Characterization of the Key Aroma Constituents in Fried Tilapia through the Sensorics Concept
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Standards
2.2. Preparation of Fried Tilapia
2.3. Accelerated Solvent Extraction and High-Vacuum Flavor Extraction Technology (ASE-HVE)
2.4. Gas Chromatography-Olfactometry (GC-O) and Aroma Extract Dilution Analysis (AEDA)
2.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.6. Quantitation of Odor-Active Compounds
2.7. Recombination and Omission Experiments
2.8. Statistical Analysis
3. Results and Discussion
3.1. GC-O and AEDA: Aroma-Active Compounds in Fried Tilapia
3.2. Quantitative Analysis of the Selected Odorants
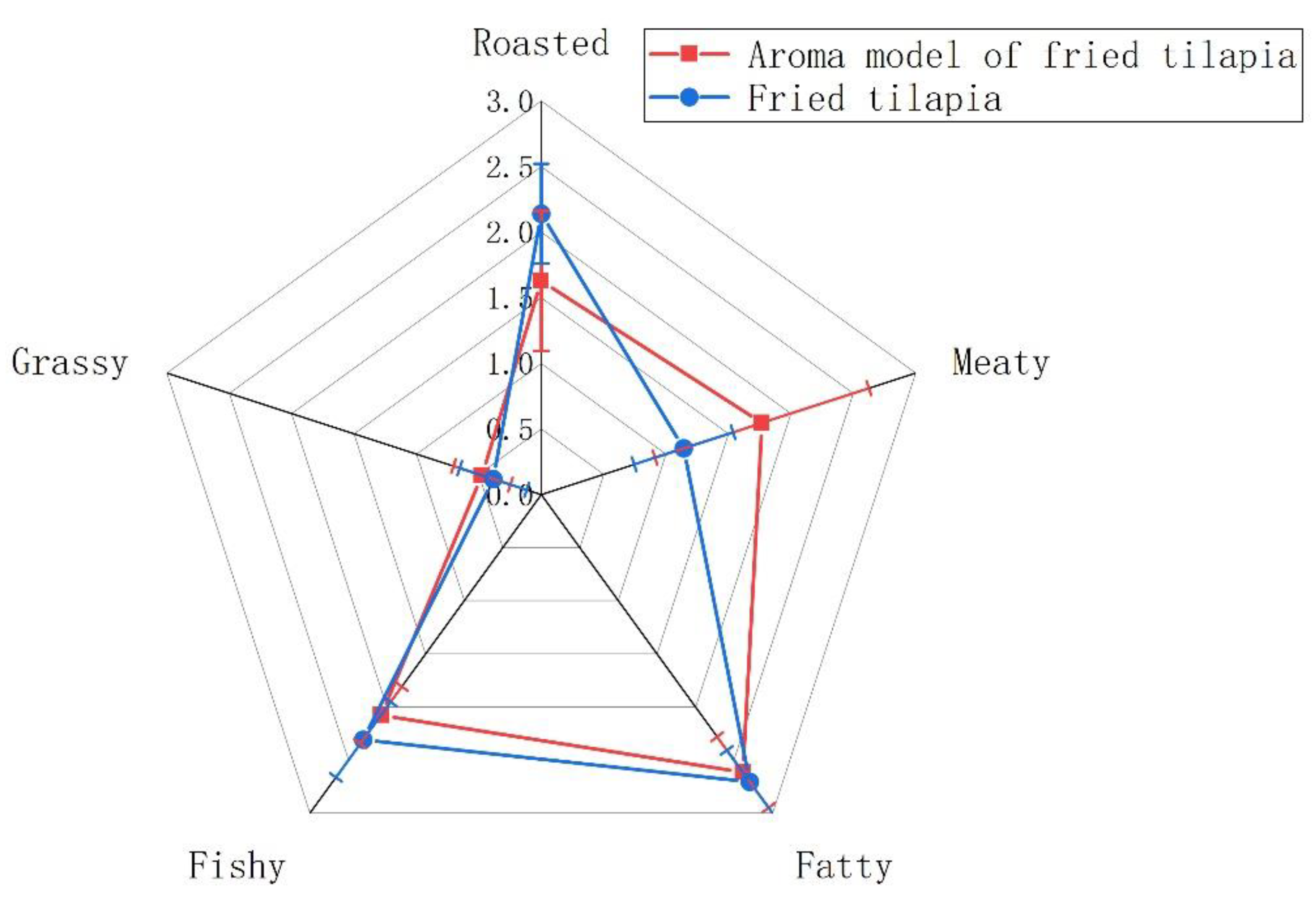
3.3. Fried Tilapia Aroma Recombination and Deletion Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Yu, K.; Ao, Q.; Tan, Y.; Fu, Q.; Jiang, H. Comparative splenic proteomic analysis of susceptible and resistant GIFT tilapia following challenge with Streptococcus agalactiae. Aquac. Int. 2021, 29, 1141–1159. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, Z.; Zhang, R.; Kang, Y.; Yu, K.; Wang, Y.; Zheng, X.; Huang, L.; Zhao, L. Spatial distribution, source identification, and risk assessment of organochlorines in wild tilapia from Guangxi, South China. Sci. Rep. 2020, 10, 15179. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, M.F.; Desta, D.T.; Alemayehu, F.R.; Kelikay, G.N.; Daba, A.K. Evaluation of fatty acid-related nutritional quality indices in fried and raw nile tilapia (Oreochromis Niloticus), fish muscles. Food Sci. Nutr. 2020, 8, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Salum, P.; Guclu, G.; Selli, S. Comparative evaluation of key aroma-active compounds in raw and cooked red mullet (Mullus barbatus) by aroma extract dilution analysis. J. Agric. Food Chem. 2017, 65, 8402–8408. [Google Scholar] [CrossRef] [PubMed]
- Mall, V.; Schieberle, P. Characterization of key aroma compounds in raw and thermally processed prawns and thermally processed lobsters by application of aroma extract dilution analysis. J. Agric. Food Chem. 2016, 64, 6433–6442. [Google Scholar] [CrossRef] [PubMed]
- Lapsongphon, N.; Yongsawatdigul, J.; Cadwallader, K.R. Identification and characterization of the aroma-impact components of Thai fish sauce. J. Agric. Food Chem. 2015, 63, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Cayhan, G.G.; Selli, S. Characterization of the key aroma compounds in cooked grey mullet (Mugil cephalus) by application of aroma extract dilution analysis. J. Agric. Food Chem. 2011, 59, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Cao, M.; Cao, A.; Zhang, W. The Effect of Magnetic Nanoparticles Plus Microwave Thawing on the Volatile Flavor Characteristics of Largemouth Bass (Micropterus salmoides) Fillets. Food Bioprocess. Technol. 2019, 12, 1340–1351. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Pan, T.; Hui, T.; Ma, J. Characterization of key aroma compounds in Beijing roasted duck by gas chromatography-olfactometry-mass spectrometry, odor-activity values, and aroma-recombination experiments. J. Agric. Food Chem. 2019, 67, 5847–5856. [Google Scholar] [CrossRef]
- Song, S.; Fan, L.; Xu, X.; Xu, R.; Jia, Q.; Feng, T. Aroma Patterns Characterization of braised pork obtained from a novel ingredient by sensory-guided analysis and gas-chromatography-olfactometry. Foods 2019, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Zhao, M.; Zhen, D.; Tan, J.; Wang, T.; Xie, J. Key aroma compounds in Chinese fried food of youtiao. Flavour Fragr. J. 2020, 35, 88–98. [Google Scholar] [CrossRef]
- Lasekan, O.; Dabaj, F. Characterization of the Key Aroma Constituents in Fry Breads by Means of the Sensomics Concept. Foods 2020, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Pueschel, V.A.; Schieberle, P. Changes in the key aroma compounds of matsutake mushroom (Tricholoma matsutake Sing.) from Canada during pan-frying elucidated by application of the sensomics approach. Eur. Food Res. Technol. 2021, 247, 51–65. [Google Scholar] [CrossRef]
- Schmidberger, P.C.; Schieberle, P. Changes in the key aroma compounds of raw shiitake mushrooms (Lentinula edodes) induced by pan-frying as well as by rehydration of dry mushrooms. J. Agric. Food Chem. 2020, 68, 4493–4506. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Lin, S.-Y.; Du, H.-T.; Qin, L.; Lei, L.-M.; Chen, D. An Insight by Molecular Sensory Science Approaches to Contributions and Variations of the Key Odorants in Shiitake Mushrooms. Foods 2021, 10, 622. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, F.; Chen, H.; Huang, M.; Xie, J.; Chen, F.; Sun, B. Analysis of volatiles in Dezhou Braised Chicken by comprehensive two-dimensional gas chromatography/high resolution-time of flight mass spectrometry. LWT-Food Sci. Technol. 2015, 60, 1235–1242. [Google Scholar] [CrossRef]
- Mahmoud, M.A.A.; Buettner, A. Characterisation of aroma-active and off-odour compounds in German rainbow trout (Oncorhynchus mykiss). Part II: Case of fish meat and skin from earthen-ponds farming. Food Chem. 2017, 232, 841–849. [Google Scholar] [CrossRef]
- Fan, M.; Xiao, Q.; Xie, J.; Cheng, J.; Sun, B.; Du, W.; Wang, Y.; Wang, T. Aroma compounds in chicken broths of Beijing Youji and commercial broilers. J. Agric. Food Chem. 2018, 66, 10242–10251. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Identification of aroma compounds of Lamiaceae species in Turkey using the purge and trap technique. Foods 2017, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- ISO 8589; Sensory Analysis-General Guidance for the Design of Test Rooms. ISO (International Organisation for Standardisation): Geneva, Switzerland, 2007.
- ISO 13299; Sensory Analysis-Methodology-General Guidance for Establishing a Sensory Profile. ISO (International Organisation for Standardisation): Geneva, Switzerland, 2016.
- Straßer, S.; Schieberle, P. Characterization of the key aroma compounds in roasted duck liver by means of aroma extract dilution analysis: Comparison with beef and pork livers. Eur. Food Res. Technol. 2014, 238, 307–313. [Google Scholar] [CrossRef]
- Feng, Y.; Su, G.; Zhao, H.; Cai, Y.; Cui, C.; Sun-Waterhouse, D.; Zhao, M. Characterisation of aroma profiles of commercial soy sauce by odour activity value and omission test. Food Chem. 2015, 167, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s chemical signatures in human olfaction: A foodborne perspective for future biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef]
- Li, H.; Kang, X.; Wang, S.; Mo, H.; Xu, D.; Zhou, W.; Hu, L. Early detection and monitoring for Aspergillus flavus contamination in maize kernels. Food Control 2021, 121, 107636. [Google Scholar] [CrossRef]
- Qian, M.; Zheng, M.; Zhao, W.; Liu, Q.; Zeng, X.; Bai, W. Effect of marinating and frying on the flavor of braised pigeon. J. Food Process. Preserv. 2021, 45, e15219. [Google Scholar] [CrossRef]
- Tuersuntuoheti, T.; Wang, Z.; Zhang, M.; Asimi, S.; Liang, S.; Wang, Z.; Ren, X.; Sohail, A. Changes of microbial diversity and volatile compounds in edible and deteriorated Qingke barley fresh noodles stored at 25 C. Int. J. Food Sci. Technol. 2021, 56, 885–896. [Google Scholar] [CrossRef]
- Yao, W.; Cai, Y.; Liu, D.; Chen, Y.; Li, J.; Zhang, M.; Chen, N.; Zhang, H. Analysis of flavor formation during production of Dezhou braised chicken using headspace-gas chromatography-ion mobility spec-trometry (HS-GC-IMS). Food Chem. 2022, 370, 130989. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xin, G.; Wei, Y.; Xu, H.; Sun, L.; Hou, Z.; Sun, B. Comparison of the umami taste and aroma of dried Suillus granulatus packed using four different packaging methods. Food Chem. 2022, 366, 130570. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Yang, X.; Liu, T.; Yang, Y.; Zhou, X.; Zhao, P.; Guo, Y. Effect of free amino acids and peptide hydrolysates from sunflower seed protein on the formation of pyrazines under different heating conditions. RSC Adv. 2021, 11, 27772–27781. [Google Scholar] [CrossRef]
- Kantono, K.; Hamid, N.; Chadha, D.; Ma, Q.; Oey, I.; Farouk, M.M. Pulsed Electric Field (PEF) Processing of Chilled and Frozen-Thawed Lamb Meat Cuts: Relationships between Sensory Characteristics and Chemical Composition of Meat. Foods 2021, 10, 1148. [Google Scholar] [CrossRef]
- Spychaj, R.; Kucharska, A.Z.; Szumny, A.; Przybylska, D.; Pejcz, E.; Piórecki, N. Potential valorization of Cornelian cherry (Cornus mas L.) stones: Roasting and extraction of bioactive and volatile compounds. Food Chem. 2021, 358, 129802. [Google Scholar] [CrossRef]
- Niu, Y.; Zhu, Q.; Xiao, Z. Characterization of perceptual interactions among ester aroma compounds found in Chinese Moutai Baijiu by gas chromatography-olfactometry, odor Intensity, olfactory threshold and odor activity value. Food Res. Int. 2020, 131, 108986. [Google Scholar] [CrossRef] [PubMed]
- Christlbauer, M.; Schieberle, P. Evaluation of the key aroma compounds in beef and pork vegetable gravies a la chef by stable isotope dilution assays and aroma recombination experiments. J. Agric. Food Chem. 2011, 59, 13122–13130. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Liu, J.; Chen, D.-W. Analysis of aroma-active compounds in bighead carp head soup and their influence on umami of a model soup. Microchem. J. 2021, 168, 106436. [Google Scholar] [CrossRef]
- Janek, K.; Niewienda, A.; Wöstemeyer, J.; Voigt, J. The cleavage specificity of the aspartic protease of cocoa beans involved in the generation of the cocoa-specific aroma precursors. Food Chem. 2016, 211, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wang, P.; Zhan, P.; Tian, H. Characterization of key aroma compounds in stewed mutton (goat meat) added with thyme (Thymus vulgaris L.) based on the combination of instrumental analysis and sensory verification. Food Chem. 2022, 371, 131111. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mei, X.; Chang, J.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Comparative characterization of key odorants of French fries and oils at the break-in, optimum, and degrading frying stages. Food Chem. 2022, 368, 130581. [Google Scholar] [CrossRef]
- Bai, J.; Baker, S.M.; Goodrich-Schneider, R.M.; Montazeri, N.; Sarnoski, P.J. Aroma profile characterization of Mahi-Mahi and Tuna for determining spoilage using purge and trap gas chromatography-mass spectrometry. J. Food Sci. 2019, 84, 481–489. [Google Scholar] [CrossRef]
- Tao, N.-P.; Wu, R.; Zhou, P.-G.; Gu, S.-Q.; Wu, W. Characterization of odor-active compounds in cooked meat of farmed obscure puffer (Takifugu obscurus) using gas chromatography-mass spectrometry-olfactometry. J. Food Drug Anal. 2014, 22, 431–438. [Google Scholar] [CrossRef]
- Wu, N.; Gu, S.; Tao, N.; Wang, X.; Ji, S. Characterization of important odorants in steamed male Chinese mitten crab (Eriocheir sinensis) using gas chromatography-mass spectrometry-olfactometry. J. Food Sci. 2014, 79, C1250–C1259. [Google Scholar] [CrossRef]
- Chang, C.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Deep-fried flavor: Characteristics, formation mechanisms, and influencing factors. Crit. Rev. Food Sci. Nutr. 2020, 60, 1496–1514. [Google Scholar] [CrossRef]
- Ding, A.; Zhu, M.; Qian, X.; Shi, L.; Huang, H.; Xiong, G.; Wang, J.; Wang, L. Effect of fatty acids on the flavor formation of fish sauce. LWT 2020, 134, 110259. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Wang, S.; Chang, Y.; Liu, B.; Chen, H.; Sun, B.; Zhang, N. Characterization of the Key Aroma-Active Compounds in Yongchuan Douchi (Fermented Soybean) by Application of the Sensomics Approach. Molecules 2021, 26, 3048. [Google Scholar] [CrossRef]
- Takakura, Y.; Osanai, H.; Masuzawa, T.; Wakabayashi, H.; Nishimura, T. Characterization of the key aroma compounds in pork soup stock by using an aroma extract dilution analysis. Biosci. Biotechnol. Biochem. 2014, 78, 124–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, A.-N.; Tan, Z.-W.; Wang, F.-S. Mechanistic studies on the formation of pyrazines by Maillard reaction between L-ascorbic acid and L-glutamic acid. LWT-Food Sci. Technol. 2013, 50, 64–71. [Google Scholar] [CrossRef]
- Da, D.; Nian, Y.; Shi, J.; Li, Y.; Zhao, D.; Zhang, G.; Li, C. Characterization of specific volatile components in braised pork with different tastes by SPME-GC/MS and electronic nose. J. Food Process. Preserv. 2021, 45, e15492. [Google Scholar] [CrossRef]
- Liu, J.; Wan, P.; Xie, C.; Chen, D.-W. Key aroma-active compounds in brown sugar and their influence on sweetness. Food Chem. 2021, 345, 128826. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yao, W.; Zhou, W. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.-J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Goris, M.; Puntervoll, P.; Rojo, D.; Claussen, J.; Larsen, Ø.; Garcia-Moyano, A.; Almendral, D.; Barbas, C.; Ferrer, M.; Bjerga, G.E.K. Use of flavin-containing monooxygenases for conversion of trimethylamine in salmon protein hydrolysates. Appl. Environ. Microbiol. 2020, 86, e02105-20. [Google Scholar] [CrossRef]
- Sun, L.L.; Zhang, K.J.; Zhang, T.Q.; Zhu, Z.W. Mechanism of fishy odor compounds metabolized by algae. In Proceedings of the Applied Mechanics and Materials, Wuhan, China, 13–14 September 2014; pp. 355–359. [Google Scholar]
- Jayasena, D.D.; Ahn, D.U.; Nam, K.C.; Jo, C. Flavour chemistry of chicken meat: A review. Asian-Australas. J. Anim. Sci. 2013, 26, 732. [Google Scholar] [CrossRef]
- Dawood, M.A.; Metwally, A.E.-S.; El-Sharawy, M.E.; Ghozlan, A.M.; Abdel-Latif, H.M.; Van Doan, H.; Ali, M.A. The influences of ferulic acid on the growth performance, haemato-immunological responses, and immune-related genes of Nile tilapia (Oreochromis niloticus) exposed to heat stress. Aquaculture 2020, 525, 735320. [Google Scholar] [CrossRef]
- Guan, D.; Sun, H.; Wang, J.; Wang, Z.; Li, Y.; Han, H.; Li, X.; Fang, T. Effects of rosiglitazone on growth and skeletal muscle glucose metabolism of GIFT tilapia based on PI3K/Akt signaling pathway. Aquac. Res. 2021, 52, 3911–3922. [Google Scholar] [CrossRef]
- Cui, H.; Yu, J.; Zhai, Y.; Feng, L.; Chen, P.; Hayat, K.; Xu, Y.; Zhang, X.; Ho, C.-T. Formation and fate of Amadori rearrangement products in Maillard reaction. Trends Food Sci. Technol. 2021, 115, 391–408. [Google Scholar] [CrossRef]
- Try, S.; Voilley, A.; Chunhieng, T.; De-Coninck, J.; Waché, Y. Aroma compounds production by solid state fermentation, importance of in situ gas-phase recovery systems. Appl. Microbiol. Biotechnol. 2018, 102, 7239–7255. [Google Scholar] [CrossRef] [PubMed]
- Varlet, V.; Fernandez, X. Sulfur-containing volatile compounds in seafood: Occurrence, odorant properties and mechanisms of formation. Food Sci. Technol. Int. 2010, 16, 463–503. [Google Scholar] [CrossRef]
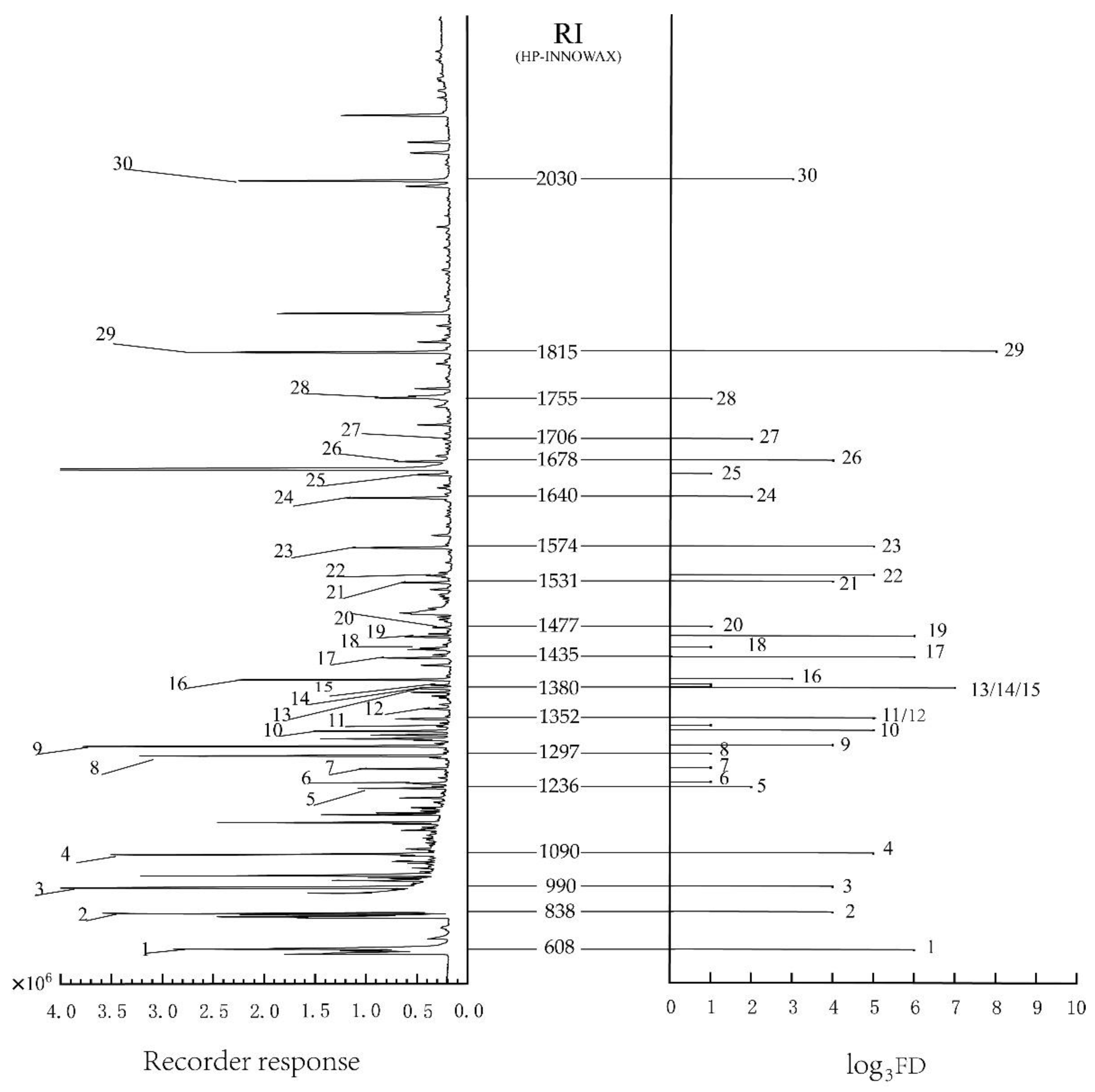
| NO. | a RI | b Compound | c CAS | d | e Description Odors |
|---|---|---|---|---|---|
| 1 | 688 | trimethylamine | 75-50-3 | 6 | fishy |
| 2 | 838 | 2-methyl-1-propanal | 78-84-2 | 4 | malty |
| 3 | 990 | diacetyl | 431-03-8 | 4 | creamy |
| 4 | 1090 | hexanal | 66-25-1 | 5 | vegetative |
| 5 | 1236 | 2-pentyl furan | 3777-69-3 | 2 | fruity |
| 6 | 1246 | amyl alcohol | 71-41-0 | 1 | balsam |
| 7 | 1274 | 2-methyl pyrazine | 109-08-0 | 1 | nutty |
| 8 | 1297 | octanal | 124-13-0 | 1 | orange peel |
| 9 | 1309 | acetone alcohol | 116-09-6 | 4 | burnt |
| 10 | 1334 | 2,6-dimethyl pyrazine | 108-50-9 | 1 | roasted |
| 11 | 1338 | 2-ethyl pyrazine | 13925-00-3 | 1 | roasted |
| 12 | 1352 | 2,3-dimethylpyrazine | 5910-89-4 | 5 | nutty |
| 13 | 1380 | dimethyl trisulfide | 3658-80-8 | 7 | meaty |
| 14 | 1391 | 2-ethyl-6-methyl pyrazine | 13925-03-6 | 1 | roasted potato |
| 15 | 1401 | nonanal | 124-19-6 | 1 | fatty |
| 16 | 1409 | 2,3,5-trimethyl pyrazine | 14667-55-1 | 3 | nutty |
| 17 | 1435 | trans-2-octenal | 2548-87-0 | 6 | cucumber oily |
| 18 | 1450 | 1-octen-3-ol | 3391-86-4 | 1 | mushroom |
| 19 | 1462 | 2,3-Dimethyl-5-ethylpyrazine | 15707-34-3 | 6 | burnt popcorn |
| 20 | 1477 | 2,3,5,6-tetramethyl pyrazine | 1124-11-4 | 1 | nutty |
| 21 | 1550 | benzaldehyde | 100-52-7 | 4 | almond |
| 22 | 1539 | (E)-2-nonenal | 18829-56-6 | 5 | cucumber |
| 23 | 1574 | 2-propyl-Pyridine | 622-39-9 | 5 | roasted |
| 24 | 1640 | gamma-butyrolactone | 96-48-0 | 2 | fatty |
| 25 | 1662 | furan-2-ylmethanol | 98-00-0 | 1 | bready |
| 26 | 1678 | 2-hexylthiophene | 18794-77-9 | 4 | floral |
| 27 | 1706 | (E,E)-2,4-nonadienal | 5910-87-2 | 2 | fatty |
| 28 | 1755 | 2-undecenal | 2463-77-6 | 1 | fruity |
| 29 | 1815 | (E,E)-2,4-decadienal | 25152-84-5 | 8 | fatty |
| 30 | 2030 | (R)-(-)-pantolactone | 599-04-2 | 3 | cotton candy |
| a RI | b Name | c Ionsd (m/z) | d Calibration Eqs | e R2 |
|---|---|---|---|---|
| 866 | trimethylamine | 58, 59, 42 | y = (x − 130,147.5252) ÷ 34,398.9676x | 0.9925 |
| 955 | 2-methyl-1-propanal | 43, 41, 72 | y = (x + 19,055.1351) ÷ 81,569.0453x | 0.9958 |
| 1014 | diacetyl | 43, 86, 42 | y = (x + 25,952.8011) ÷ 78,372.0369x | 0.9985 |
| 1091 | hexanal | 44, 56, 41 | y = (x + 64,514.4616) ÷ 16,148.8895x | 0.9931 |
| 1309 | acetone alcohol | 43, 31, 74 | y = (x + 117,988.5619) ÷ 89,266.6282x | 0.9977 |
| 1349 | 2,3-dimethylpyrazine | 67, 108, 40 | y = (x + 19,338.4727) ÷ 79,528.563618x | 0.9975 |
| 1394 | dimethyl trisulfide | 126, 45, 79 | y = (x + 91,851.5539) ÷ 90,629.4553x | 0.9983 |
| 1406 | 2,3,5-trimethyl pyrazine | 42, 122, 39 | y = (x + 25,713.2766) ÷ 82,365.0994x | 0.9968 |
| 1435 | trans-2-octenal | 41, 55, 29 | y = (x + 15,351.2721) ÷ 14,161.1804x | 0.9963 |
| 1462 | 2,3-Dimethyl-5-ethylpyrazine | 135, 136, 108 | y = (x + 2351.1146) ÷ 9868.1848x | 0.9962 |
| 1531 | benzaldehyde | 77, 106, 105 | y = (x + 49,893.6100) ÷ 57,871.0904x | 0.9971 |
| 1539 | (E)-2-nonenal | 43, 55, 70 | y = (x + 26,133.6678) ÷ 23,108.7456x | 0.9969 |
| 1574 | Pyridine, 2-propyl- | 93, 106, 120 | y = (x + 25,621.8714) ÷ 20,115.1258x | 0.9961 |
| 1678 | 2-hexylthiophene | 97, 98, 168 | y = (x + 15,988.1955) ÷ 13,139.4778x | 0.9959 |
| 1811 | (E,E)-2,4-decadienal | 81, 41, 39 | y = (x − 607,760.6942) ÷ 82,363.2326x | 0.9914 |
| 2030 | (R)-(-)-pantolactone | 71, 43, 41 | y = (x + 233,527.6726) ÷ 48,525.7271x | 0.9950 |
| RI | Name | a FD | CAS | b Concentration (mg/kg) | c Olfactory Threshold (mg/kg) |
|---|---|---|---|---|---|
| 866 | trimethylamine | 729 | 75-50-3 | 78.6441 ± 9.4903 | 0.1079 ± 0.0228 |
| 955 | 2-methyl-1-propanal | 81 | 78-84-2 | 10.9421 ± 1.8490 | 0.1351 ± 0.0228 |
| 1014 | diacetyl | 81 | 431-03-8 | 16.6671 ± 2.7772 | 0.2058 ± 0.0343 |
| 1091 | hexanal | 243 | 66-25-1 | 78.6330 ± 3.1470 | 0.3236 ± 0.0130 |
| 1309 | acetone alcohol | 81 | 116-09-6 | 95.4288 ± 5.6692 | 1.1781 ± 0.0278 |
| 1349 | 2,3-dimethylpyrazine | 243 | 5910-89-4 | 3.9575 ± 0.7406 | 0.0163 ± 0.0030 |
| 1394 | dimethyl trisulfide | 2187 | 3658-80-8 | 5.3529 ± 0.5088 | 0.0024 ± 0.0002 |
| 1406 | 2,3,5-trimethyl pyrazine | 27 | 14667-55-1 | 41.2071 ± 3.3091 | 1.5262 ± 0.1226 |
| 1435 | trans-2-octenal | 729 | 2548-87-0 | 42.1350 ± 4.0492 | 0.0578 ± 0.0056 |
| 1462 | 2,3-Dimethyl-5-ethylpyrazine | 729 | 15707-34-3 | 89.9714 ± 3.8457 | 0.1234 ± 0.0053 |
| 1531 | benzaldehyde | 81 | 100-52-7 | 9.5011 ± 0.6848 | 0.1173 ± 0.0085 |
| 1539 | (E)-2-nonenal | 243 | 18829-56-6 | 4.5697 ± 0.2256 | 0.0188 ± 0.0009 |
| 1574 | Pyridine, 2-propyl- | 243 | 2294-76-0 | 93.4565 ± 9.3617 | 0.3846 ± 0.0263 |
| 1678 | 2-hexylthiophene | 81 | 18794-77-9 | 99.9169 ± 7.9401 | 1.2335 ± 0.1645 |
| 1811 | (E,E)-2,4-decadienal | 6561 | 25152-84-5 | 120.3953 ± 1.5619 | 0.0184 ± 0.0002 |
| 2030 | (R)-(-)-pantolactone | 27 | 599-04-2 | 94.1932 ± 8.8211 | 3.4886 ± 0.3267 |
| No. | Compound(s) Omitted | Significance |
|---|---|---|
| 1 | trimethylamine | *** |
| 2 | 2-methyl-1-propanal | NS |
| 3 | diacetyl | NS |
| 4 | hexanal | * |
| 5 | acetone alcohol | NS |
| 6 | 2,3-dimethylpyrazine | ** |
| 7 | dimethyl trisulfide | *** |
| 8 | 2,3,5-trimethyl pyrazine | NS |
| 9 | trans-2-octenal | * |
| 10 | 2,3-Dimethyl-5-ethylpyrazine | ** |
| 11 | benzaldehyde | NS |
| 12 | (E)-2-nonenal | * |
| 13 | 2-propyl-pyridine | ** |
| 14 | 2-hexylthiophene | * |
| 15 | (E,E)-2,4-decadienal | *** |
| 16 | (R)-(-)-pantolactone | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhao, X.; Zhao, M.; Liu, X.; Pang, Y.; Zhang, M. Characterization of the Key Aroma Constituents in Fried Tilapia through the Sensorics Concept. Foods 2022, 11, 494. https://doi.org/10.3390/foods11040494
Liu M, Zhao X, Zhao M, Liu X, Pang Y, Zhang M. Characterization of the Key Aroma Constituents in Fried Tilapia through the Sensorics Concept. Foods. 2022; 11(4):494. https://doi.org/10.3390/foods11040494
Chicago/Turabian StyleLiu, Mingyuan, Xiaoying Zhao, Mouming Zhao, Xiaoling Liu, Yiyang Pang, and Meishuo Zhang. 2022. "Characterization of the Key Aroma Constituents in Fried Tilapia through the Sensorics Concept" Foods 11, no. 4: 494. https://doi.org/10.3390/foods11040494
APA StyleLiu, M., Zhao, X., Zhao, M., Liu, X., Pang, Y., & Zhang, M. (2022). Characterization of the Key Aroma Constituents in Fried Tilapia through the Sensorics Concept. Foods, 11(4), 494. https://doi.org/10.3390/foods11040494






