco-friendlier biocatalytic systems. Sci. Total Environ. 2020, 722, 137903. [Google Scholar] [CrossRef] [PubMed]
- Girelli, A.M.; Astolfi, M.L.; Scuto, F.R. Agro-industrial wastes as potential cariers for enzyme immobilization: A review. Chemosphere 2020, 244, 125368. [Google Scholar] [CrossRef]
- Federsel, H.-J.; Moody, T.S.; Taylor, S.J.C. Recent trends in Enzyme Immobilization—Concepts for Expanding the Biocatalysis Toolbox. Molecules 2021, 26, 2822. [Google Scholar] [CrossRef]
- Houde, A.; Kademi, A.; Leblanc, D. Lipases and their industrial applications. Appl. Biochem. Biotechnol. 2004, 118, 155–170. [Google Scholar] [CrossRef]
- Andualema, B.; Gessesse, A. Microbial lipases and their industrial applications: Review. Biotechnol. 2012, 11, 100–118. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Thakur, S.; Bhatt, A.K. Microbial Lipases: Industrial Applications and Properties (A Review). Int. Res. J. Biol. Sci. 2012, 1, 88–92. [Google Scholar]
- Guerrand, D. Lipases industrial applications: Focus on food and agroindustries. Oilseeds Fats Crops Lipids 2017, 24, D403. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Gerits, L.R.; Pareyt, B.; Decamps, K.; Delcour, J.A. Lipases and their functionality in the production of wheat-based food systems. Compr. Rev. Food Sci. Food Saf. 2014, 13, 978–989. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Wang, S.; Xiang, X.; Shi, J.; Huang, J.; Deng, Q.; Huang, F.; Xiao, J.-Y. Facile preparation of magnetic carbon nanotubes-immobilized lipase for highly efficient synthesis of 1,3-dioleoyl-2- palmitoylglycerol-rich human milk fat substitutes. Food Chem. 2017, 228, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Şahin-Yeşilçubuk, N.; Akoh, C.C. Biotechnological and novel approaches for designing structured lipids intended for infant nutrition. J. Am. Oil Chem. Soc. 2017, 94, 1005–1034. [Google Scholar] [CrossRef]
- Ray, J.; Nagy, Z.K.; Smith, K.W.; Bhaggan, K.; Stapley, A.G.F. Kinetic study of the acidolysis of high oleic sunflower oil with stearic-palmitic acid mixtures catalysed by immobilised Rhizopus oryzae lipase. Biochem. Eng. J. 2013, 73, 17–28. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zheng, L.; Jin, Q.; Wang, X. Synthesis of 1,3-distearoyl-2-oleoylglycerol by enzymatic acidolysis in a solvent-free system. Food Chem. 2017, 228, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Atabani, A.E.; Mercimek, S.M.; Arvindnarayan, S.; Shobana, S.; Kumar, G.; Cadir, M.; Al-Muhatseb, A.H. Valorization of spent coffee grounds recycling as a potential alternative fuel resource in Turkey: An experimental study. J. Air Waste Manag. Assoc. 2018, 68, 196–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazani, S.M.; Marangoni, A.G. Facile lipase-catalyzed synthesis of a chocolate fat mimetic. Sci. Rep. 2018, 8, 15271. [Google Scholar] [CrossRef] [Green Version]
- SÁ, A.G.A.; de Meneses, A.C.; de Araújo, P.H.H.; de Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Palomo, J.M.; Mateo, C.; Munilla, R.; Ortiz, C.; Cabrera, Z.; Guisan, J.M.; Fernandez-Lafuente, R. Glutaraldehyde cross-Linking of lipases adsorbed on aminated supports in the presence of detergents leads to improved performance. Biomacromolecules 2006, 7, 2610–2615. [Google Scholar] [CrossRef]
- Souza, L.T.A.; Moreno-Perez, S.; Fernández Lorente, G.; Cipolatti, E.P.; de Oliveira, D.; Resende, R.R.; Pessela, B.C. Immobilization of Moniliella spathulata R25L270 lipase on ionic, hydrophobic and covalent supports: Functional properties and hydrolysis of sardine oil. Molecules 2017, 22, 1508. [Google Scholar] [CrossRef] [Green Version]
- Adulkar, T.V.; Rathod, V.K. Pre-treatment of high-fat content dairy wastewater using different commercial lipases. Desalination Water Treat. 2013, 53, 2450–2455. [Google Scholar] [CrossRef]
- Kanmani, P.; Kumaresan, K.; Aravind, J. Pretreatment of coconut mill effluent using celite-immobilized hydrolytic enzyme preparation from Staphylococcus pasteuri and its impact on anaerobic digestion. Biotechnol. Prog. 2015, 31, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Olaniran, A.O. Treatment of lipid-rich wastewater using a mixture of free or immobilized bioemulsifier and hydrolytic enzymes from indigenous bacterial isolates. Desalination Water Treat. 2018, 132, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Franssen, M.C.R.; Steunenberg, P.; Scott, E.L.; Zuilhof, H.; Sandrers, J.P.M. Immobilised enzymes in biorenewables production. Chem. Soc. Rev. 2013, 42, 6491–6533. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Denčić, I.; Tibhe, J.; Pedraza, C.A.S.; Wang, Q.; Nöel, T.; Meuldijk, J.; de Croon, M.; Hessel, V.; Weizenmann, N.; et al. Threonine aldolase immobilization on different supports for engineering, cost-efficient enzymatic microreactors. Chem. Eng. J. 2012, 207–208, 564–576. [Google Scholar] [CrossRef]
- Denčić, I.; de Vaan, S.; Noël, T.; Meuldijke, J.; de Croon, M.; Hessel, V. Lipase-based biocatalytic flow process in a packed-bed microreactor. Ind. Eng. Chem. Res. 2013, 52, 10951–10960. [Google Scholar] [CrossRef]
- Hessel, V. Novel process windows—Gate to maximizing process intensification via flow chemistry. Chem. Eng. Technol. 2009, 32, 1655–1681. [Google Scholar] [CrossRef]
- Hessel, V.; Tibhe, J.; Noël, T.; Wang, Q. Biotechnical micro-flow processing at the EDGE—Lessons to be learnt for a young discipline. Chem. Biochem. Eng. Q. 2014, 28, 167–188. [Google Scholar] [CrossRef]
- Budžaki, S.; Ostojčić, M.; Strelec, I. Heterogeni Biokatalizatori na Bazi Otpada/Nusproizvoda Prehrambene Industrije za Održivu Proizvodnju Biodizela. In Neke Mogućnosti Iskorištenja Nusproizvoda Prehrambene Industrije; Šubarić, D., Babić, J., Eds.; Josip Juraj Strossmayer University of Osijek, Faculty of Food Technology Osijek: Osijek, Croatia, 2017; pp. 241–258. [Google Scholar]
- Vaz, R.P.; Filho, E.X.F. Ion Exchange Chromatography for Enzyme Immobilization. In Applications of Ion Exchange Materials in Biomedical Industries; Inamuddin, I., Ed.; Springer: Chem, Switzerland, 2019; pp. 13–27. [Google Scholar]
- Mateo, C.; Abian, O.; Fernandez-Lafuente, R.; Guisan, J.M. Reversible enzyme immobilization via a very strong and nondistorting ionic adsorption on support– polyethylenimine composites. Biotechnol. Bioeng. 2000, 68, 98–105. [Google Scholar] [CrossRef]
- Torres-Salas, P.; del Monte-Martinez, A.; Cutiño-Avila, B.; Rodriguez-Colinas, B.; Alcalde, M.; Ballesteros, A.O.; Plou, F.J. Immobilized Biocatalysts: Novel Approaches and Tools for Binding Enzymes to Supports. Adv. Mater. 2011, 23, 5275–5282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ondul, E.; Dizge, N.; Albayrak, N. Immobilization of Candida antarctica A and Thermomyces lanuginosus lipases on cotton terry cloth fibrils using polyethyleneimine. Colloids Surf. B Biointerfaces 2012, 95, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of Enzymes: A Literature Survey. In Immobilization of Enzymes and Cells: Methods in Molecular Biology; Guisan, J.M., Ed.; Springer Science+Business Media: New York, NY, USA, 2013; pp. 15–31. [Google Scholar]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Corici, L.; Ferrario, V.; Pellis, A.; Ebert, C.; Lotteria, S.; Catone, S.; Voinovich, D.; Gardossi, L. Large scale application of immobilized enzymes call for sustainable and inexpensive solutions: Rice husk as renewable alternative to fossil-based organic resins. RSC Adv. 2016, 6, 63256–63270. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.Y. Enzyme immobilization on cellulose matrixes. J. Bioact. Compat. Polym. 2016, 31, 553–567. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Virgen-Ortiz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, A.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7690. [Google Scholar] [CrossRef] [Green Version]
- Cespugli, M.; Lotteria, S.; Navarini, L.; Lonzarich, V.; Del Terra, L.; Vita, F.; Zweyer, M.; Baldini, G.; Ferrario, V.; Ebert, C.; et al. Rice Husk as an Inexpensive Renewable Immobilization Carrier for Biocatalysts Employed in the Food, Cosmetic and Polymer Sector. Catalysts 2018, 8, 471. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Lee, O.K.; Le, E.Y. Nano-immobilized biocatalysts for biodiesel production from renewable and sustainable resources. Catalysts 2018, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I. An overview of integration opportunities for sustainable bioethanol production from first- and second-generation sugar-based feedstocks. J. Clean. Prod. 2020, 245, 118857. [Google Scholar] [CrossRef]
- Roukas, T.; Kotzekidou, P. Rotary biofilm reactor: A new tool for long-term bioethanol production from non-sterilized beet molasses by Saccharomyces cerevisiae in repeated-batch fermentation. J. Clean. Prod. 2020, 257, 120519. [Google Scholar] [CrossRef]
- Martinez, O.; Sanchez, A.; Font, X.; Barrena, R. Valorization of sugarcane bagasse and sugar beet molasses using Kulyveromyces marxianus for producing value-added aroma compounds via solid-state fermentation. J. Clean. Prod. 2017, 158, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Moayedi, H.; Aghel, B.; Abdullahi, M.M.; Nguyen, H.; Rashid, S.A. Applications of rice husk ash as green and sustainable biomass. J. Clean. Prod. 2019, 237, 117851. [Google Scholar] [CrossRef]
- Nunes, L.A.; Silva, M.L.S.; Gerber, J.Z.; Kalid, R.d.A. Waste green coconut shells: Diagnosis of the disposal and applications for use in other products. J. Clean. Prod. 2020, 255, 120169. [Google Scholar] [CrossRef]
- Arevalo-Gallegos, A.; Ahmad, Z.; Asgher, M.; Parra-Saldivar, R.; Iqbal, H.M.N. Lignocellulose: A sustainable material to produce value-added products with a zero waste approach—A review. Int. J. Biol. Macromol. 2017, 99, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Kessi, E.; Arias, J.L. Using Natural Waste Material as a Matrix for the Immobilization of Enzymes: Chicken Eggshell Membrane Powder for β-Galactosidase Immobilization. Appl. Biochem. Biotechnol. 2018, 187, 101–115. [Google Scholar] [CrossRef]
- Mignardi, S.; Archilletti, L.; Medeghini, L.; De Vito, C. Valorization of Eggshell Biowaste for Sustainable Environmental Remediation. Sci. Rep. 2020, 10, 2436. [Google Scholar] [CrossRef] [Green Version]
- Waheed, M.; Butt, M.S.; Shehzad, A.; Adzahan, N.M.; Shabbir, M.A.; Suleria, H.A.R.; Aadil, R.M. Eggshell calcium: A cheap alternative to expensive supplements. Trends Food Sci. Technol. 2019, 91, 219–230. [Google Scholar] [CrossRef]
- Nys, Y.; Gautron, J. Structure and Formation of the Eggshell. In Bioactive Egg Compounds; Huopalahti, R., López-Fandiño, R., Anton, M., Schade, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 99–102. [Google Scholar]
- Athanasiadou, D.; Jiang, W.; Goldbaum, D.; Saleem, A.; Basu, K.; Pacella, M.S.; Böhm, C.F.; Chromik, R.R.; Hincke, M.T.; Rodríguez-Navarro, A.B.; et al. Nanostructure, osteopontin, and mechanical properties of calcitic avian eggshell. Sci. Adv. 2018, 4, eaar3219. [Google Scholar] [CrossRef] [Green Version]
- Makkar, H.P.S.; Sharma, O.P. Egg Shell as a Carrier for Enzyme Immobilization. Biotechnol. Bioeng. 1983, 25, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, G.; Banerjee, R.; Bhattacharyya, B.C. Immobilization of lipase using egg shell and alginate as carriers: Optimization of reaction conditions. Bioprocess. Eng. 1998, 19, 111–114. [Google Scholar] [CrossRef]
- Pundir, C.S.; Bhambi, M.; Chauhan, N.S. Chemical activation of egg shell membrane for covalent immobilization of enzymes and its evaluation as inter support in urinary oxalate determination. Talanta 2009, 77, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.F.; Kumar, J.; Jha, S.K.; Kubal, B.S. Immobilization of the urease on eggshell membrane and its application in biosensor. Mater. Sci. Eng. C 2013, 33, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.G.; Roy, S. Eggshell membrane: A natural substrate for immobilization and detection of DNA. Mater. Sci. Eng. C 2016, 59, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Salleh, S.; Serri, N.A.; Hena, S.; Tajarudin, H.A. Preliminary studies on immobilization of lipase using chicken eggshell. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012026. [Google Scholar] [CrossRef] [Green Version]
- Norouzian, D.; Akbarzadeh, A.; Mirdamadi, S.; Khetami, S.; Farhanghi, A. Immobilization of mushroom tyrosinase by different methods in order to transform L-tyrosine to L-3,4 dihydroxyphenylalanine (L-dopa). Biotechnology 2007, 6, 436–439. [Google Scholar] [CrossRef]
- Strelec, I.; Ostojćić, M.; Budžaki, S. Transformacija Ljuske Kokošjih Jaja u Prizvode Dodane Vrijednosti. In Neke Mogućnosti Iskorištenja Nusproizvoda Prehrambene Industrije-Knjiga 3, 1st ed.; Šubarić, D., Miličević, B., Eds.; Josip Juraj Strossmayer University of Osijek, Faculty of Food Technology Osijek: Osijek, Croatia, 2021; pp. 303–327. [Google Scholar]
- Ribeiro, G.C.A.; Fernandes, P.; de Assis, S.A. Production, characterization, and immobilization of inulinase produced by Pseudozyma sp. (CCMB 306). Chem. Eng. Commun. 2018, 205, 1060–1068. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sen, R. A comparative performance evaluation of jute and eggshell matrices to immobilize pancreatic lipase. Process. Biochem. 2012, 47, 749–757. [Google Scholar] [CrossRef]
- Venkaiah, B.; Kumar, A. Process for the recovery and immobilization of starch phosphorylase from starch based industrial waste-water. Biotechnol. Appl. Biochem. 1995, 21, 77–85. [Google Scholar]
- Siemiradzka, W.; Dolińska, B.; Ryszka, F. An ecological and multi valuable raw material for obtaining effective calcium preparations. IOSR J. Biotechnol. Biochem. 2019, 5, 50–57. [Google Scholar]
- Thakur, R.J.; Shaikh, H.; Gat, Y.; Waghmare, R.B. Effect of calcium chloride extracted from eggshell in maintaining quality of selected fresh-cut fruits. Int. J. Recycl. Org. Waste Agric. 2019, 8, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhou, K.; Li, D.; Guyonnet, V.; Hincke, M.T.; Mine, Y. Avian Eggshell Membrane as a Novel Biomaterial: A Review. Foods 2021, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Tembe, S.; Kubal, B.S.; Karve, M.; D’Souza, S.F. Glutaraldehyde activated eggshell membrane for immobilization of tyrosinase from Amorphophallus companulatus: Application in construction of electrochemical biosensor for dopamine. Anal. Chim. Acta 2008, 612, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, R.; Sanny, S.A.; Derman, E. Stability studies of immobilized lipase on rice husk and eggshell membrane. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012032. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Huang, M.; Feng, J.-X. Immobilization of α-amylase on eggshell membrane and Ag-nanoparticle-decorated eggshell membrane for the biotransformation of starch. Starch 2017, 69, 1600352. [Google Scholar] [CrossRef]
- Choi, M.M.F.; Pang, W.S.H.; Xiao, D.; Wu, X. An optical glucose biosensor with eggshell membrane as an enzyme immobilisation platform. Analyst 2001, 126, 1558–1563. [Google Scholar] [CrossRef]
- Xiao, D.; Choi, M.M.F. Aspartame Optical Biosensor with Bienzyme-Immobilized Eggshell Membrane and Oxygen-Sensitive Optode Membrane. Anal. Chem. 2002, 74, 863–870. [Google Scholar] [CrossRef]
- Choi, M.M.F.; Yiu, T.P. Immobilization of beef liver catalase on eggshell membrane for fabrication of hydrogen peroxide biosensor. Enzym. Microb. Technol. 2004, 34, 41–47. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, G.; Shuang, S.; Choi, M.M.F. Biosensors for determination of glucose with glucose oxidase immobilized on an eggshell membrane. Talanta 2004, 64, 546–553. [Google Scholar] [CrossRef]
- Choi, M.M.F. Application of a long shelf-life biosensor for the analysis of L-lactate in dairy products and serum samples. Food Chem. 2005, 92, 575–581. [Google Scholar] [CrossRef]
- Choi, M.M.F.; Liang, M.M.K.; Lee, A.W.M. A biosensing method with enzyme-immobilized eggshell membranes for determination of total glucosinolates in vegetables. Enzym. Microb. Technol. 2005, 36, 91–99. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, D.; Shuang, S.; Choi, M.M.F. A homocysteine biosensor with eggshell membrane as an enzyme immobilization platform. Sens. Actuators B 2006, 114, 936–942. [Google Scholar] [CrossRef]
- Joshi, P.; Joshi, H.C.; Sanghi, S.K.; Kundu, S. Immobilization of monoamine oxidase on eggshell membrane and its application in designing an amperometric biosensor for dopamine. Microchim. Acta 2010, 169, 383–388. [Google Scholar] [CrossRef]
- Aini, B.N.; Siddiquee, S.; Ampon, K.; Rodrigues, K.F.; Suryani, S. Development of glucose biosensor based on ZnO nanoparticles film and glucose oxidase-immobilized eggshell membrane. Sens. Bio Sens. Res. 2015, 4, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Roychoudhury, A.; Jha, S.K. Reusable Glucose Sensor Based on Enzyme Immobilized Egg-shell Membrane. Anal. Sci. 2016, 32, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Torres-Mansilla, A.C.; Delgado-Mejía, E. Influence of Separation Techniques with Acid Solutions on the Composition of Eggshell Membrane. Int. J. Polutry Sci. 2017, 16, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Pasarin, D.; Rovinaru, C. Separation Methods of the Eggshell Membranes from Eggshell. Proceedings 2019, 29, 122. [Google Scholar] [CrossRef] [Green Version]
- Scully, D.S.; Jaiswal, A.K.; Abu-Ghannam, N. An investigation into spent coffee waste as a renewable source of bioactive compounds and industrially important sugars. Bioengineering 2016, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of industrial onion wastes (Allium cepa L.): Dietary fibre and bioactive compounds. Plant. Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations—FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 4 March 2021).
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Esteban, R.M. Onion Products: Source of Healthy Compounds; Nova Science Publishers, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Choi, I.S.; Cho, E.J.; Moon, J.H.; Bae, H.J. Onion skin waste as a valorization resource for the by-products quercetin and biosugar. Food Chem. 2015, 188, 537–542. [Google Scholar] [CrossRef]
- Jaime, L.; Martín-Cabrejas, M.A.; Mollá, E.; López-Andréu, F.J.; Esteban, R.M. Effect of storage on fructan and fructooligosaccharide of onion (Allium cepa L.). J. Agric. Food Chem. 2011, 49, 982–988. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Fereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Ng, A.; Smith, A.C.; Waldron, K.W. Effect of tissue type and variety on cell wall chemistry of onion (Allium cepa L.). Food Chem. 1998, 63, 17–24. [Google Scholar] [CrossRef]
- Vardon, D.R.; Moser, B.R.; Zheng, W.; Witkin, K.; Evangelista, R.L.; Strathmann, T.J.; Rajagopalan, K.; Sharma, B.K. Complete Utilization of Spent Coffee Grounds to Produce Biodiesel, Bio-Oil, and Biochar. ACS Sustain. Chem. Eng. 2013, 1, 1286–1294. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q.S. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Tongcumpou, C.; Usapein, P.; Tuntiwiwattanapun, N. Complete utilization of wet spent coffee grounds waste as a novel feedstock for antioxidant, biodiesel, and bio-char production. Ind. Crops Prod. 2010, 138, 111484. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Nile, S.H.; Lee, E.T.; Leea, Y.R. Economical and environment-friendly approaches for usage of onion (Allium cepa L.) wastes. Food Funct. 2016, 7, 3354–3369. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, H.S.; Hassan, N.M.M.; El Khalek, M.H.A. The effect of using onion skin powder as a source of dietary fiber and antioxidants on properties of dried and fried noodles. Curr. Sci. Int. 2014, 3, 468–475. [Google Scholar]
- Banerjee, A.; Singh, V.; Solanki, K.; Mukherjee, J.; Gupta, M.N. Combi-protein coated microcrystals of lipases for production of biodiesel from oil from spent coffee grounds. Sustain. Chem. Process. 2013, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Yücel, Y. Biodiesel production from pomace oil by using lipase immobilized onto olive pomace. Bioresour. Technol. 2011, 102, 3977–3980. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Y. Optimization of immobilization conditions of Thermomyces lanuginosus lipase on olive pomace using response surface methodology. Biocatal. Agric. Biotechnol. 2012, 1, 39–44. [Google Scholar] [CrossRef]
- Yücel, Y. Optimization of biocatalytic biodiesel production from pomace oil using response surface technology. Fuel Processing Technol. 2012, 99, 97–102. [Google Scholar] [CrossRef]
- Ittrat, P.; Chacho, T.; Pholprayoon, J.; Suttiwarayanon, N.; Charoenpanich, J. Application of agriculture waste as a support for lipase immobilization. Biocatal. Agric. Biotechnol. 2014, 3, 77–82. [Google Scholar] [CrossRef]
- Brigida, A.I.S.; Pinheiro, A.D.T.; Ferreira, A.L.O.; Gonçalves, L.R.B. Immobilization of Candida antarctica Lipase B by Adsorption of Green Coconut Fiber. Appl. Biochem. Biotechnol. 2008, 146, 173–187. [Google Scholar] [CrossRef]
- Gong, R.; Zhang, J.; Zhu, J.; Wang, J.; Lai, Q.; Jiang, B. Loofah sponge activated by periodate oxidation as a carrier for covalent immobilization of lipase. Korean J. Chem. Eng. 2013, 30, 1620–1625. [Google Scholar] [CrossRef]
- Kumar, V.R.; Pundir, C.S. Covalent immobilization of lipase onto onion membrane affixed on plastic surface: Kinetic properties and application in milk fat hydrolysis. Indian J. Biotechnol. 2007, 6, 479–484. [Google Scholar]
- Kumar, J.; D’Souza, S.F. Inner epidermis of onion bulb scale: As natural support for immobilization of glucose oxidase and its application in dissolved oxygen based biosensor. Biosens. Bioelectron. 2009, 24, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yao, J.; Russel, M.; Chen, H.; Chen, K.; Zhou, Y.; Ceccanti, B.; Zaray, G.; Choi, M.M.F. Development and analytical application of a glucose biosensor based on glucose oxidase/O-(2-hydroxyl)propyl-3-trimethylammonium chitosan chloride nanoparticle-immobilized onion inner epidermis. Biosens. Bioelectron. 2010, 25, 2238–2243. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-I.; Lo, Y.-C.; Liu, C.-W.; Yu, R.-C.; Chou, C.-C.; Cheng, K.-C. Enrichment of two isoflavone aglycones in black soymilk by using spent coffee grounds as an immobiliser for b-glucosidase. Food Chem. 2013, 139, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Buntić, A.V.; Pavlović, M.D.; Šiler-Marinković, S.S.; Miljković, M.G.; Davidović, S.Z.; Mihajlovski, K.R.; Dimitrijević-Branković, S.I. Screening for Factors Affecting Cellulase Adsorption from Solutions by Modified Coffee Residues. In Proceedings of the International Conference on Civil, Biological and Environmental Engineering (CBEE-2014), Istanbul, Turkey, 27–28 May 2014. [Google Scholar]
- Buntić, A.V.; Pavlović, M.D.; Antonović, D.G.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Utilization of spent coffee grounds for isolation and stabilization of Paenibacillus chitinolyticus CKS1 cellulase by immobilization. Helyon 2016, 2, e00146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buntić, A.; Pavlović, M.; Antonović, D.; Pavlović, V.; Vrućinić, D.; Šiler-Marinković, S.; Dimitrijević-Branković, S. Customizing the spent coffee for Trichoderma reesei cellulaseimmobilization by modification with activating agents. Int. J. Biol. Macromol. 2018, 107, 1856–1863. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budžaki, S.; Velić, N.; Ostojčić, M.; Stjepanović, M.; Rajs, B.B.; Šereš, Z.; Maravić, N.; Stanojev, J.; Hessel, V.; Strelec, I. Waste Management in the Agri-Food Industry: The Conversion of Eggshells, Spent Coffee Grounds, and Brown Onion Skins into Carriers for Lipase Immobilization. Foods 2022, 11, 409. https://doi.org/10.3390/foods11030409
Budžaki S, Velić N, Ostojčić M, Stjepanović M, Rajs BB, Šereš Z, Maravić N, Stanojev J, Hessel V, Strelec I. Waste Management in the Agri-Food Industry: The Conversion of Eggshells, Spent Coffee Grounds, and Brown Onion Skins into Carriers for Lipase Immobilization. Foods. 2022; 11(3):409. https://doi.org/10.3390/foods11030409
Chicago/Turabian StyleBudžaki, Sandra, Natalija Velić, Marta Ostojčić, Marija Stjepanović, Blanka Bilić Rajs, Zita Šereš, Nikola Maravić, Jovana Stanojev, Volker Hessel, and Ivica Strelec. 2022. "Waste Management in the Agri-Food Industry: The Conversion of Eggshells, Spent Coffee Grounds, and Brown Onion Skins into Carriers for Lipase Immobilization" Foods 11, no. 3: 409. https://doi.org/10.3390/foods11030409







