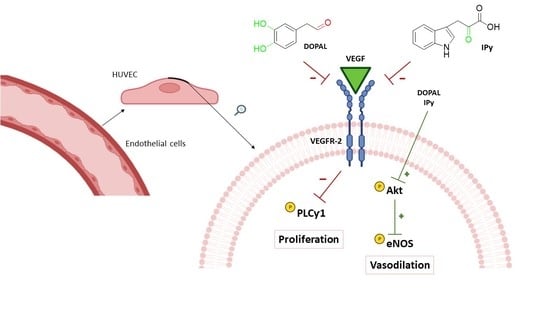
Anti-VEGF Effect of Bioactive Indolic Compounds and Hydroxytyrosol Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Treatment of HUVECs
2.4. Phosphorilated VEGFR-2 (ELISA Assay)
2.5. Western Blot Analysis for PLCγ1, Akt and eNOS
2.6. Statistical Analysis
3. Results
3.1. Anti-Angiogenic Effect by Inhibition of VEGFR-2 Activation in the Presence of Hydroxytyrosol Metabolites and Indolic Compounds
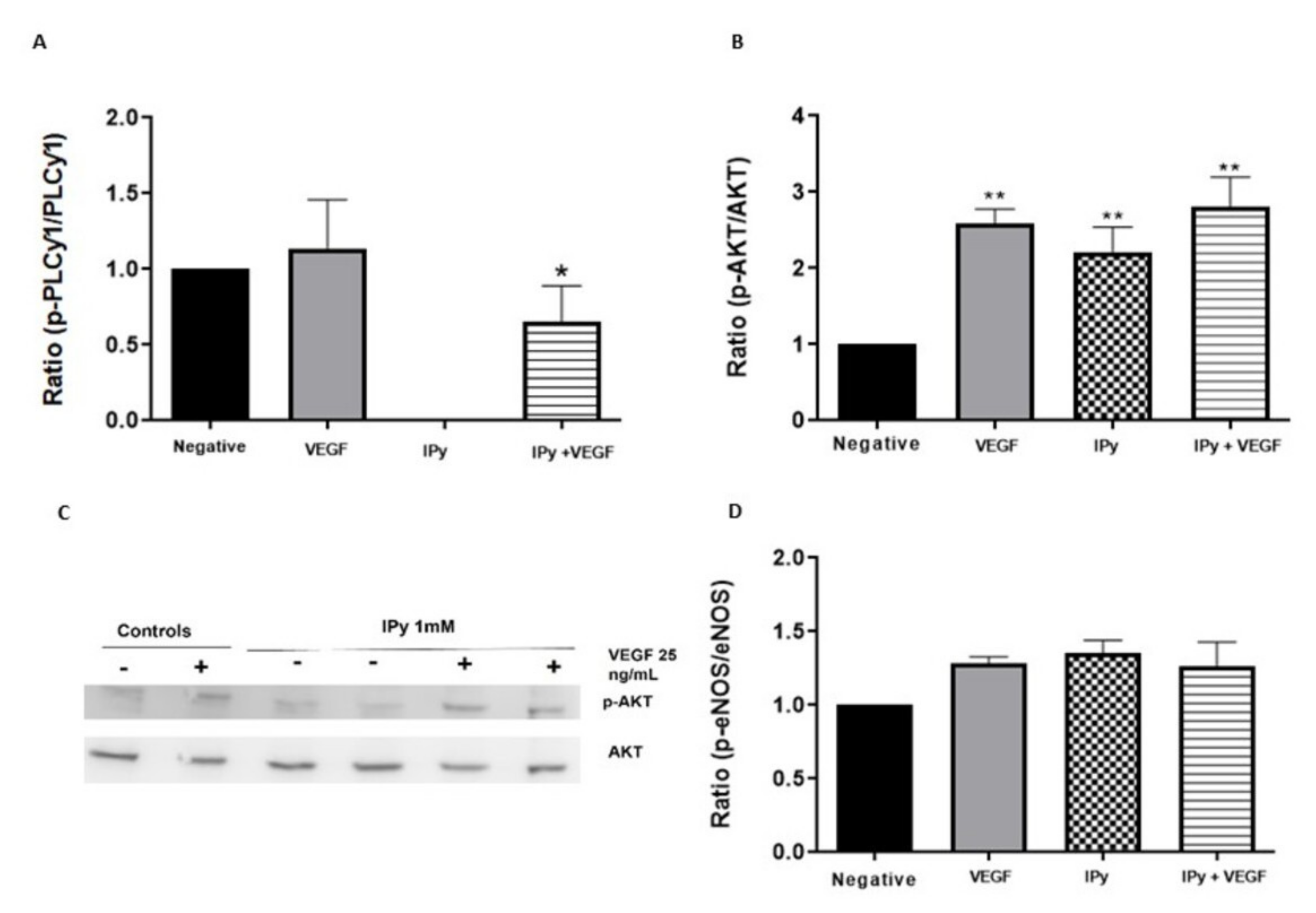
3.2. Effects of DOPAL and 3-Indolpiruvic Acid on PLCγ1, Akt and eNOS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baluk, P.; Hashizume, H.; McDonald, D.M. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005, 15, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Yehya, A.H.S.; Asif, M.; Petersen, S.H.; Subramaniam, A.V.; Kono, K.; Majid, A.M.S.A.; Oon, C.E. Angiogenesis: Managing the culprits behind tumorigenesis and metastasis. Medicina 2018, 54, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, E.S.; Khankin, E.V.; Karumanchi, S.A.; Humphreys, B.D. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: Mechanisms and potential use as a biomarker. Semin. Nephrol. 2010, 30, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N. VEGF and the Quest for Tumor Angiogenesis Factors. Nat. Rev. Cancer 2002, 2, 795–803. [Google Scholar] [CrossRef]
- Cébe-Suarez, S.; Zehnder-Fjällman, A.; Ballmer-Hofer, K. The Role of VEGF Receptors in Angiogenesis; Complex Partnerships. Cell. Mol. Life Sci. 2006, 63, 601–615. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Kroetz, D.L. Bevacizumab-induced hypertension: Clinical presentation and molecular understanding. Pharmacol. Ther. 2018, 182, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Touyz, R.M.; Herrmann, S.; Herrmann, J. Vascular toxicities with VEGF inhibitor therapies focus on hypertension and arterial thrombotic events. J. Am. Soc. Hypertens. 2018, 12, 409–425. [Google Scholar] [CrossRef]
- Wu, S.; Chen, J.J.; Kudelka, A.; Lu, J.; Zhu, X. Incidence and risk of hypertension with sorafenib in patients with cancer: A systematic review and meta-analysis. Lancet Oncol. 2008, 9, 117–123. [Google Scholar] [CrossRef]
- Moyle, C.W.A.; Cerezo, A.B.; Winterbone, M.S.; Hollands, W.J.; Alexeev, Y.; Needs, P.W.; Kroon, P.A. Potent Inhibition of VEGFR-2 Activation by Tight Binding of Green Tea Epigallocatechin Gallate and Apple Procyanidins to VEGF: Relevance to Angiogenesis. Mol. Nutr. Food Res. 2015, 59, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Cerezo, A.B.; Winterbone, M.S.; Moyle, C.W.A.; Needs, P.W.; Kroon, P.A. Molecular Structure-Function Relationship of Dietary Polyphenols for Inhibiting VEGF-Induced VEGFR-2 Activity. Mol. Nutr. Food Res. 2015, 59, 2119–2131. [Google Scholar] [CrossRef] [Green Version]
- Igura, K.; Ohta, T.; Kuroda, Y.; Kaji, K. Resveratrol and Quercetin Inhibit Angiogenesis in Vitro. Cancer Lett. 2001, 171, 11–16. [Google Scholar] [CrossRef]
- Liao, H.F.; Chen, Y.Y.; Liu, J.J.; Hsu, M.L.; Shieh, H.J.; Liao, H.J.; Shieh, C.J.; Shiao, M.S.; Chen, Y.J. Inhibitory Effect of Caffeic Acid Phenethyl Ester on Angiogenesis, Tumor Invasion, and Metastasis. J. Agric. Food Chem. 2003, 51, 7907–7912. [Google Scholar] [CrossRef]
- Sala, R.; Mena, P.; Savi, M.; Brighenti, F.; Crozier, A.; Miragoli, M.; Stilli, D.; Del Rio, D. Urolithins at Physiological Concentrations Affect the Levels of Pro-Inflammatory Cytokines and Growth Factor in Cultured Cardiac Cells in Hyperglucidic Conditions. J. Funct. Foods 2015, 15, 97–105. [Google Scholar] [CrossRef]
- Sartippour, M.R.; Seeram, N.P.; Rao, J.Y.; Moro, A.; Harris, D.M.; Henning, S.M.; Firouzi, A.; Rettig, M.B.; Aronson, W.J.; Pantuck, A.J.; et al. Ellagitannin-Rich Pomegranate Extract Inhibits Angiogenesis in Prostate Cancer in Vitro and in Vivo. Int. J. Oncol. 2008, 32, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin Inhibits Proliferation, Invasion, Angiogenesis and Metastasis of Different Cancers through Interaction with Multiple Cell Signaling Proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Labrador, M.; Gutiérrez, A.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcia-Parrilla, M.C. Anti-VEGF Signalling Mechanism in HUVECs by Melatonin, Serotonin, Hydroxytyrosol and Other Bioactive Compounds. Nutrients 2019, 11, 2421. [Google Scholar] [CrossRef] [Green Version]
- Cerezo, A.B.; Hornedo-Ortega, R.; Álvarez-Fernández, M.A.; Troncoso, A.M.; Garcia-Parrilla, M.C. Inhibition of VEGF-Induced VEGFR-2 Activation and HUVEC Migration by Melatonin and Other Bioactive Indolic Compounds. Nutrients 2017, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Xu, Z.; Xiang, C.; Liu, J.; Zhou, L.; Li, T.; Yang, Z.; Ding, C. Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason. Sonochem. 2017, 37, 328–334. [Google Scholar] [CrossRef]
- Sánchez, C.S.; González, A.T.; García-Parrilla, M.C.; Granados, J.Q.; De La Serrana, H.L.G.; Martínez, M.L. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- Plastina, P.; Benincasa, C.; Perri, E.; Fazio, A.; Augimeri, G.; Poland, M.; Witkamp, R.; Meijerink, J. Identification of hydroxytyrosyl oleate, a derivative of hydroxytyrosol with anti-inflammatory properties, in olive oil by-products. Food Chem. 2019, 279, 105–113. [Google Scholar] [CrossRef]
- Wang, S.T.; Le, J.; Peng, R.; Li, Y. Efficient extraction and sensitive LC-MS quantification of hydroxytyrosol in wine, oil and plasma. Food Chem. 2020, 323, 126803. [Google Scholar] [CrossRef]
- Proestos, C.; Bakogiannis, A.; Psarianos, C.; Koutinas, A.A.; Kanellaki, M.; Komaitis, M. High Performance Liquid Chromatography Analysis of Phenolic Substances in Greek Wines. Food Control 2005, 16, 319–323. [Google Scholar] [CrossRef]
- Boselli, E.; Minardi, M.; Giomo, A.; Frega, N.G. Phenolic Composition and Quality of White d.o.c. Wines from Marche (Italy). Anal. Chim. Acta 2006, 563, 93–100. [Google Scholar] [CrossRef]
- Minuti, L.; Pellegrino, R.M.; Tesei, I. Simple Extraction Method and Gas Chromatography-Mass Spectrometry in the Selective Ion Monitoring Mode for the Determination of Phenols in Wine. J. Chromatogr. A 2006, 1114, 263–268. [Google Scholar] [CrossRef]
- Dudley, J.I.; Lekli, I.; Mukherjee, S.; Das, M.; Bertelli, A.A.A.; Das, D.K. Does White Wine Qualify for French Paradox? Comparison of the Cardioprotective Effects of Red and White Wines and Their Constituents: Resveratrol, Tyrosol, and Hydroxytyrosol. J. Agric. Food Chem. 2012, 60, 2767. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Cantos-Villar, E.; Palma, M.; Puertas, B. Direct Liquid Chromatography Method for the Simultaneous Quantification of Hydroxytyrosol and Tyrosol in Red Wines. J. Agric. Food Chem. 2011, 59, 11683–11689. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive Oil Phenols Are Absorbed in Humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Miro-Casas, E.; Covas, M.I.; Farre, M.; Fito, M.; Ortuno, J.; Weinbrenner, T.; Roset, P.; De La Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [Green Version]
- De la Torre, R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 2008, 16, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Protective Effects of Hydroxytyrosol against α-Synuclein Toxicity on PC12 cells and Fibril Formation. Food Chem. Toxicol. 2018, 120, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Boronat, A.; Mateus, J.; Soldevila-Domenech, N.; Guerra, M.; Rodríguez-Morató, J.; Varon, C.; Muñoz, D.; Barbosa, F.; Morales, J.C.; Gaedigk, A.; et al. Data on the Endogenous Conversion of Tyrosol into Hydroxytyrosol in Humans. Data Br. 2019, 27, 104787. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic Disposition and Biological Significance of Simple Phenols of Dietary Origin: Hydroxytyrosol and Tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [Green Version]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, Oxidative Stress and Protein–Quinone Modifications in Parkinson’s and Other Neurodegenerative Diseases. Angew. Chem. Int. Ed. 2019, 58, 6512–6527. [Google Scholar] [CrossRef]
- Muñoz, P.; Huenchuguala, S.; Paris, I.; Segura-Aguilar, J. Dopamine Oxidation and Autophagy. Parkinson’s Dis. 2012, 2012, 920953. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; Garcia-Parrilla, M.C. Melatonin and Derived L-Tryptophan Metabolites Produced during Alcoholic Fermentation by Different Wine Yeast Strains. Food Chem. 2017, 217, 431–437. [Google Scholar] [CrossRef]
- Liang, H.; Dai, Z.; Liu, N.; Ji, Y.; Chen, J.; Zhang, Y.; Yang, Y.; Li, J.; Wu, Z.; Wu, G. Dietary L-tryptophan modulates the structural and functional composition of the intestinal microbiome in weaned piglets. Front. Microbiol. 2018, 9, 1736. [Google Scholar] [CrossRef]
- Chung, K.T.; Anderson, G.M.; Fulk, G.E. Formation of Indoleacetic Acid by Intestinal Anaerobes. J. Bacteriol. Res. 1975, 124, 573–575. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Fernández, M.A.; Carafa, I.; Vrhovsek, U.; Arapitsas, P. Modulating Wine Aromatic Amino Acid Catabolites by Using Torulaspora Delbrueckii in Sequentially Inoculated Fermentations or Saccharomyces Cerevisiae Alone. Microorganisms 2020, 8, 1349. [Google Scholar] [CrossRef]
- Álvarez-Fernández, M.A.; Fernández-Cruz, E.; Garcia-Parrilla, M.C.; Troncoso, A.M.; Mattivi, F.; Vrhovsek, U.; Arapitsas, P. Saccharomyces Cerevisiae and Torulaspora Delbrueckii Intra- and Extra-Cellular Aromatic Amino Acids Metabolism. J. Agric. Food Chem. 2019, 67, 7942–7953. [Google Scholar] [CrossRef]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Special Section on Drug Metabolism and the Microbiome—Minireview Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef] [Green Version]
- Morita, I.; Kawamoto, M.; Yoshida, H. Difference in the Concentration of Tryptophan Metabolites between Maternal and Umbilical Foetal Blood. J. Chromatogr. B Biomed. Appl. 1992, 576, 334–339. [Google Scholar] [CrossRef]
- Burke, W.J.; Chung, H.D.; Li, S.W. Quantitation of 3,4-Dihydroxyphenylacetaldehyde and 3,4-Dihydroxyphenylglycolaldehyde, the Monoamine Oxidase Metabolites of Dopamine and Noradrenaline, in Human Tissues by Microcolumn High-Performance Liquid Chromatography. Anal. Biochem. 1999, 273, 111–116. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Learnings from quantitative structure–activity relationship (QSAR) studies with respect to food protein-derived bioactive peptides: A review. RSC Adv. 2016, 6, 75400–75413. [Google Scholar] [CrossRef]
- She, X.; Song, X. Cytokinin- and Auxin-Induced Stomatal Opening Is Related to the Change of Nitric Oxide Levels in Guard Cells in Broad Bean. Physiol. Plant. 2006, 128, 569–579. [Google Scholar] [CrossRef]
- Zhu, X.; Stergiopoulos, K.; Wu, S. Risk of Hypertension and Renal Dysfunction with an Angiogenesis Inhibitor Sunitinib: Systematic Review and Meta-Analysis. Acta Oncol. 2009, 48, 9–17. [Google Scholar] [CrossRef]
- Qi, W.X.; Lin, F.; Sun, Y.J.; Tang, L.N.; He, A.N.; Yao, Y.; Shen, Z. Incidence and Risk of Hypertension with Pazopanib in Patients with Cancer: A Meta-Analysis. Cancer Chemother. Pharmacol. 2013, 71, 431–439. [Google Scholar] [CrossRef]
- Qi, W.X.; He, A.N.; Shen, Z.; Yao, Y. Incidence and Risk of Hypertension with a Novel Multi-Targeted Kinase Inhibitor Axitinib in Cancer Patients: A Systematic Review and Meta-Analysis. Br. J. Clin. Pharmacol. 2013, 76, 348–357. [Google Scholar] [CrossRef] [Green Version]
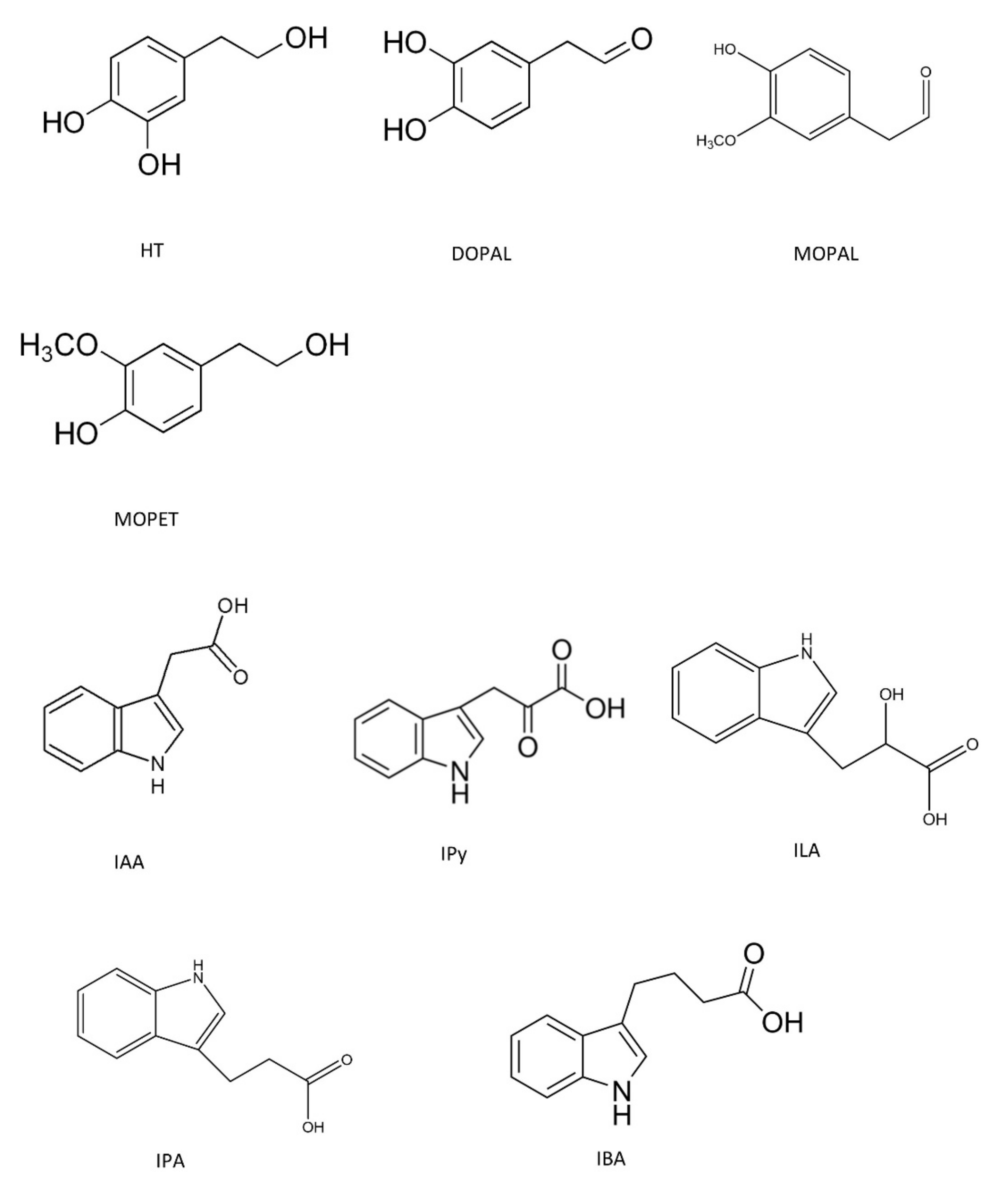


| Compounds | Concentrations | Inhibition (%) | IC50 |
|---|---|---|---|
| DOPAL | 50 µM | 5 ± 7 | 119 µM |
| 100 µM | 46 ± 8 | (95.49–148.1) | |
| MOPAL | 100 µM | NI | ND |
| MOPET | 100 µM | NI | ND |
| IPy | 0.1 mM | 4 ± 10 | 1.037 mM |
| 1 mM | 56 ± 0.7 | (0.9061–1.171) | |
| IPA | 0.1 mM | NI | ND |
| 1 mM | NI | ||
| ILA | 0.1 mM | NI | ND |
| 1 mM | NI | ||
| IBA | 0.1 mM | NI | ND |
| 1 mM | NI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo-Fernández, M.; Cerezo, A.B.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcia-Parrilla, M.C. Anti-VEGF Effect of Bioactive Indolic Compounds and Hydroxytyrosol Metabolites. Foods 2022, 11, 526. https://doi.org/10.3390/foods11040526
Gallardo-Fernández M, Cerezo AB, Hornedo-Ortega R, Troncoso AM, Garcia-Parrilla MC. Anti-VEGF Effect of Bioactive Indolic Compounds and Hydroxytyrosol Metabolites. Foods. 2022; 11(4):526. https://doi.org/10.3390/foods11040526
Chicago/Turabian StyleGallardo-Fernández, Marta, Ana B. Cerezo, Ruth Hornedo-Ortega, Ana M. Troncoso, and M. Carmen Garcia-Parrilla. 2022. "Anti-VEGF Effect of Bioactive Indolic Compounds and Hydroxytyrosol Metabolites" Foods 11, no. 4: 526. https://doi.org/10.3390/foods11040526