Effect of Extracts Derived from Brown Algae (Sargassum horneri) on the Gel Property and Moisture Distribution of Hairtail Surimi Gel (Trichiurus haumela)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Phlorotannins Extracts
2.3. Phlorotannins Extracts Identification
2.3.1. Chromatographic Conditions
2.3.2. Mass Spectrometry Conditions
2.4. Preparation of Surimi Gel
2.5. Texture Analysis of Surimi Gel
2.6. Scanning Electron Microscopy (SEM) of Surimi Gel
2.7. Dielectric Properties Determination of Surimi Gel
2.8. Nuclear Magnetic Resonance (NMR) Analysis of Surimi Gel
2.9. Magnetic Resonance Imaging (MRI) Analysis of Surimi Gel
2.10. Statistical Analysis
3. Results and Discussion
3.1. PE Identification
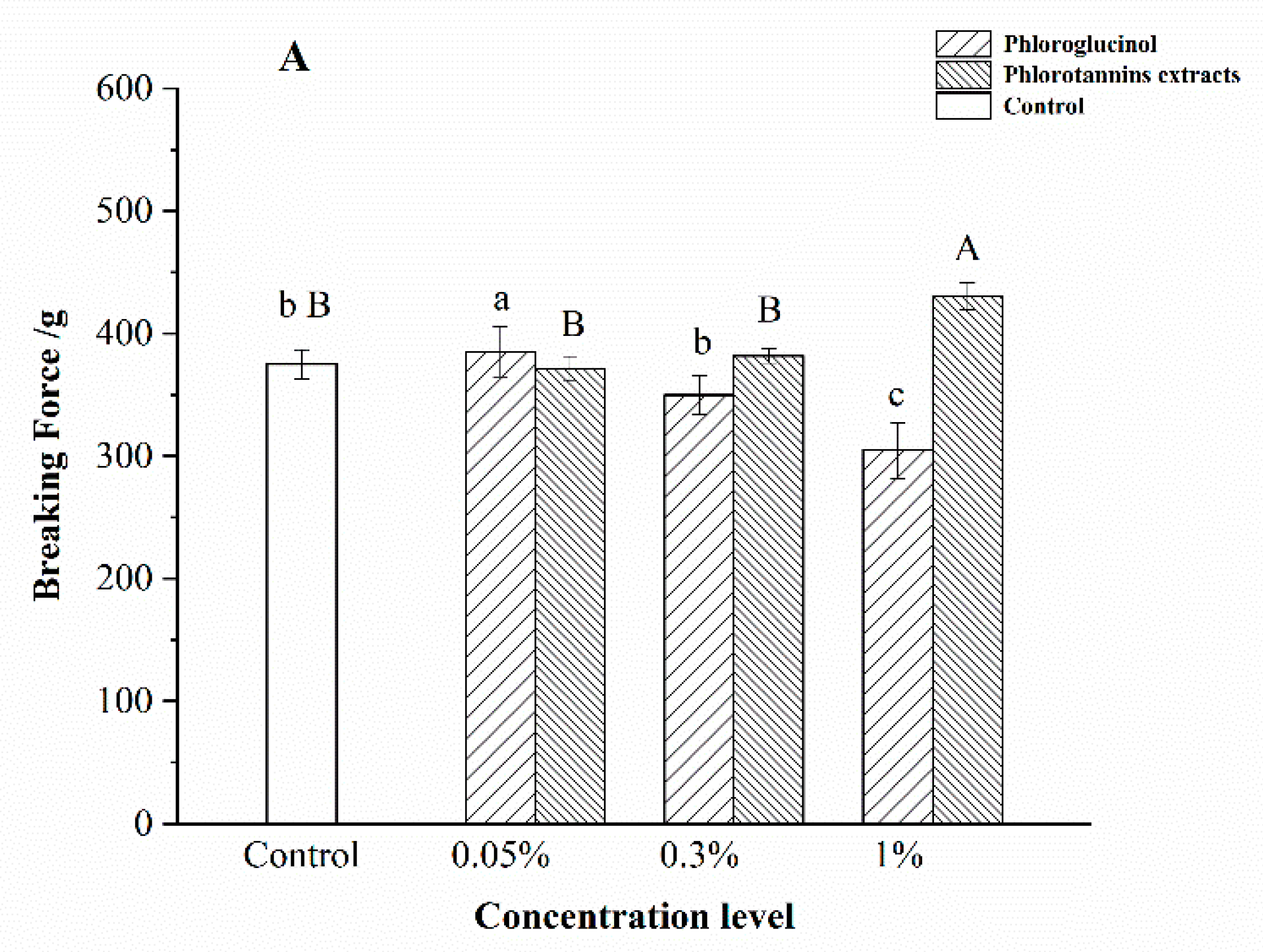
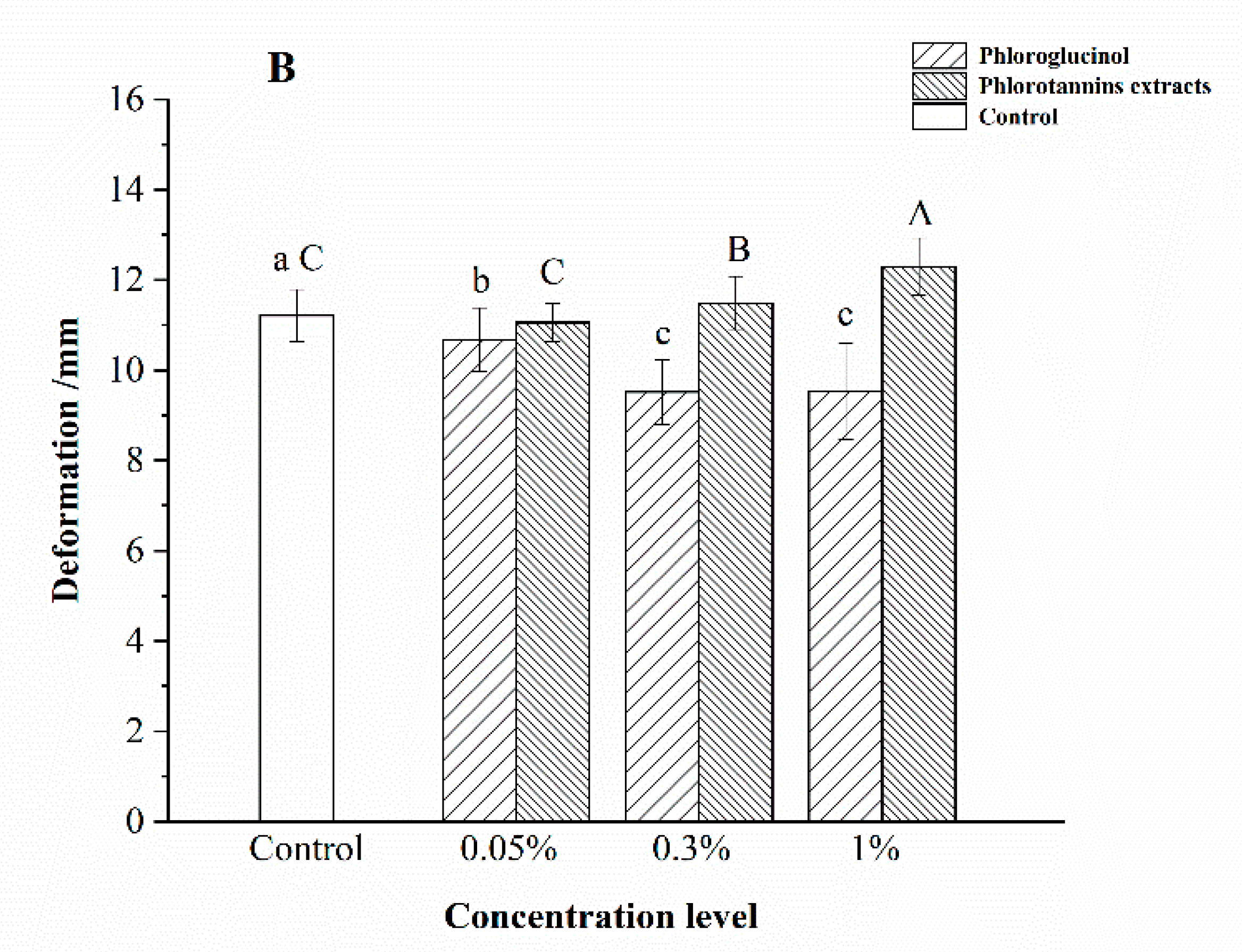
3.2. Gel Property of Surimi Gel
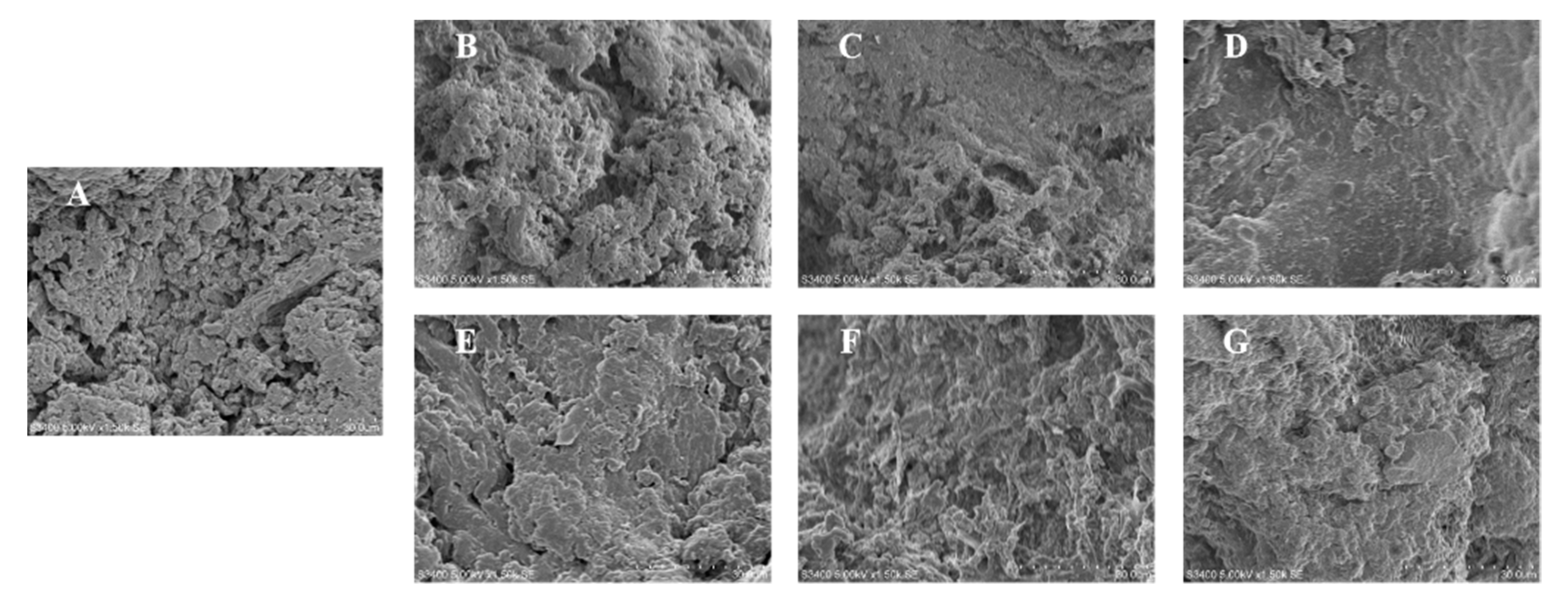
3.3. The Microstructure of Surimi Gels with Phlorotannins Extraction and Phloroglucinol
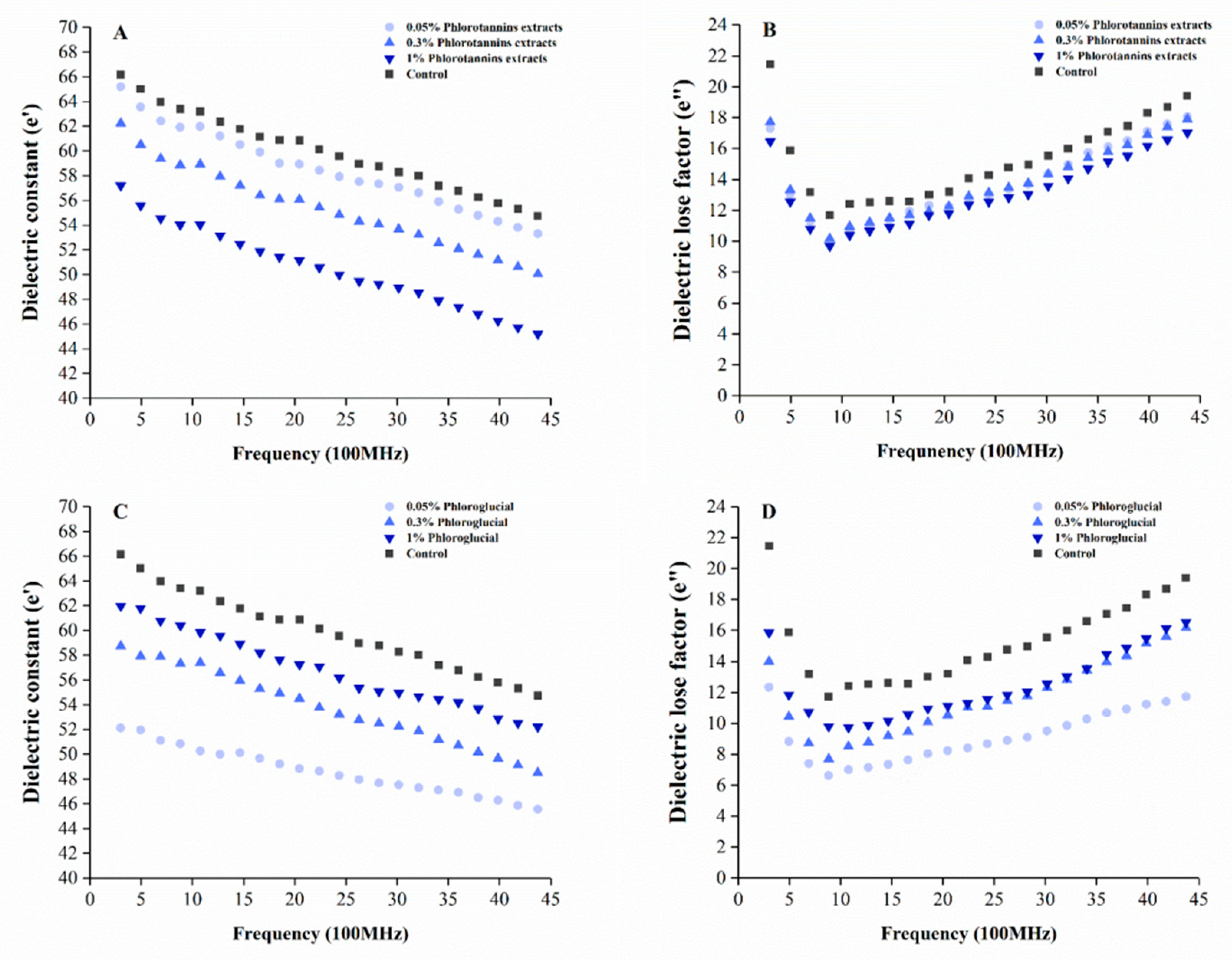
3.4. Dielectric Properties of Surimi Gel
3.5. The Water Relaxation Time of Surimi Gel
3.6. Water Distribution of Surimi Gel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takeda, H.; Seki, N. Enzyme-catalyzed Cross-linking and Degradation of Myosin Heavy Chain in Walleye Pollack Surimi Paste during Setting. Fish. Sci. 1996, 62, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Flores, R.; Cando, D.; Borderías, A.J.; Moreno, H.M. Importance of salt and temperature in myosin polymerization during surimi gelation. Food Chem. 2018, 239, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Hz, A.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar]
- Zhang, R.; Zhang, T.; Hu, M.; Xue, Y.; Xue, C. Effects of oleogels prepared with fish oil and beeswax on the gelation behaviors of protein recovered from Alaska Pollock. LWT 2020, 137, 110423. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, Y.; Wu, C.; Yuan, C.; Ishimura, G.; Liu, W.; Chen, S. γ-PGA and MTGase improve the formation of ε-(γ-glutamyl) lysine cross-links within hairtail (Trichiurus haumela) surimi protein. Food Chem. 2018, 242, 330–337. [Google Scholar] [CrossRef]
- Spanos, G.A.; Wrolstad, R.E. Phenolics of apple, pear, and white grape juices and their changes with processing and storage. A review. J. Agric. Food Chem. 1992, 40, 1478–1487. [Google Scholar] [CrossRef]
- Strauss, G.; Gibson, S.M. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food in-gredients. Food Hydrocoll. 2004, 18, 81–89. [Google Scholar] [CrossRef]
- Balange, A.K.; Benjakul, S. Effect of oxidised phenolic compounds on the gel property of mackerel (Rastrelliger kanagurta) surimi. LWT-Food Sci. Technol. 2009, 42, 1059–1064. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S. Effect of ethanolic coconut husk extract and pre-emulsification on properties and stability of surimi gel fortified with seabass oil during refrigerated storage. LWT 2019, 108, 160–167. [Google Scholar] [CrossRef]
- Cho, S.; Shimizu, M. Natural Sleep Aids and Polyphenols as Treatments for Insomnia. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Elsevier: Amsterdam, The Netherlands, 2015; pp. 141–151. [Google Scholar]
- Senapati, S.R.; Singh, C.B.; Hassan, M.A.; Vignaesh, D.; MartinXavier, K.A.; Balange, A.K. Effect of differentsolvents on total phenolics and antioxidant activity of extracts from Sargassum tenerrium (J. Agardh, 1848). J. Environ. Bio.-Sci. 2016, 30, 415–419. [Google Scholar]
- Jiang, D.; Shen, P.; Pu, Y.; Jin, M.; Yu, C.; Qi, H. Enhancement of gel properties of Scomberomorus niphonius myofibrillar protein using phlorotannin extracts under UVA irradiation. J. Food Sci. 2020, 85, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Shitole, S.S.; Balange, A.K.; Gangan, S.S. Use of Seaweed (Sargassum tenerrimum) extract as gel enhancer for lesser sardine (Sardinella brachiosoma) surimi. Int. Aquat. Res. 2014, 6, 55. [Google Scholar] [CrossRef]
- Shitole, S.; Balange, A. Enhancement of gel strength of surimi from Japanese threadfin bream (Nemipterus japonicus Bloch, 1791) using seaweed extract. Fish. Technol. 2014, 51, 102–105. [Google Scholar]
- Wang, Y.; Tian, X.; Li, J.; Tu, L.; Tan, S.; Wu, W.; Bao, B. Extraction Optimization Using Response Surface Methodology and Structure Identification of Phlorotannins from Sargassum horneri. Sci. Technol. Food Ind. 2018, 39, 143–149. [Google Scholar]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MSn: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [Green Version]
- Julavittayanukul, O.; Benjakul, S.; Visessanguan, W. Effect of phosphate compounds on gel-forming ability of surimi from bigeye snapper (Priacanthus tayenus). Food Hydrocoll. 2006, 20, 1153–1163. [Google Scholar] [CrossRef]
- Oujifard, A.; Benjakul, S.; Ahmad, M.; Seyfabadi, J. Effect of bambara groundnut protein isolate on autolysis and gel properties of surimi from threadfin bream (Nemipterus bleekeri). LWT 2012, 47, 261–266. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Zhu, Y.; Zhang, R.; Cheng, Y.; Jin, Y. Effects of sucrose and glucose on the dielectric properties of minced Antarctic krill. Sci. Technol. Food Ind. 2017, 24, 58–64. [Google Scholar]
- Kroll, J.; Rawel, H.M.; Rohn, S. Reactions of Plant Phenolics with Food Proteins and Enzymes under Special Consideration of Covalent Bonds. Food Sci. Technol. Res. 2003, 9, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W. Surimi and Surimi Seafood; CRC Press LLC: Baton Rouge, LA, USA, 2013. [Google Scholar]
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein-phenolic compound interactions. Int. J. Food Sci. Technol. 2016, 52, 573–585. [Google Scholar] [CrossRef]
- Haslam, E. Plant Polyphenols: Vegetable Tannins Revisited; CUP Archive: Cambridge, UK, 1989; p. 230. [Google Scholar]
- Balange, A.K.; Benjakul, S. Effect of oxidised tannic acid on the gel properties of mackerel (Rastrelliger kanagurta) mince and surimi prepared by different washing processes. Food Hydrocoll. 2009, 23, 1693–1701. [Google Scholar] [CrossRef]
- Ngo, V.P.; Morioka, K.; Itoh, Y. Microstructure of white croaker surimi protein gels set at low temperature under the in-hibition of the polymerization and degradation of protein. J. Biol. Sci. 2010, 10, 499–506. [Google Scholar]
- Shen, P.; Gao, Z.; Xu, M.; Rao, J.; Chen, B. Physicochemical and structural properties of proteins extracted from dehulled industrial hempseeds: Role of defatting process and precipitation pH. Food Hydrocoll. 2020, 108, 106065. [Google Scholar] [CrossRef]
- Balange, A.K.; Benjakul, S. Use of kiam wood extract as gel enhancer for mackerel (Rastrelliger kanagurta) surimi. Int. J. Food Sci. Technol. 2009, 44, 1661–1669. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging Role of Phenolic Compounds as Natural Food Additives in Fish and Fish Products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef]
- Frazier, R.A.; Papadopoulou, A.; Mueller-Harvey, I.; Kissoon, D.; Green, R.J. Probing Protein−Tannin Interactions by Isothermal Titration Microcalorimetry. J. Agric. Food Chem. 2003, 51, 5189–5195. [Google Scholar] [CrossRef]
- Nunes, A.; Bohigas, X.; Tejada, J. Dielectric study of milk for frequencies between 1 and 20GHz. J. Food Eng. 2006, 76, 250–255. [Google Scholar] [CrossRef]
- Zhu, X.H.; Guo, W.C. A Review of Affecting Factors and Their Mechanisms of the Radio Frequency-Microwave Dielectric Properties of Foods. Food Sci. 2010, 31, 410–414. [Google Scholar]
- Schwan, H.P. Biological effects of non-ionizing radiations: Cellular properties and interactions. Ann. Biomed. Eng. 1988, 16, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Mabrook, M.; Petty, M. A novel technique for the detection of added water to full fat milk using single frequency admittance measurements. Sens. Actuators B Chem. 2003, 96, 215–218. [Google Scholar] [CrossRef]
- Lizhi, H.; Toyoda, K.; Ihara, I. Dielectric properties of edible oils and fatty acids as a function of frequency, temperature, moisture and composition. J. Food Eng. 2008, 88, 151–158. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Heating surimi products using microwave combined with steam methods: Study on energy saving and quality. Innov. Food Sci. Emerg. Technol. 2018, 47, 231–240. [Google Scholar] [CrossRef]
- Ryynänen, S. The electromagnetic properties of food materials: A review of the basic principles. J. Food Eng. 1995, 26, 409–429. [Google Scholar] [CrossRef]
- Damez, J.-L.; Clerjon, S. Quantifying and predicting meat and meat products quality attributes using electromagnetic waves: An overview. Meat Sci. 2013, 95, 879–896. [Google Scholar] [CrossRef]
- Clerjon, S.; Daudin, J.-D.; Damez, J.-L. Water activity and dielectric properties of gels in the frequency range 200 MHz–6 GHz. Food Chem. 2003, 82, 87–97. [Google Scholar] [CrossRef]
- Tananuwong, K.; Reid, D. DSC and NMR relaxation studies of starch–water interactions during gelatinization. Carbohydr. Polym. 2004, 58, 345–358. [Google Scholar] [CrossRef]
- Han, M.; Wang, P.; Xu, X.; Zhou, G. Low-field NMR study of heat-induced gelation of pork myofibrillar proteins and its relationship with microstructural characteristics. Food Res. Int. 2014, 62, 1175–1182. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Yang, K.; Yin, X.; Ma, J.; Li, Z.; Sun, W.; Han, M. Effect of low-frequency magnetic field on the gel properties of pork myofibrillar proteins. Food Chem. 2019, 274, 775–781. [Google Scholar] [CrossRef]
- Ducel, V.; Pouliquen, D.; Richard, J.; Boury, F. 1H NMR relaxation studies of protein–polysaccharide mixtures. Int. J. Biol. Macromol. 2008, 43, 359–366. [Google Scholar] [CrossRef]
- Mu, Y.; Sun, J.; Obadi, M.; Chen, Z.; Xu, B. Effects of saccharides on the rheological and gelling properties and water mobility of egg white protein. Food Hydrocoll. 2020, 108, 106038. [Google Scholar] [CrossRef]
- Hills, B.; Takacs, S.; Belton, P. The effects of proteins on the proton N.M.R. transverse relaxation times of water. Mol. Phys. 1989, 67, 903–918. [Google Scholar] [CrossRef]
- Kuntz, I., Jr.; Kauzmann, W. Hydration of proteins and polypeptides. In Advances in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 1974; pp. 239–345. [Google Scholar]
- Chinachoti, P.; White, V.A.; Lo, L.; Stengle, T.R. Application of high-resolution carbon-13, oxygen-17, and sodium-23 nuclear magnetic resonance to study the influences of water, sucrose, and sodium chloride on starch gelatinization. Cereal Chem. 1991, 68, 238–244. [Google Scholar]
- Dolata, W.; Piotrowska, E.; Wajdzik, J.; Tritt-Goc, J. The use of the MRI technique in the evaluation of water distribution in tumbled porcine muscle. Meat Sci. 2004, 67, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Gianferri, R.; D’Aiuto, V.; Curini, R.; Delfini, M.; Brosio, E. Proton NMR transverse relaxation measurements to study water dynamic states and age-related changes in Mozzarella di Bufala Campana cheese. Food Chem. 2007, 105, 720–726. [Google Scholar] [CrossRef]
- Kudre, T.; Benjakul, S.; Kishimura, H. Effects of protein isolates from black bean and mungbean on proteolysis and gel properties of surimi from sardine (Sardinella albella). LWT-Food Sci. Technol. 2013, 50, 511–518. [Google Scholar] [CrossRef]


| Number | Retention Time/min | m/z | Average Molecular Weight | Chemical Formula |
|---|---|---|---|---|
| 1 | 2.620 | 182.96170 | 182.64745 | |
| 2 | 2.628 | 124.99404 | 124.38192 | |
| 3 | 2.647 | 101.00841 | 100.37207 | |
| 4 | 2.657 | 110.00857 | 109.35370 | |
| 5 | 2.690 | 112.96627 | 112.07419 | |
| 6 | 2.720 | 198.93934 | 198.38203 | C4H6OS4 |
| 7 | 2.721 | 116.98560 | 116.00102 | |
| 8 | 2.729 | 128.94901 | 128.02619 | C4OS2 |
| 9 | 2.734 | 328.89734 | 328.38389 | C10H4N2OS5 |
| 10 | 2.737 | 458.85542 | 458.34203 | C13H2N2O7S5 |
| 11 | 2.742 | 474.83270 | 474.04785 | |
| 12 | 2.744 | 214.91687 | 213.99547 | C9HCl3 |
| 13 | 2.746 | 344.87480 | 344.02586 | |
| 14 | 2.746 | 140.91655 | 139.98820 | |
| 15 | 3.179 | 256.04484 | 233.19057 | C9H7N5O3 |
| 16 | 3.183 | 421.11386 | 420.30871 | C13H20N6O8S |
| 17 | 3.187 | 302.04970 | 301.19281 | C11H11NO9 |
| 18 | 3.190 | 329.18305 | 328.33031 | C14H24N4O5 |
| 19 | 3.195 | 405.13736 | 404.63680 | C22H28OS3 |
| 20 | 3.199 | 345.1552 | 344.48908 | C17H28O3S2 |
| 21 | 3.2 | 172.09316 | 171.15199 | C11H24N6O4S |
| 22 | 3.22 | 337.16384 | 336.30625 | C8H5N5O |
| 23 | 3.226 | 188.05795 | 187.14249 | C14H28N2O4S |
| 24 | 3.234 | 321.18461 | 320.64899 | C6H13N3OS2 |
| 25 | 3.271 | 208.05698 | 207.04968 | C8H6ClN5O |
| 26 | 3.277 | 224.0342 | 223.56814 | C14H21ClN6O4 |
| 27 | 3.281 | 373.13822 | 372.7625 | C8H7N5O5S |
| 28 | 3.352 | 286.02447 | 285.20045 | C17H26N2O3 |
| 29 | 3.361 | 307.20102 | 306.34703 | C11H24N6O4S |
| 30 | 3.361 | 270.04693 | 269.46882 | C9H19NS4 |
| 31 | 3.368 | 208.05658 | 207.21412 | C5H9N3O6 |
| 32 | 3.369 | 158.09690 | 157.15869 | C11H11N |
| 33 | 3.433 | 224.03409 | 223.49965 | C8H6ClN5O |
| 34 | 3.437 | 373.13812 | 372.74777 | C13H25ClN2O8 |
| 35 | 3.445 | 172.09212 | 171.15013 | C4H9N7O |
| 36 | 3.448 | 337.16171 | 336.31311 | C13H24N2O8 |
| 37 | 3.451 | 188.05770 | 187.1416 | C8H5N5O |
| 38 | 3.503 | 321.18443 | 321.28052 | C16H30Cl2N2 |
| 39 | 3.528 | 235.07545 | 234.48632 | C9H10N6S |
| 40 | 3.529 | 251.05280 | 250.17086 | C8H6N6O4 |
| 41 | 3.821 | 166.10571 | 165.19649 | C6H15NO4 |
| 42 | 3.870 | 194.13675 | 193.27074 | C8H19NO4 |
| 43 | 3.932 | 307.20089 | 306.32947 | C17H26N2O3 |
| 44 | 4.099 | 150.17505 | 149.23387 | |
| 45 | 4.143 | 337.16147 | 336.30020 | C14H20N6O4 |
| 46 | 4.291 | 307.20132 | 306.34264 | C17H26N2O3 |
| 47 | 4.914 | 176.12795 | 175.23936 | C8H17NO3 |
| 48 | 4.914 | 194.13837 | 193.23043 | C8H19NO4 |
| 49 | 5.809 | 303.18921 | 302.29168 | C10H22N8O3 |
| 50 | 8.410 | 123.05531 | 122.12213 | C6H6N2O |
| 51 | 9.828 | 260.14681 | 237.31662 | C10H23NO5 |
| 52 | 10.798 | 194.13863 | 193.24705 | C8H19NO4 |
| 53 | 10.816 | 324.24887 | 323.39376 | C14H33N3O5 |
| 54 | 10.816 | 411.31749 | 410.31017 | C18H42N4O6 |
| 55 | 10.825 | 455.34358 | 454.61986 | C20H46N4O7 |
| 56 | 10.825 | 352.24382 | 351.41140 | C15H33N3O6 |
| 57 | 10.827 | 238.16453 | 237.30869 | C10H23NO5 |
| 58 | 10.827 | 176.12787 | 175.25413 | C8H17NO3 |
| 59 | 10.832 | 368.27506 | 367.46745 | C16H37N3O6 |
| 60 | 10.841 | 499.37012 | 498.6614 | C22H50N4O8 |
| 61 | 11.108 | 102.1278 | 101.18727 | C6H15N |
| 62 | 11.180 | 304.17291 | 281.31975 | C12H27NO6 |
| 63 | 11.181 | 113.03499 | 112.14134 | C4H4N2O2 |
| 64 | 11.324 | 116.07051 | 115.11642 | C5H9NO2 |
| 65 | 11.339 | 200.0885 | 199.18191 | C5H9N7O2 |
| 66 | 11.422 | 314.09103 | 313.32248 | C11H15N5O4S |
| 67 | 11.518 | 136.06170 | 135.12716 | C5H5N5 |
| 68 | 11.632 | 323.16347 | 322.45909 | C13H26N2O5S |
| 69 | 11.680 | 127.05003 | 109.07874 | C5H3NO2 |
| 70 | 12.116 | 116.07110 | 115.12176 | C5H9NO2 |
| 71 | 12.117 | 233.14945 | 232.27569 | C10H20N2O4 |
| 72 | 12.488 | 228.06569 | 227.26876 | C6H9N7OS |
| 73 | 12.496 | 174.05757 | 173.21876 | C7H11NO2S |
| 74 | 12.506 | 172.09389 | 171.15761 | C4H9N7O |
| 75 | 12.992 | 217.10404 | 194.21772 | C8H18O5 |
| 76 | 13.006 | 162.11199 | 161.22416 | C7H15NO3 |
| 77 | 13.008 | 277.17483 | 276.31391 | C12H24N2O5 |
| 78 | 13.135 | 251.05470 | 250.22581 | C12H10O6 |
| 79 | 13.178 | 321.20715 | 320.41285 | C20H24N4 |
| 80 | 13.735 | 321.20217 | 320.36389 | C14H28N2O6 |
| 81 | 13.898 | 133.08577 | 132.16099 | C6H12O3 |
| 82 | 13.900 | 239.14873 | 238.28483 | C10H22O6 |
| 83 | 13.940 | 136.06160 | 135.12835 | C5H5N5 |
| 84 | 13.942 | 298.09683 | 297.30583 | C11H15N5O3S |
| 85 | 14.077 | 109.02839 | 108.07940 | C6H4O2 |
| 86 | 14.357 | 197.11603 | 196.24830 | C11H16O3 |
| 87 | 14.358 | 141.05368 | 140.13538 | C7H8O3 |
| 88 | 14.358 | 179.10562 | 178.20740 | C11H14O2 |
| 89 | 14.359 | 237.10761 | 214.23068 | C7H14N6O2 |
| 90 | 14.359 | 133.10010 | 132.28006 | C10H12 |
| 91 | 14.369 | 172.09028 | 171.25490 | C7H13N3S |
| 92 | 14.507 | 130.15875 | 129.27435 | C8H19N |
| 93 | 14.634 | 283.17414 | 282.32968 | C12H26O7 |
| 94 | 14.637 | 133.08560 | 132.21589 | C6H12O3 |
| 95 | 14.762 | 161.10666 | 160.20811 | C10H12N2 |
| 96 | 14.802 | 375.07051 | 374.30363 | C18H14O9 |
| 97 | 15.243 | 327.20070 | 326.37497 | C11H30N6OS2 |
| 98 | 15.256 | 205.13307 | 204.23334 | C12H16N2O |
| 99 | 15.609 | 205.13317 | 204.24578 | C12H16N2O |
| 100 | 15.779 | 388.25120 | 370.41506 | C12H30N6O7 |
| 101 | 16.179 | 236.16439 | 235.31530 | C14H21NO2 |
| 102 | 16.250 | 432.27712 | 414.47561 | C14H34N6O8 |
| 103 | 16.306 | 330.16396 | 329.38774 | C18H23N3OS |
| 104 | 16.321 | 360.23156 | 359.39133 | C25H29NO |
| 105 | 16.337 | 209.14770 | 208.28387 | C8H20N2O4 |
| 106 | 16.343 | 133.09741 | 132.20609 | C5H12N2O2 |
| 107 | 16.593 | 212.11430 | 211.24952 | C8H13N5O2 |
| 108 | 16.666 | 476.30475 | 458.52634 | C16H38N6O9 |
| 109 | 16.757 | 219.14800 | 218.34457 | C13H18N2O |
| 110 | 16.922 | 207.09448 | 206.25775 | C11H14N2S |
| 111 | 17.042 | 520.33041 | 502.58621 | C18H42N6O10 |
| 112 | 17.070 | 248.14593 | 247.25591 | C7H17N7O3 |
| 113 | 17.301 | 350.23023 | 349.45141 | C16H27N7O2 |
| 114 | 17.384 | 564.35756 | 546.65633 | C21H42N10O7 |
| 115 | 17.699 | 608.38428 | 607.72716 | C23H49N11O8 |
| 116 | 17.733 | 542.25877 | 541.55646 | C23H31N11O5 |
| 117 | 17.799 | 123.11549 | 122.21653 | C9H14 |
| 118 | 17.801 | 207.13640 | 206.28160 | C9H14N6 |
| 119 | 17.828 | 447.09129 | 446.36504 | C21H18O11 |
| 120 | 17.894 | 270.12425 | 269.74843 | C14H20ClNO2 |
| 121 | 18.366 | 321.09245 | 320.28310 | C11H16N2O9 |
| 122 | 18.378 | 219.10016 | 218.36085 | C9H18 |
| 123 | 18.381 | 524.24689 | 506.59667 | C30H34O5S |
| 124 | 18.596 | 588.40820 | 565.78566 | C29H59NO9 |
| 125 | 18.784 | 388.28160 | 387.57821 | C20H33N7O |
| 126 | 18.830 | 135.11497 | 134.24089 | C10H14 |
| 127 | 18.831 | 197.11686 | 196.23207 | C11H16O3 |
| 128 | 18.831 | 133.10030 | 132.20364 | C10H12 |
| 129 | 18.835 | 179.10436 | 178.22762 | C7H10N6 |
| 130 | 18.872 | 384.28912 | 383.56288 | C25H37NO2 |
| 131 | 18.919 | 365.11881 | 364.31631 | C13H20N2O10 |
| 132 | 18.971 | 406.21052 | 405.94931 | C18H32ClN3O5 |
| 133 | 19.134 | 218.20622 | 217.33160 | C8H23N7 |
| 134 | 19.146 | 701.49011 | 700.91185 | C32H68N4O12 |
| 135 | 19.150 | 679.50780 | 678.95367 | C31H66N8O8 |
| 136 | 19.235 | 186.21576 | 185.37423 | |
| 137 | 19.295 | 508.32521 | 507.66241 | C25H37N11O |
| 138 | 19.366 | 538.37259 | 537.69005 | C27H43N11O |
| 139 | 19.380 | 197.11712 | 196.22811 | C11H16O3 |
| 140 | 19.544 | 559.37285 | 558.74173 | C29H42N12 |
| 141 | 19.560 | 242.28420 | 241.44480 | C16H35N |
| 142 | 19.670 | 274.27228 | 273.44389 | C12H31N7 |
| 143 | 19.703 | 318.30000 | 317.50362 | C18H39NO3 |
| 144 | 19.73 | 362.32583 | 361.56617 | C20H43NO4 |
| 145 | 19.879 | 256.25624 | 255.43859 | |
| 146 | 19.886 | 237.14136 | 236.34064 | C13H20N2S |
| 147 | 20.188 | 302.30455 | 301.49885 | C18H39NO2 |
| 148 | 20.229 | 346.33073 | 345.57569 | C20H43NO3 |
| 149 | 20.364 | 337.19662 | 336.42614 | C14H28N2O7 |
| 150 | 20.938 | 386.32592 | 385.57069 | C22H43NO4 |
| 151 | 20.947 | 330.33617 | 329.55028 | C20H43NO2 |
| 152 | 21.010 | 374.36291 | 373.62298 | C22H47NO3 |
| 153 | 21.085 | 301.13816 | 300.28852 | C13H20N2 |
| 154 | 21.088 | 149.02325 | 148.11612 | C8H4O3 |
| 155 | 21.340 | 388.34156 | 387.57253 | C22H45NO4 |
| 156 | 21.389 | 234.96051 | 234.05388 | C9H2N2O2S2 |
| 157 | 21.463 | 415.21075 | 414.49322 | C24H30O6 |
| 158 | 21.466 | 586.29929 | 585.66389 | C28H39N7O7 |
| 159 | 22.618 | 358.36684 | 357.62209 | C22H47NO |
| Material | Total Phenol Content/% | Phlorotannins Content/% | Purity/% |
|---|---|---|---|
| Brown algae dry powder | 7.80 | 6.46 | — |
| Phlorotannins extracts | 34.07 | 25.16 | 73.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Wang, Y.; Chu, Q.; Bao, B. Effect of Extracts Derived from Brown Algae (Sargassum horneri) on the Gel Property and Moisture Distribution of Hairtail Surimi Gel (Trichiurus haumela). Foods 2022, 11, 411. https://doi.org/10.3390/foods11030411
Han Q, Wang Y, Chu Q, Bao B. Effect of Extracts Derived from Brown Algae (Sargassum horneri) on the Gel Property and Moisture Distribution of Hairtail Surimi Gel (Trichiurus haumela). Foods. 2022; 11(3):411. https://doi.org/10.3390/foods11030411
Chicago/Turabian StyleHan, Qiuyu, Yuxin Wang, Qianqian Chu, and Bin Bao. 2022. "Effect of Extracts Derived from Brown Algae (Sargassum horneri) on the Gel Property and Moisture Distribution of Hairtail Surimi Gel (Trichiurus haumela)" Foods 11, no. 3: 411. https://doi.org/10.3390/foods11030411
APA StyleHan, Q., Wang, Y., Chu, Q., & Bao, B. (2022). Effect of Extracts Derived from Brown Algae (Sargassum horneri) on the Gel Property and Moisture Distribution of Hairtail Surimi Gel (Trichiurus haumela). Foods, 11(3), 411. https://doi.org/10.3390/foods11030411







