How Healthy Are Non-Traditional Dietary Proteins? The Effect of Diverse Protein Foods on Biomarkers of Human Health
Abstract
1. Introduction
Scope of Review
2. Materials and Methods
2.1. Search Strategy
2.2. Screening and Study Selection
- publication date 2000 or later;
- study focus specifically on human health;
- studies examining alternative processing, or ‘raising’, protocols were included only where the outcome was directly focussed on the human health properties of the protein food.
- relating only to animal health;
- reporting only compositional analysis;
- examining consumer acceptance of non-traditional proteins/foods;
- examining specific non-protein food components (e.g., oils, fibre);
- reporting food frequency data, whole dietary patterns or retrospective dietary analysis;
- reporting the same (or overlapping) data from the same research group;
- conducted in silico;
- review papers, book chapters, or editorials.
3. Results
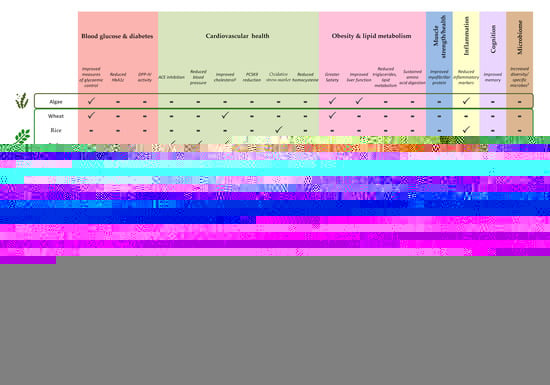
3.1. Algal Proteins
3.2. Cereal Proteins
3.2.1. Wheat
3.2.2. Rice
3.2.3. Barley, Rye and Buckwheat
3.3. Fresh Fruit and Vegetable Proteins
3.4. Insect and Snail Proteins
3.4.1. Cricket
3.4.2. Mealworm
3.4.3. Silkworm
3.4.4. Termites
3.4.5. Snails
3.5. Mycoprotein
3.6. Nut and Oil Seed Protein
3.6.1. Nuts
3.6.2. Oil Seeds
3.7. Non-Soy Legume Proteins
3.7.1. Peas
3.7.2. Lentil
3.7.3. Lupin
3.7.4. Mixed Legumes
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strings
References
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Zaharudin, N.; Tullin, M.; Pekmez, C.T.; Sloth, J.J.; Rasmussen, R.R.; Dragsted, L.O. Effects of brown seaweeds on postprandial glucose, insulin and appetite in humans—A randomized, 3-way, blinded, cross-over meal study. Clin. Nutr. 2021, 40, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Abbasalizad Farhangi, M.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Beneficial effects of supplementation with microalgae Chlorella vulgaris: A double-blind placebo-controlled randomized clinical trial. Clin. Nutr. 2017, 36, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef]
- Allsopp, P.; Crowe, W.; Bahar, B.; Harnedy, P.A.; Brown, E.S.; Taylor, S.S.; Smyth, T.J.; Soler-Vila, A.; Magee, P.J.; Gill, C.I.; et al. The effect of consuming Palmaria palmata-enriched bread on inflammatory markers, antioxidant status, lipid profile and thyroid function in a randomised placebo-controlled intervention trial in healthy adults. Eur. J. Nutr. 2016, 55, 1951–1962. [Google Scholar] [CrossRef]
- Teas, J.; Braverman, L.E.; Kurzer, M.S.; Pino, S.; Hurley, T.G.; Hebert, J.R. Seaweed and soy: Companion foods in Asian cuisine and their effects on thyroid function in American women. J. Med. Food 2007, 10, 90–100. [Google Scholar] [CrossRef]
- Tanna, B.; Yadav, S.; Mishra, A. Anti-proliferative and ROS-inhibitory activities reveal the anticancer potential of Caulerpa species. Mol. Biol. Rep. 2020, 47, 7403–7411. [Google Scholar] [CrossRef]
- Abou El Azm, N.; Fleita, D.; Rifaat, D.; Mpingirika, E.Z.; Amleh, A.; El-Sayed, M.M.H. Production of Bioactive Compounds from the Sulfated Polysaccharides Extracts of Ulva lactuca: Post-Extraction Enzymatic Hydrolysis Followed by Ion-Exchange Chromatographic Fractionation. Molecules 2019, 24, 2132. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Nutritional and Functional Bioactivity Value of Selected Azorean Macroalgae: Ulva compressa, Ulva rigida, Gelidium microdon, and Pterocladiella capillacea. J. Food Sci. 2017, 82, 1757–1764. [Google Scholar] [CrossRef]
- Szabo, N.J.; Matulka, R.A.; Chan, T. Safety evaluation of Whole Algalin Protein (WAP) from Chlorella protothecoides. Food Chem. Toxicol. 2013, 59, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Aluko, R.E.; Hossain, M.; Rai, D.K.; Hayes, M. Potential of a renin inhibitory peptide from the red seaweed Palmaria palmata as a functional food ingredient following confirmation and characterization of a hypotensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2014, 62, 8352–8356. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Lorini, C.; Garamella, G.; Bonaccorsi, G. Seaweeds as a “Palatable” Challenge between Innovation and Sustainability: A Systematic Review of Food Safety. Sustainability 2021, 13, 7652. [Google Scholar] [CrossRef]
- Rzymski, P.; Budzulak, J.; Niedzielski, P.; Klimaszyk, P.; Proch, J.; Kozak, L.; Poniedziałek, B. Essential and toxic elements in commercial microalgal food supplements. J. Appl. Phycol. 2019, 31, 3567–3579. [Google Scholar] [CrossRef]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.-H.; Show, P.L. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Saris, W.H.; Kruijshoop, M.; Wagenmakers, A.J. Maximizing postexercise muscle glycogen synthesis: Carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am. J. Clin. Nutr. 2000, 72, 106–111. [Google Scholar] [CrossRef]
- Van Loon, L.J.; Kruijshoop, M.; Verhagen, H.; Saris, W.H.; Wagenmakers, A.J. Ingestion of protein hydrolysate and amino acid-carbohydrate mixtures increases postexercise plasma insulin responses in men. J. Nutr. 2000, 130, 2508–2513. [Google Scholar] [CrossRef]
- Gorissen, S.H.; Horstman, A.M.; Franssen, R.; Crombag, J.J.; Langer, H.; Bierau, J.; Respondek, F.; van Loon, L.J. Ingestion of Wheat Protein Increases In Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomized Trial. J. Nutr. 2016, 146, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Vidgen, E.; Augustin, L.S.; Parker, T.; Faulkner, D.; Vieth, R.; Vandenbroucke, A.C.; Josse, R.G. Effect of high vegetable protein diets on urinary calcium loss in middle-aged men and women. Eur J. Clin. Nutr. 2003, 57, 376–382. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Vidgen, E.; Augustin, L.S.; van Erk, M.; Geelen, A.; Parker, T.; Faulkner, D.; Vuksan, V.; Josse, R.G.; et al. High-protein diets in hyperlipidemia: Effect of wheat gluten on serum lipids, uric acid, and renal function. Am. J. Clin. Nutr. 2001, 74, 57–63. [Google Scholar] [CrossRef]
- Stoeger, V.; Lieder, B.; Riedel, J.; Schweiger, K.; Hoi, J.; Ruzsanyi, V.; Klieber, M.; Rust, P.; Hans, J.; Ley, J.P.; et al. Wheat Protein Hydrolysate Fortified With l-Arginine Enhances Satiation Induced by the Capsaicinoid Nonivamide in Moderately Overweight Male Subjects. Mol. Nutr. Food Res. 2019, 63, e1900133. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Shi, L.; Webb, D.L.; Hellström, P.M.; Risérus, U.; Landberg, R. Effects of whole-grain rye porridge with added inulin and wheat gluten on appetite, gut fermentation and postprandial glucose metabolism: A randomised, cross-over, breakfast study. Br. J. Nutr. 2016, 116, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Calame, W.; Siemensma, A.D.; van Baak, M.A.; Saris, W.H. The effect of different protein hydrolysate/carbohydrate mixtures on postprandial glucagon and insulin responses in healthy subjects. Eur J. Clin. Nutr. 2009, 63, 48–56. [Google Scholar] [CrossRef]
- Rein, D.; Ternes, P.; Demin, R.; Gierke, J.; Helgason, T.; Schön, C. Artificial intelligence identified peptides modulate inflammation in healthy adults. Food Funct. 2019, 10, 6030–6041. [Google Scholar] [CrossRef]
- Joy, J.M.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; De Souza, E.O.; Wilson, S.M.; Kalman, D.S.; Dudeck, J.E.; Jäger, R. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr. J. 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.M.; Ratliff, K.M.; Blumkaitis, J.C.; Harty, P.S.; Zabriskie, H.A.; Stecker, R.A.; Currier, B.S.; Jagim, A.R.; Jäger, R.; Purpura, M.; et al. Effects of daily 24-gram doses of rice or whey protein on resistance training adaptations in trained males. J. Int. Soc. Sports Nutr. 2020, 17, 60. [Google Scholar] [CrossRef]
- Saracino, P.G.; Saylor, H.E.; Hanna, B.R.; Hickner, R.C.; Kim, J.S.; Ormsbee, M.J. Effects of Pre-Sleep Whey vs. Plant-Based Protein Consumption on Muscle Recovery Following Damaging Morning Exercise. Nutrients 2020, 12, 2049. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Srichaikul, K.; Wong, J.M.; Kendall, C.W.; Bashyam, B.; Vidgen, E.; Lamarche, B.; Rao, A.V.; Jones, P.J.; Josse, R.G.; et al. Supplemental barley protein and casein similarly affect serum lipids in hypercholesterolemic women and men. J. Nutr. 2010, 140, 1633–1637. [Google Scholar] [CrossRef]
- Misan, A.; Petelin, A.; Stubelj, M.; Mandic, A.; Simurina, O.; Pojic, M.; Milovanovic, I.; Jakus, T.; Filipcev, B.; Praznikar, Z.J. Buckwheat—Enriched instant porridge improves lipid profile and reduces inflammation in participants with mild to moderate hypercholesterolemia. J. Funct. Foods 2017, 36, 186–194. [Google Scholar] [CrossRef]
- Valdez-Meza, E.E.; Raymundo, A.; Figueroa-Salcido, O.G.; Ramírez-Torres, G.I.; Fradinho, P.; Oliveira, S.; de Sousa, I.; Suárez-Jiménez, M.; Cárdenas-Torres, F.I.; Islas-Rubio, A.R.; et al. Pasta Enrichment with an Amaranth Hydrolysate Affects the Overall Acceptability while Maintaining Antihypertensive Properties. Foods 2019, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Siow, P.C.; Peh, E.; Henry, C.J. Influence of rice, pea and oat proteins in attenuating glycemic response of sugar-sweetened beverages. Eur J. Nutr. 2018, 57, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, R.; Li, Y.M.; Liang, N.; Zhao, Y.; Zhu, H.; He, Z.; Liu, J.; Hao, W.; Jiao, R.; et al. Cholesterol-Lowering Activity of Tartary Buckwheat Protein. J. Agric. Food Chem. 2017, 65, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Ahn, J.; Jung, C.H.; Ha, T.Y. Cholesterol-lowering Effect of Rice Protein by Enhancing Fecal Excretion of Lipids in Rats. Prev. Nutr. Food Sci. 2013, 18, 210–213. [Google Scholar] [CrossRef][Green Version]
- Escudero, N.L.; Zirulnik, F.; Gomez, N.N.; Mucciarelli, S.I.; Giménez, M.S. Influence of a protein concentrate from Amaranthus cruentus seeds on lipid metabolism. Exp. Biol. Med. 2006, 231, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. Utilization of high-pressure homogenization of potato protein isolate for the production of dairy-free yogurt-like fermented product. Food Hydrocoll. 2021, 113, 106442. [Google Scholar] [CrossRef]
- Acheson, K.J.; Blondel-Lubrano, A.; Oguey-Araymon, S.; Beaumont, M.; Emady-Azar, S.; Ammon-Zufferey, C.; Monnard, I.; Pinaud, S.; Nielsen-Moennoz, C.; Bovetto, L. Protein choices targeting thermogenesis and metabolism1–3. Am. J. Clin. Nutr. 2011, 93, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Dougkas, A.; Östman, E. Comparable effects of breakfast meals varying in protein source on appetite and subsequent energy intake in healthy males. Eur. J. Nutr. 2018, 57, 1097–1108. [Google Scholar] [CrossRef]
- He, T.; Spelbrink, R.E.; Witteman, B.J.; Giuseppin, M.L. Digestion kinetics of potato protein isolates in vitro and in vivo. Int. J. Food Sci. Nutr. 2013, 64, 787–793. [Google Scholar] [CrossRef]
- Lorinczova, H.T.; Deb, S.; Begum, G.; Renshaw, D.; Zariwala, M.G. Comparative Assessment of the Acute Effects of Whey, Rice and Potato Protein Isolate Intake on Markers of Glycaemic Regulation and Appetite in Healthy Males Using a Randomised Study Design. Nutrients 2021, 13, 2157. [Google Scholar] [CrossRef]
- Oikawa, S.Y.; Bahniwal, R.; Holloway, T.M.; Lim, C.; McLeod, J.C.; McGlory, C.; Baker, S.K.; Phillips, S.M. Potato Protein Isolate Stimulates Muscle Protein Synthesis at Rest and with Resistance Exercise in Young Women. Nutrients 2020, 12, 1235. [Google Scholar] [CrossRef]
- Dougkas, A.; Östman, E. Protein-Enriched Liquid Preloads Varying in Macronutrient Content Modulate Appetite and Appetite-Regulating Hormones in Healthy Adults. J. Nutr. 2016, 146, 637–645. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Ellis, V. The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br. J. Nutr. 2010, 104, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, O.T.; Ajayi, K. Consumption pattern and acceptability of winged termites (Macroterme bellicosus)-enriched infant complementary foods in Ekiti State, Nigeria. Int. J. Trop. Insect Sci. 2021, 41, 2039–2050. [Google Scholar] [CrossRef]
- Ojinnaka, M.C.; Ofoelo, M.U.; Ezenwa, L.I. Nutritional evaluation of wheat cakes enriched with edible African termites (Macrotermes nigeriensis). Agro-Science 2013, 12, 35–42. [Google Scholar] [CrossRef][Green Version]
- Vangsoe, M.T.; Thogersen, R.; Bertram, H.C.; Heckmann, L.H.L.; Hansen, M. Ingestion of Insect Protein Isolate Enhances Blood Amino Acid Concentrations Similar to Soy Protein in A Human Trial. Nutrients 2018, 10, 1357. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover Trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef]
- Bergmans, R.S.; Nikodemova, M.; Stull, V.J.; Rapp, A.; Malecki, K.M.C. Comparison of cricket diet with peanut-based and milk-based diets in the recovery from protein malnutrition in mice and the impact on growth, metabolism and immune function. PLoS ONE 2020, 15, e0234559. [Google Scholar] [CrossRef]
- Hall, F.; Liceaga, A. Effect of microwave-assisted enzymatic hydrolysis of cricket (Gryllodes sigillatus) protein on ACE and DPP-IV inhibition and tropomyosin-IgG binding. J. Funct. Foods 2020, 64, 103634. [Google Scholar] [CrossRef]
- Zielinska, E.; Baraniak, B.; Karas, M. Antioxidant and Anti-Inflammatory Activities of Hydrolysates and Peptide Fractions Obtained by Enzymatic Hydrolysis of Selected Heat-Treated Edible Insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.R.; Choi, R.Y.; Lee, Y.; Lee, M.K. Effects of Edible Insect Tenebrio molitor Larva Fermentation Extract as a Substitute Protein on Hepatosteatogenesis and Proteomic Changes in Obese Mice Induced by High-Fat Diet. Int. J. Mol. Sci. 2021, 22, 3615. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Schwarz, A.; Meyer, S.; Wen, G. Insect meal as alternative protein source exerts pronounced lipid-lowering effects in hyperlipidemic obese Zucker rats. J. Nutr. 2019, 149, 566–577. [Google Scholar] [CrossRef]
- Seo, M.; Goo, T.W.; Chung, M.Y.; Baek, M.; Hwang, J.S.; Kim, M.A.; Yun, E.Y. Tenebrio molitor Larvae Inhibit Adipogenesis through AMPK and MAPKs Signaling in 3T3-L1 Adipocytes and Obesity in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2017, 18, 518. [Google Scholar] [CrossRef]
- Kar, S.K.; Jansman, A.J.M.; Schokker, D.; Kruijt, L.; Harms, A.C.; Wells, J.M.; Smits, M.A. Amine Metabolism Is Influenced by Dietary Protein Source. Front. Nutr. 2017, 4, 41. [Google Scholar] [CrossRef]
- Yoon, S.; Wong, N.A.K.; Chae, M.; Auh, J.H. Comparative Characterization of Protein Hydrolysates from Three Edible Insects: Mealworm Larvae, Adult Crickets, and Silkworm Pupae. Foods 2019, 8, 563. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Teran, I.D.; Fogliano, V.; Wichers, H.J. Investigation into the potential of commercially available lesser mealworm (A-diaperinus) protein to serve as sources of peptides with DPP-IV inhibitory activity. Int. J. Food Sci. Technol. 2019, 54, 696–704. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Evaluation of the potential of dietary proteins as precursors of dipeptidyl peptidase (DPP)-IV inhibitors by an in silico approach. J. Funct. Foods 2012, 4, 403–422. [Google Scholar] [CrossRef]
- Zielinska, E.; Baraniak, B.; Karas, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar] [CrossRef]
- Miguens-Gomez, A.; Grau-Bove, C.; Sierra-Cruz, M.; Jorba-Martin, R.; Caro, A.; Rodriguez-Gallego, E.; Beltran-Debon, R.; Blay, M.T.; Terra, X.; Ardevol, A.; et al. Gastrointestinally Digested Protein from the Insect Alphitobius diaperinus Stimulates a Different Intestinal Secretome than Beef or Almond, Producing a Differential Response in Food Intake in Rats. Nutrients 2020, 12, 2366. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Hong, K.S.; Song, M.Y.; Yun, S.M.; Ji, S.D.; Son, J.G.; Kim, E.H. Hepatoprotective Effects of Steamed and Freeze-Dried Mature Silkworm Larval Powder against Ethanol-Induced Fatty Liver Disease in Rats. Foods 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Mishyna, M.; Glumac, M. So different, yet so alike Pancrustacea: Health benefits of insects and shrimps. J. Funct. Foods 2021, 76, 104316. [Google Scholar] [CrossRef]
- Agengo, F.B.; Onyango, A.N.; Serrem, C.A.; Okoth, J. Efficacy of compositing with snail meat powder on protein nutritional quality of sorghum-wheat buns using a rat bioassay. J. Sci. Food Agric. 2020, 100, 2963–2970. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.W.; Park, J.B.; Pyo, S.E.; Hong, Y.K.; Ku, S.K.; Kim, M.R. Blood glycemia-modulating effects of melanian snail protein hydrolysates in mice with type II diabetes. Int. J. Mol. Med. 2017, 39, 1437–1451. [Google Scholar] [CrossRef][Green Version]
- Radzki, R.P.; Bienko, M.; Polak, P.; Szkucik, K.; Ziomek, M.; Ostapiuk, M.; Bienias, J. Is the consumption of snail meat actually healthy? An analysis of the osteotropic influence of snail meat as a sole source of protein in growing rats. J. Anim. Physiol. Anim. Nutr. 2018, 102, E885–E891. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ma, M.F.; Chow, C.J.; Tsai, Y.H. Angiotensin I-converting enzyme inhibitory and hypocholesterolemic activities: Effects of protein hydrolysates prepared from Achatina fulica snail foot muscle. Int. J. Food Prop. 2017, 20, 3102–3111. [Google Scholar] [CrossRef]
- Monteyne, A.J.; Dunlop, M.V.; Machin, D.J.; Coelho, M.O.C.; Pavis, G.F.; Porter, C.; Murton, A.J.; Abdelrahman, D.R.; Dirks, M.L.; Stephens, F.B.; et al. A mycoprotein-based high-protein vegan diet supports equivalent daily myofibrillar protein synthesis rates compared with an isonitrogenous omnivorous diet in older adults: A randomised controlled trial. Br. J. Nutr. 2021, 126, 674–684. [Google Scholar] [CrossRef]
- Monteyne, A.J.; Coelho, M.O.C.; Porter, C.; Abdelrahman, D.R.; Jameson, T.S.O.; Jackman, S.R.; Blackwell, J.R.; Finnigan, T.J.A.; Stephens, F.B.; Dirks, M.L.; et al. Mycoprotein ingestion stimulates protein synthesis rates to a greater extent than milk protein in rested and exercised skeletal muscle of healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 112, 318–333. [Google Scholar] [CrossRef]
- Monteyne, A.J.; Coelho, M.O.C.; Porter, C.; Abdelrahman, D.R.; Jameson, T.S.O.; Finnigan, T.J.A.; Stephens, F.B.; Dirks, M.L.; Wall, B.T. Branched-Chain Amino Acid Fortification Does Not Restore Muscle Protein Synthesis Rates following Ingestion of Lower- Compared with Higher-Dose Mycoprotein. J. Nutr. 2020, 150, 2931–2941. [Google Scholar] [CrossRef]
- Coelho, M.O.C.; Monteyne, A.J.; Dirks, M.L.; Finnigan, T.J.A.; Stephens, F.B.; Wall, B.T. Daily mycoprotein consumption for 1 week does not affect insulin sensitivity or glycaemic control but modulates the plasma lipidome in healthy adults: A randomised controlled trial. Br. J. Nutr. 2021, 125, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.O.C.; Monteyne, A.J.; Dunlop, M.V.; Harris, H.C.; Morrison, D.J.; Stephens, F.B.; Wall, B.T. Mycoprotein as a possible alternative source of dietary protein to support muscle and metabolic health. Nutr. Rev. 2020, 78, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.V.; Kilroe, S.P.; Bowtell, J.L.; Finnigan, T.J.A.; Salmon, D.L.; Wall, B.T. Mycoprotein represents a bioavailable and insulinotropic non-animal-derived dietary protein source: A dose-response study. Br. J. Nutr. 2017, 118, 673–685. [Google Scholar] [CrossRef]
- Bottin, J.H.; Swann, J.R.; Cropp, E.; Chambers, E.S.; Ford, H.E.; Ghatei, M.A.; Frost, G.S. Mycoprotein reduces energy intake and postprandial insulin release without altering glucagon-like peptide-1 and peptide tyrosine-tyrosine concentrations in healthy overweight and obese adults: A randomised-controlled trial. Br. J. Nutr. 2016, 116, 360–374. [Google Scholar] [CrossRef]
- Williamson, D.A.; Geiselman, P.J.; Lovejoy, J.; Greenway, F.; Volaufova, J.; Martin, C.K.; Arnett, C.; Ortego, L. Effects of consuming mycoprotein, tofu or chicken upon subsequent eating behaviour, hunger and safety. Appetite 2006, 46, 41–48. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; McMillan, B. The impact of mycoprotein on blood cholesterol levels: A pilot study. Br. Food J. 2010, 112, 1092–1101. [Google Scholar] [CrossRef]
- Wang, X.M.; Chen, H.X.; Li, S.Q.; Zhou, J.C.; Xu, J.T. Physico-chemical properties, antioxidant activities and antihypertensive effects of walnut protein and its hydrolysate. J. Sci. Food Agric. 2016, 96, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Kang, X.Y.; Shi, C.C.; Li, Y.J.; Majumder, K.; Ning, Z.X.; Ren, J.Y. Moderation of hyperuricemia in rats via consuming walnut protein hydrolysate diet and identification of new antihyperuricemic peptides. Food Funct. 2018, 9, 107–116. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Liu, C.L.; Fang, L.; Min, W.H. Protein Hydrolyzates from Changbai Mountain Walnut (Juglans mandshurica Maxim.) Boost Mouse Immune System and Exhibit Immunoregulatory Activities. Evid. Based Complement. Altern. Med. 2018, 2018. [Google Scholar] [CrossRef]
- Chen, D.S.; Ni, Z. Effect of functional activity of walnut protein peptide on fatigue recovery after sports training. Prog. Nutr. 2020, 22. [Google Scholar] [CrossRef]
- Li, W.Z.; Zhao, T.T.; Zhang, J.N.; Wu, C.P.; Zhao, M.M.; Su, G.W. Comparison of Neuroprotective and Cognition-Enhancing Properties of Hydrolysates from Soybean, Walnut, and Peanut Protein. J. Chem. 2016, 2016, 9358285. [Google Scholar] [CrossRef]
- Xu, D.F.; Wang, W.Q.; Liao, J.M.; Liao, L.; Li, C.H.; Zhao, M.M. Walnut protein hydrolysates, rich with peptide fragments of WSREEQEREE and ADIYTEEAGR ameliorate UV-induced photoaging through inhibition of the NF-kappa B/MMP-1 signaling pathway in female rats. Food Funct. 2020, 11, 10601–10616. [Google Scholar] [CrossRef]
- Food Standards Australia and New Zealand. Australian Food Composition Database—Release 1; Food Standards Australia and New Zealand: Canberra, ACT, Australia, 2019.
- Liu, R.L.; Ge, X.L.; Gao, X.Y.; Zhan, H.Y.; Shi, T.; Su, N.; Zhang, Z.Q. Two angiotensin-converting enzyme-inhibitory peptides from almond protein and the protective action on vascular endothelial function. Food Funct. 2016, 7, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Je, J.Y.; Cho, Y.S.; Yada, R.Y. Almond protein hydrolysate fraction modulates the expression of proinflammatory cytokines and enzymes in activated macrophages. Food Funct. 2013, 4, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.Y.; Wang, P.; Liu, C.L.; Wang, J.; Liu, X.Q.; Liu, J.S.; Min, W.H. Hazelnut protein-derived peptide LDAPGHR shows anti-inflammatory activity on LPS-induced RAW264.7 macrophage. J. Funct. Foods 2018, 46, 449–455. [Google Scholar] [CrossRef]
- Liv, D.D.; Regenstein, J.M.; Diao, Y.; Qiu, J.Q.; Zhang, H.; Li, J.T.; Zhao, H.T.; Wang, Z.Y. Antidiabetic effects of water-soluble Korean pine nut protein on type 2 diabetic mice. Biomed. Pharmacother. 2019, 117, 108989. [Google Scholar] [CrossRef]
- Malomo, S.A.; Nwachukwu, I.D.; Girgih, A.T.; Idowu, A.O.; Aluko, R.E.; Fagbemi, T.N. Antioxidant and Renin-Angiotensin System Inhibitory Properties of Cashew Nut and Fluted-Pumpkin Protein Hydrolysates. Pol. J. Food Nutr. Sci. 2020, 70, 275–289. [Google Scholar] [CrossRef]
- Jacques, H.; Leblanc, N.; Papineau, R.; Richard, D.; Cote, C.H. Peanut protein reduces body protein mass and alters skeletal muscle contractile properties and lipid metabolism in rats. Br. J. Nutr. 2010, 103, 1331–1339. [Google Scholar] [CrossRef]
- Bos, C.; Airinei, G.; Mariotti, F.; Benamouzig, R.; Berot, S.; Evrard, J.; Fenart, E.; Tome, D.; Gaudichon, C. The poor digestibility of rapeseed protein is balanced by its very high metabolic utilization in humans. J. Nutr. 2007, 137, 594–600. [Google Scholar] [CrossRef]
- Fleddermann, M.; Fechner, A.; Rößler, A.; Bähr, M.; Pastor, A.; Liebert, F.; Jahreis, G. Nutritional evaluation of rapeseed protein compared to soy protein for quality, plasma amino acids, and nitrogen balance—A randomized cross-over intervention study in humans. Clin. Nutr. 2013, 32, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, A.J.; He, R.; Malomo, S.A.; Girgih, A.T.; Aluko, R.E. Blood pressure lowering effects of Australian canola protein hydrolysates in spontaneously hypertensive rats. Food Res. Int. 2014, 55, 281–287. [Google Scholar] [CrossRef]
- Volk, C.; Brandsch, C.; Schlegelmilch, U.; Wensch-Dorendorf, M.; Hirche, F.; Simm, A.; Gargum, O.; Wiacek, C.; Braun, P.G.; Kopp, J.F.; et al. Postprandial Metabolic Response to Rapeseed Protein in Healthy Subjects. Nutrients 2020, 12, 2270. [Google Scholar] [CrossRef]
- Girgih, A.T.; Alashi, A.; He, R.; Malomo, S.; Aluko, R.E. Preventive and treatment effects of a hemp seed (Cannabis sativa L.) meal protein hydrolysate against high blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2014, 53, 1237–1246. [Google Scholar] [CrossRef]
- Malomo, S.A.; Onuh, J.O.; Girgih, A.T.; Aluko, R.E. Structural and Antihypertensive Properties of Enzymatic Hemp Seed Protein Hydrolysates. Nutrients 2015, 7, 7616–7632. [Google Scholar] [CrossRef]
- Samsamikor, M.; Mackay, D.; Mollard, R.C.; Aluko, R.E. A double-blind, randomized, crossover trial protocol of whole hemp seed protein and hemp seed protein hydrolysate consumption for hypertension. Trials 2020, 21, 354. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Bhattacharyya, D.K. Nutritional quality of sesame seed protein fraction extracted with isopropanol. J. Agric. Food Chem. 2001, 49, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Dhar, P.; Ghosh, S. Antihyperlipidemic Effect of Sesame (Sesamum indicum L.) Protein Isolate in Rats Fed a Normal and High Cholesterol Diet. J. Food Sci. 2010, 75, H274–H279. [Google Scholar] [CrossRef]
- Aondona, M.M.; Ikya, J.K.; Ukeyima, M.T.; Gborigo, T.; Aluko, R.E.; Girgih, A.T. In vitro antioxidant and antihypertensive properties of sesame seed enzymatic protein hydrolysate and ultrafiltration peptide fractions. J. Food Biochem. 2021, 45, e13587. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Bhattacharyya, D.K. Nutritional quality of sunflower seed protein fraction extracted with isopropanol. Plant Foods Hum. Nutr. 2000, 55, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Bhattacharyya, D.K. Hypolipidemic effect of enzymatically extracted sunflower seed protein fraction. J. Sci. Food Agric. 2001, 81, 347–352. [Google Scholar] [CrossRef]
- Diepvens, K.; Häberer, D.; Westerterp-Plantenga, M. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J. Obes. 2008, 32, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Abou-Samra, R.; Keersmaekers, L.; Brienza, D.; Mukherjee, R.; Macé, K. Effect of different protein sources on satiation and short-term satiety when consumed as a starter. Nutr. J. 2011, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Mollard, R.C.; Luhovyy, B.L.; Anderson, G.H. The effect of yellow pea protein and fibre on short-term food intake, subjective appetite and glycaemic response in healthy young men. Br. J. Nutr. 2012, 108 (Suppl. 1), S74–S80. [Google Scholar] [CrossRef]
- Mollard, R.C.; Luhovyy, B.L.; Smith, C.; Anderson, G.H. Acute effects of pea protein and hull fibre alone and combined on blood glucose, appetite, and food intake in healthy young men—A randomized crossover trial. Appl. Physiol. Nutr. Metab. 2014, 39, 1360–1365. [Google Scholar] [CrossRef]
- Chauhan, S.; Kerr, A.; Keogh, B.; Nolan, S.; Casey, R.; Adelfio, A.; Murphy, N.; Doherty, A.; Davis, H.; Wall, A.M.; et al. An Artificial-Intelligence-Discovered Functional Ingredient, NRT_N0G5IJ, Derived from Pisum sativum, Decreases HbA1c in a Prediabetic Population. Nutrients 2021, 13, 1635. [Google Scholar] [CrossRef]
- Li, H.; Prairie, N.; Udenigwe, C.C.; Adebiyi, A.P.; Tappia, P.S.; Aukema, H.M.; Jones, P.J.; Aluko, R.E. Blood pressure lowering effect of a pea protein hydrolysate in hypertensive rats and humans. J. Agric. Food Chem. 2011, 59, 9854–9860. [Google Scholar] [CrossRef] [PubMed]
- Teunissen-Beekman, K.F.; Dopheide, J.; Geleijnse, J.M.; Bakker, S.J.; Brink, E.J.; de Leeuw, P.W.; Serroyen, J.; van Baak, M.A. Differential effects of proteins and carbohydrates on postprandial blood pressure-related responses. Br. J. Nutr. 2014, 112, 600–608. [Google Scholar] [CrossRef]
- Weinborn, V.; Pizarro, F.; Olivares, M.; Brito, A.; Arredondo, M.; Flores, S.; Valenzuela, C. The Effect of Plant Proteins Derived from Cereals and Legumes on Heme Iron Absorption. Nutrients 2015, 7, 8977–8986. [Google Scholar] [CrossRef] [PubMed]
- Fabek, H.; Rajadurai, A.; Arshad, M.U.; Smith, C.E.; Bailo, B.G.; Kubant, R.; Anderson, G.H. Acute Effects of Lentil Fractions on Satiety and Glycemic Responses Before and After a Meal in Healthy Young Men. FASEB J. 2016, 30, 893–894. [Google Scholar]
- Weisse, K.; Brandsch, C.; Zernsdorf, B.; Nkengfack Nembongwe, G.S.; Hofmann, K.; Eder, K.; Stangl, G.I. Lupin protein compared to casein lowers the LDL cholesterol:HDL cholesterol-ratio of hypercholesterolemic adults. Eur. J. Nutr. 2010, 49, 65–71. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Krämer, J.; Kiehntopf, M.; Jahreis, G. Lupin protein positively affects plasma LDL cholesterol and LDL:HDL cholesterol ratio in hypercholesterolemic adults after four weeks of supplementation: A randomized, controlled crossover study. Nutr. J. 2013, 12, 107. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Kiehntopf, M.; Jahreis, G. Consuming a mixed diet enriched with lupin protein beneficially affects plasma lipids in hypercholesterolemic subjects: A randomized controlled trial. Clin. Nutr. 2015, 34, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, C.; Lammi, C.; Ruscica, M.; Bosisio, R.; Mombelli, G.; Zanoni, C.; Calabresi, L.; Sirtori, C.R.; Magni, P.; Arnoldi, A. Effects of a lupin protein concentrate on lipids, blood pressure and insulin resistance in moderately dyslipidaemic patients: A randomised controlled trial. J. Funct. Foods 2017, 37, 8–15. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Álvarez-Ríos, A.I.; Santos-Sánchez, G.; Pedroche, J.; Millán, F.; Carrera Sánchez, C.; Fernández-Pachón, M.S.; Millán-Linares, M.C.; Martínez-López, A.; et al. Safety and Efficacy of a Beverage Containing Lupine Protein Hydrolysates on the Immune, Oxidative and Lipid Status in Healthy Subjects: An Intervention Study (the Lupine-1 Trial). Mol. Nutr. Food Res. 2021, 65, e2100139. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, J.C.; Calvo, M.A.; Hancke, J.L.; Burgos, R.A.; Riva, A.; Morazzoni, P.; Ponzone, C.; Magni, C.; Duranti, M. Hypoglycemic effect of lupin seed γ-conglutin in experimental animals and healthy human subjects. Fitoterapia 2011, 82, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.D.; Bendsen, N.T.; Christensen, S.M.; Astrup, A.; Raben, A. Meals based on vegetable protein sources (beans and peas) are more satiating than meals based on animal protein sources (veal and pork)—A randomized cross-over meal test study. Food Nutr. Res. 2016, 60, 32634. [Google Scholar] [CrossRef]
- Nielsen, L.V.; Kristensen, M.D.; Klingenberg, L.; Ritz, C.; Belza, A.; Astrup, A.; Raben, A. Protein from Meat or Vegetable Sources in Meals Matched for Fiber Content has Similar Effects on Subjective Appetite Sensations and Energy Intake-A Randomized Acute Cross-Over Meal Test Study. Nutrients 2018, 10, 96. [Google Scholar] [CrossRef]
- Hosseinpour-Niazi, S.; Mirmiran, P.; Fallah-Ghohroudi, A.; Azizi, F. Non-soya legume-based therapeutic lifestyle change diet reduces inflammatory status in diabetic patients: A randomised cross-over clinical trial. Br. J. Nutr. 2015, 114, 213–219. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.M.; Berrue, F.; McGinn, P.J.; MacQuarrie, S.P.; Puttaswamy, A.; Patelakis, S.; Schmidt, D.; Melanson, R.; MacKenzie, S.E. A Rat Study to Evaluate the Protein Quality of Three Green Microalgal Species and the Impact of Mechanical Cell Wall Disruption. Foods 2020, 9, 1531. [Google Scholar] [CrossRef]
- Ertl, P.; Knaus, W.; Zollitsch, W. An approach to including protein quality when assessing the net contribution of livestock to human food supply. Animal 2016, 10, 1883–1889. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein Digestibility-Corrected Amino Acid Scores and Digestible Indispensable Amino Acid Scores Differentially Describe Protein Quality in Growing Male Rats. J. Nutr. 2014, 145, 372–379. [Google Scholar] [CrossRef]
- Joye, I. Protein Digestibility of Cereal Products. Foods 2019, 8, 199. [Google Scholar] [CrossRef]
- Stone, A.K.; Tanaka, T.; Nickerson, M.T. Protein quality and physicochemical properties of commercial cricket and mealworm powders. J. Food Sci. Technol. 2019, 56, 3355–3363. [Google Scholar] [CrossRef]
- Oibiokpa, F.I.; Olufunmilayo, H.A.; Jigam, A.A.; Saidu, A.N.; Egwim, E.C. Protein quality of four indigenous edible insect species in Nigeria. Food Sci. Hum. Wellness 2018, 7, 175–183. [Google Scholar] [CrossRef]
- Longvah, T.; Mangthya, K.; Ramulu, P. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chem. 2011, 128, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.G.; Cummings, J.H. The protein quality of mycoprotein. Proc. Nutr. Soc. 2010, 69, E331. [Google Scholar] [CrossRef]
- Lackey, K.A.; Fleming, S.A. Brief Research Report: Estimation of the Protein Digestibility-Corrected Amino Acid Score of Defatted Walnuts. Front. Nutr. 2021, 8, 702857. [Google Scholar] [CrossRef] [PubMed]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; House, J.D. Factors Influencing the Quality of Dietary Proteins: Implications for Pulses. Cereal Chem. 2017, 94, 49–57. [Google Scholar] [CrossRef]
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a sustainable source of protein in human diets. Glob. Food Secur. Agric. Policy Econ. Environ. 2021, 28, 100520. [Google Scholar] [CrossRef]
- Van Huis, A.; van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P.; Fabek, H. Edible Insects. Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013. [Google Scholar]
- Jongema, Y. List of Edible Insect Species of the World. Available online: https://www.wur.nl/en/Research-Results/Chair-groups/Plant-Sciences/Laboratory-of-Entomology/Edible-insects/Worldwide-species-list.htm (accessed on 1 October 2021).
- De Gier, S.; Verhoeckx, K. Insect (food) allergy and allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Reyes, R.; Lessard, B. Edible Insects. A Roadmap for the Strategic Growth of an Emerging Australian Industry; CSIRO: Canberra, ACT, Australia, 2021.
- Rumpold, B.A.; Fröhling, A.; Reineke, K.; Knorr, D. Comparison of volumetric and surface decontamination techniques for innovative processing of mealworm larvae (Tenebrio molitor). Innov. Food Sci. Emerg. Technol. 2014, 26, 232–241. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Jia, J.Q.; Yan, H.; Du, J.J.; Gui, Z.Z. A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: Biochemical characterization and molecular docking study. Peptides 2015, 68, 17–24. [Google Scholar] [CrossRef]
- Cavuoto, P.; Fenech, M.F. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat. Rev. 2012, 38, 726–736. [Google Scholar] [CrossRef] [PubMed]

| Protein Source | Human Studies (#) | PDCAAS * (In Vitro) | Potential Health Effects | Knowledge Gaps and Future Directions |
|---|---|---|---|---|
| Algae | 0 | 0.29–0.64 [121] | Improved glycaemic status Appetite control Improved liver function in NAFLD Modulate antioxidant, anti-inflammatory and ACE inhibition actions for disease prevention | Human clinical and protein bioavailability data needed Optimised algal growth conditions Protein extraction protocols Safety & toxicity testing Testing and selection of optimal species for human consumption |
| Cereal, Barley | 1 | 0.59–0.76 [122,123] | Comparable with casein with respect LDL cholesterol, inflammation (CRP), oxidative stress and blood pressure | More human clinical data needed to determine whether effects are specific to barley as a whole food or protein fraction. |
| Cereal, Buckwheat | 1 | 0.041–0.5 [123] | Lipid profile and inflammatory markers improved in a cohort with mild/moderate hypercholesterolemia | More human clinical data needed to determine whether effects are specific to whole food or protein fraction. |
| Cereal, Oat | 1 | 0.67 [124] | Hunger/appetite suppression Increased plasma insulin | More human clinical data needed to determine whether effects are specific to whole food or protein fraction. |
| Cereal, Rice | 7 | 0.51–0.62 [124] | Reduced pro-inflammatory cytokines. Compares well with whey for body composition with exercise | Further analysis needed for different processing methods and |
| Cereal, Rye | 1 | 0.59 [122] | Improved satiety Improved biomarkers of glycaemic control | More human clinical data needed |
| Cereal, Wheat | 7 | 0.42–0.54 [123,125] | Increased glycogen synthesis Supported exercise and muscle performance and reduced exercise-induced inflammation Myofibrillar protein synthesis lower than whey or casein Improve blood lipid profile and anti-hypertensive effects Assist with energy balance and improve satiety Elicits insulin response | More human clinical data needed including myofibrillar synthesis and satiety Greater clarity whether effects are due to protein alone, or whole food |
| Fresh fruit | 0 | n/a | No studies identified | |
| Fresh vegetable, potato | 4 | 0.87–1.0 [123] | Potato protein augments effect of myofibrillar protein synthesis Increased glucose control | More human clinical data needed |
| Insect, Cricket (ground) Gryllus assimilis | 1 | 0.65–0.73 [126,127] | Improved gut microbiome Reduced inflammation Reduced LDL cholesterol Bioactives (antioxidants, anti-inflammatories, ACE inhibition, DPP-IV inhibition) | Human clinical data needed Scalability and consistency of production Production cost Overcoming the ‘yuck’ factor |
| Insect, Mealworm (ground) Tenebrio molitor | 1 | 0.54 [126] | Slower, sustained amino acid digestion Improved glucose tolerance Improved lipid metabolism Potential anti-obesity effects Reduced homocysteine Bioactives (antioxidants, anti-inflammatories, ACE inhibition, DPP-IV inhibition) | Human clinical data needed Scalability and consistency of production Production cost Overcoming the ‘yuck’ factor |
| Silkworm Bombyx mori | 0 | N/A for B. mori (Samia ricinii 0.86 [128]) | Improved lipid metabolism Improved fatty liver disease Anti-inflammatory factors | Human clinical data needed Scalability and consistency of production |
| Termites Macrotermes nigeriensis | 2 | 0.42 [127] | Rich in protein Rich in minerals (Mg, Ca, K, P) Well tolerated and accepted for infant food supplementation | Human clinical data needed Scalability and consistency of production |
| Snails | 0 | N/A | Improved glycaemic control and diabetic complications Improved malnutrition Bioactives, including ACE inhibitor Deleterious effect on bone mineralisation and strength | Human clinical data needed Scalability and consistency of production More bone health studies needed |
| Myco- protein | 9 | 1.0 [129] | Improved myofibrillar protein synthesis and gene expression Stimulated post exercise tissue remodelling Comparable to milk, fish and meat protein for glycaemic control Improved satiety Improved total and LDL cholesterol, lipid metabolism Slower, sustained amino acid release | Larger cohorts needed to confirm effects |
| Nuts | 0 | 0.22 (almonds) 0.81 (roasted pistachios) (rat bioassay) [130] | Improved cognition and memory (walnuts, pine nuts) Bioactives (antioxidant, antihypertensive, antiinflammation) | Human clinical data needed. Low-moderate PDCAAS scores. |
| Oil Seeds | 3 | 0.5–0.6 (hemp seed) (rat bioassay) [131] | Improved hypoglycaemic response (canola/rapeseed) Improved hypotensive response (hemp seed, sesame seeds) Improved satiety Improved cholesterol (sesame seed) Improved antioxidant capacity | More human studies needed. |
| Legumes, Beans | 3 (in mixed legume diet) | Fava bean 0.56 [126] Cooked beans 0.54–0.75 Extruded beans 0.58–0.69 Baked beans 0.47–0.66 ** [132] | Improved satiety Reduced inflammatory cytokines (CRP, IL6, TNFα) | Specific clinical studies on bean proteins not available. More human studies required |
| Legumes, Peas | 14 | Yellow pea 0.59 [126] Cooked (0.69–0.72) Extruded (0.65–0.73) Baked (0.69–0.75) [132] | Improved satiety Reduced postprandial diastolic blood pressure (pea protein isolate) and systolic blood pressure in longer term (hydrolysate) Reduced postprandial blood glucose and HbA1c (hydrolysate) Reduced inflammatory cytokines (CRP, IL6, TNFα) | Lack of consistency in satiety and blood glucose outcomes. More studies required to confirm effects on blood pressure and inflammatory biomarkers |
| Legumes, Lentils | 3 | 0.68–0.80 [123] | Improved satiety Reduced postprandial blood glucose Heme-iron absorption maintained Reduced inflammatory cytokines (CRP, IL6, TNFα) | Limited clinical data available on lentil proteins |
| Legumes, Chickpeas | 1 | 0.69–0.77 [123] | Reduced inflammatory cytokines (CRP, IL6, TNFα) | No clinical data on purified chickpea proteins |
| Legumes, Lupin | 7 | 0.6 [133] | Improved hyperglycaemia (conglutin) Reduced LDL cholesterol and LDL:HDL ratio, especially in hypercholesterolemic subjects Reduced PCSK9 expression (improve lipid and cholesterol management) Reduced inflammatory cytokines and Th1-cell activation Increased antioxidant capacity of PBMCs | Insufficient clinical data available on glycaemic and immune responses |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bull, C.; Belobrajdic, D.; Hamzelou, S.; Jones, D.; Leifert, W.; Ponce-Reyes, R.; Terefe, N.S.; Williams, G.; Colgrave, M. How Healthy Are Non-Traditional Dietary Proteins? The Effect of Diverse Protein Foods on Biomarkers of Human Health. Foods 2022, 11, 528. https://doi.org/10.3390/foods11040528
Bull C, Belobrajdic D, Hamzelou S, Jones D, Leifert W, Ponce-Reyes R, Terefe NS, Williams G, Colgrave M. How Healthy Are Non-Traditional Dietary Proteins? The Effect of Diverse Protein Foods on Biomarkers of Human Health. Foods. 2022; 11(4):528. https://doi.org/10.3390/foods11040528
Chicago/Turabian StyleBull, Caroline, Damien Belobrajdic, Sara Hamzelou, Darren Jones, Wayne Leifert, Rocío Ponce-Reyes, Netsanet Shiferaw Terefe, Gemma Williams, and Michelle Colgrave. 2022. "How Healthy Are Non-Traditional Dietary Proteins? The Effect of Diverse Protein Foods on Biomarkers of Human Health" Foods 11, no. 4: 528. https://doi.org/10.3390/foods11040528
APA StyleBull, C., Belobrajdic, D., Hamzelou, S., Jones, D., Leifert, W., Ponce-Reyes, R., Terefe, N. S., Williams, G., & Colgrave, M. (2022). How Healthy Are Non-Traditional Dietary Proteins? The Effect of Diverse Protein Foods on Biomarkers of Human Health. Foods, 11(4), 528. https://doi.org/10.3390/foods11040528