High-Performance Thin-Layer Chromatography-Immunostaining as a Technique for the Characterization of Whey Protein Enrichment in Edam Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
2.1.2. Preparation of Cheese Samples
2.1.3. Antibodies
2.1.4. HPTLC Plates
2.2. Methods
2.2.1. Sample Preparation
Protein Extraction
Hydrolysis by Trypsin
Hydrolysis by Pronase E
2.2.2. High-Performance Thin-Layer Chromatography (HPTLC)
Separation of the Tryptic Hydrolysates
Fluorescamine Staining
Immunostaining
2.2.3. o-Phthalaldehyde Assay (OPA Assay)
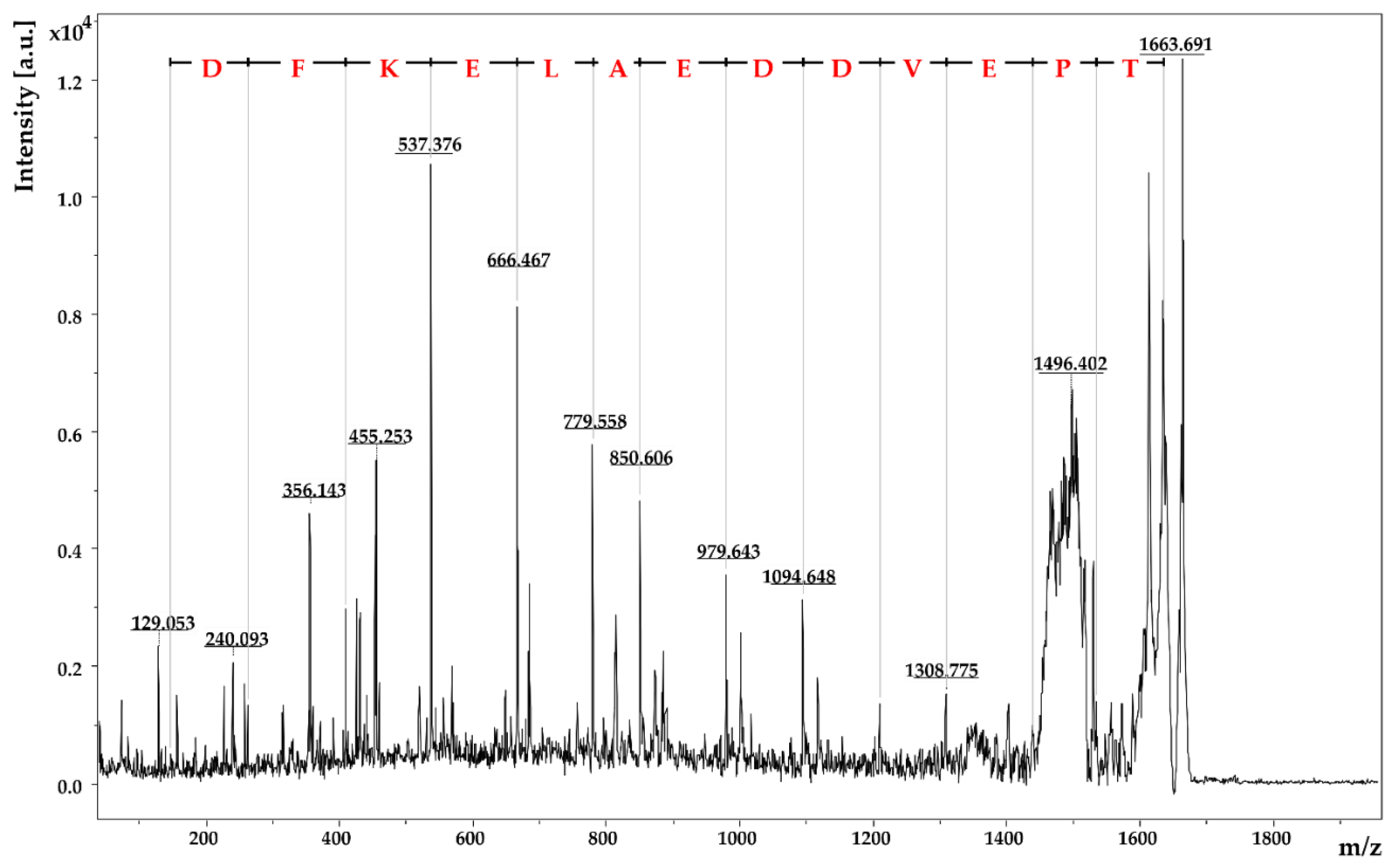
2.2.4. Identification of Amino Acid Sequences by MALDI-TOF-MS/MS
3. Results and Discussion
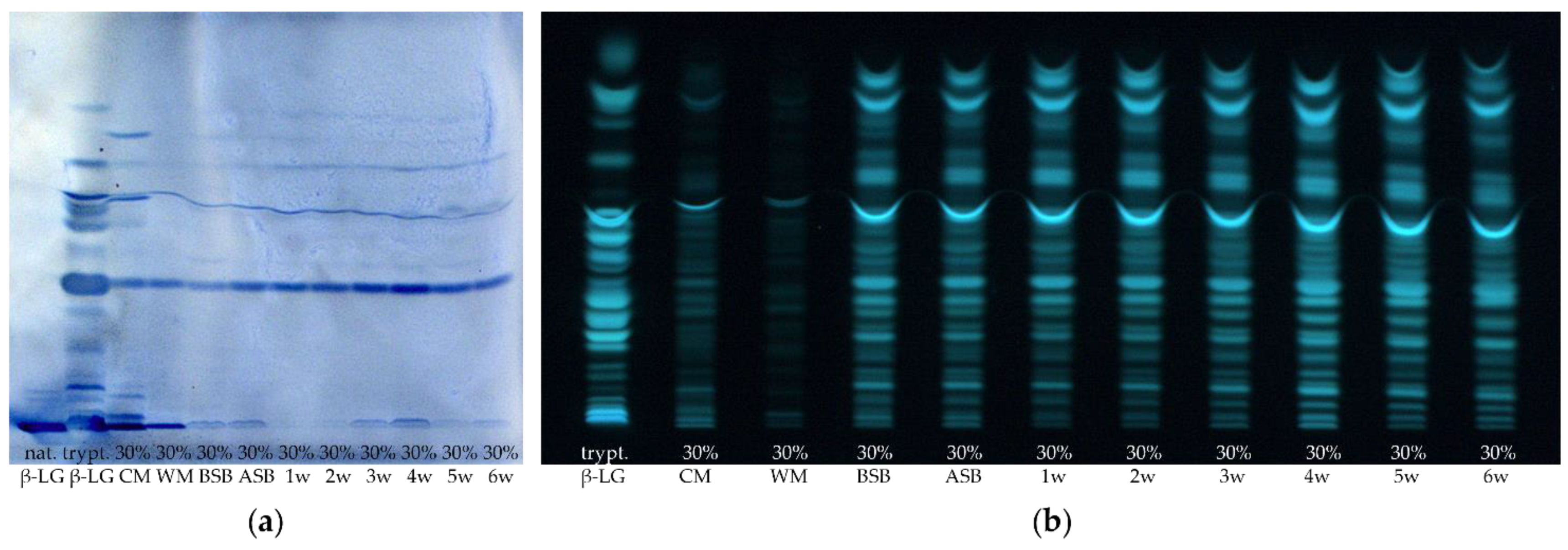
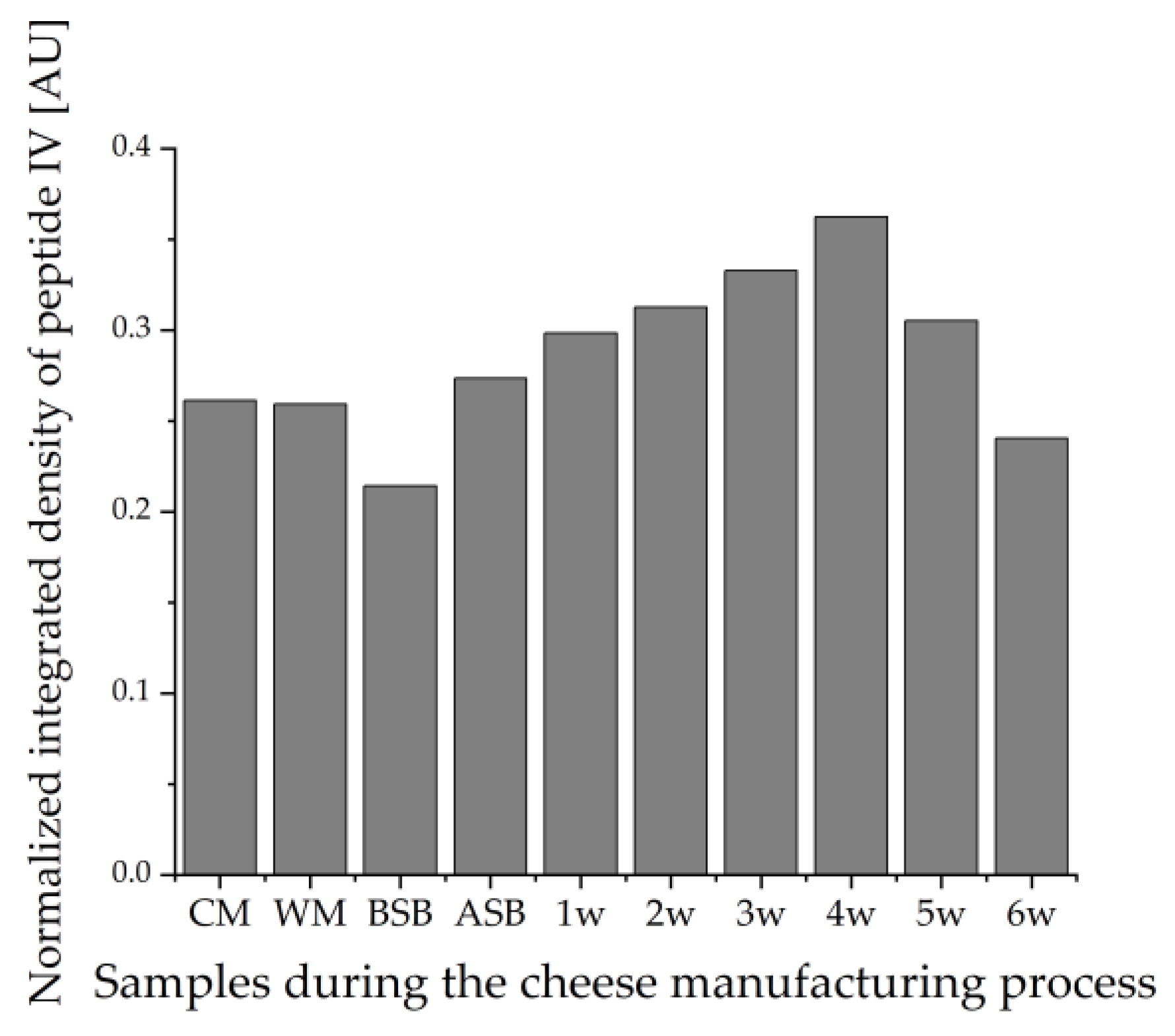
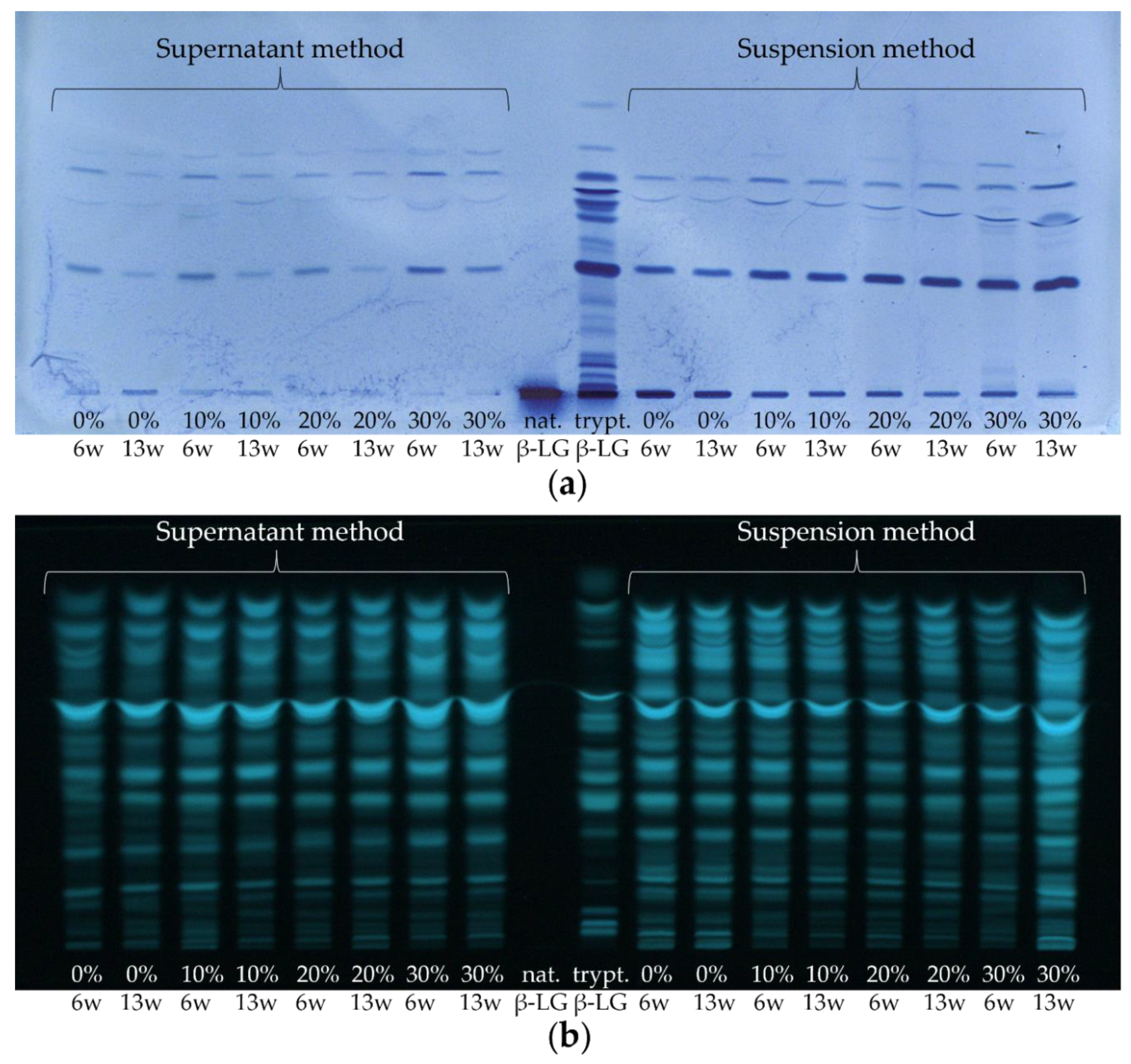
3.1. Proteolysis of β-Lactoglobulin during the Ripening of Whey Protein-Enriched Cheese
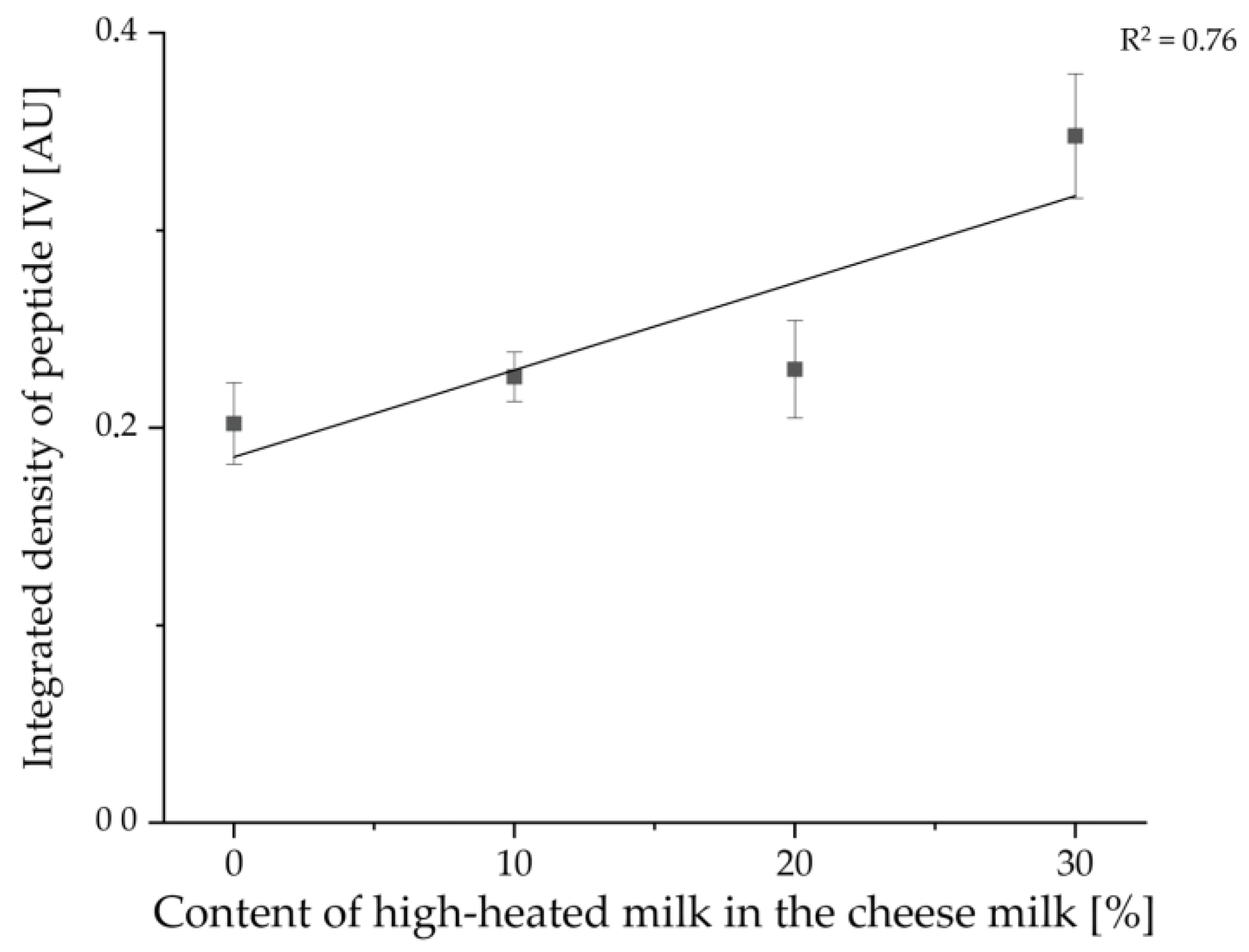
3.2. Enrichment of β-Lactoglobulin in Whey Protein-Enriched Cheese
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Masotti, F.; Cattaneo, S.; Stuknytė, M.; De Noni, I. Technological tools to include whey proteins in cheese: Current status and perspectives. Trends Food Sci. Technol. 2017, 64, 102–114. [Google Scholar] [CrossRef]
- Abd El-Gawad, M.A.M.; Ahmed, N.S. Cheese yield as affected by some parameters review. Acta Sci. Pol. Technol. Aliment. 2011, 10, 131–153. [Google Scholar]
- Mulvihill, D.M.; Donovan, M. Whey proteins and their thermal denaturation—A review. Irish J. Food Sci. Technol. 1987, 11, 43–75. [Google Scholar]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Wagner, J.; Biliaderis, C.G.; Moschakis, T. Whey proteins: Musings on denaturation, aggregate formation and gelation. Crit. Rev. Food Sci. Nutr. 2020, 60, 3793–3806. [Google Scholar] [CrossRef]
- Schingoethe, D.J. Whey Utilization in Animal Feeding: A Summary and Evaluation. J. Dairy Sci. 1976, 59, 556–570. [Google Scholar] [CrossRef]
- Le Maux, S.; Bouhallab, S.; Giblin, L.; Brodkorb, A.; Croguennec, T. Bovine β-lactoglobulin/fatty acid complexes: Binding, structural, and biological properties. Dairy Sci. Technol. 2014, 94, 409–426. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P.J.B.; Jameson, G.B. Structure and Stability of Whey Proteins, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128152515. [Google Scholar]
- Petit, J.; Herbig, A.; Moreau, A.; Delaplace, G. Influence of calcium on ß-lactoglobulin denaturation kinetics: Implications in unfolding and aggreagtion mechanisms. J. Dairy Sci. 2011, 94, 5794–5810. [Google Scholar] [CrossRef] [Green Version]
- Hinrichs, J. Incorporation of whey proteins in cheese. Int. Dairy J. 2001, 11, 495–503. [Google Scholar] [CrossRef]
- Lelievre, J. Whey proteins in cheese—An overview. Adv. Exp. Med. Biol. 1995, 367, 359–365. [Google Scholar]
- Chromik, C.; Partschefeld, C.; Jaros, D.; Henle, T.; Rohm, H. Adjustment of vat milk treatment to optimize whey protein transfer into semi-hard cheese: A case study. J. Food Eng. 2010, 100, 496–503. [Google Scholar] [CrossRef]
- Hickey, C.D.; Auty, M.A.E.; Wilkinson, M.G.; Sheehan, J.J. The influence of cheese manufacture parameters on cheese microstructure, microbial localisation and their interactions during ripening: A review. Trends Food Sci. Technol. 2015, 41, 135–148. [Google Scholar] [CrossRef]
- Guinee, T.P.; Pudja, P.D.; Reville, W.J.; Harrington, D.; Mulholland, E.O.; Cotter, M.; Cogan, T.M. Composition, microstructure and maturation of semi-hard cheeses from high protein ultrafiltered milk retentates with different levels of denatured whey protein. Int. Dairy J. 1995, 5, 543–568. [Google Scholar] [CrossRef]
- Donato, L.; Guyomarc’h, F. Formation and properties of the whey protein/κ-casein complexes in heated skim milk—A review. Dairy Sci. Technol. 2009, 89, 3–29. [Google Scholar] [CrossRef]
- Van den Berg, G.; Exterkate, F.A. Technological parameters involved in cheese ripening. Int. Dairy J. 1993, 3, 485–507. [Google Scholar] [CrossRef]
- Niero, G.; Sturaro, A.; Trentin, A.R.; Masi, A.; De Marchi, M.; Cassandro, M. Effect of cheesemaking with microparticulated whey proteins on the concentration of low molecular thiols in cheese. Acta Agrar. Kaposváriensis 2014, 18, 103–108. [Google Scholar]
- Ardö, Y.; McSweeney, P.L.H.; Magboul, A.A.A.; Upadhyay, V.K.; Fox, P.F. Biochemistry of Cheese Ripening: Proteolysis, 4th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 1, ISBN 9780122636530. [Google Scholar]
- Creamer, L.K.; Zoerb, H.F.; Olson, N.F.; Richardson, T. Surface Hydrophobicity of αs1 -I, αs1 -Casein A and B and Its Implications in Cheese Structure. J. Dairy Sci. 1982, 65, 902–906. [Google Scholar] [CrossRef]
- Afshari, R.; Pillidge, C.J.; Dias, D.A.; Osborn, A.M.; Gill, H. Cheesomics: The future pathway to understanding cheese flavour and quality. Crit. Rev. Food Sci. Nutr. 2020, 60, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Effect of ripening and in vitro digestion on the evolution and fate of bioactive peptides in Parmigiano-Reggiano cheese. Int. Dairy J. 2020, 105, 104668. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H. Application of proteomics to the areas of milk production, processing and quality control—A review. Int. J. Dairy Technol. 2014, 67, 153–166. [Google Scholar] [CrossRef]
- Enright, E.; Patricia Bland, A.; Needs, E.C.; Kelly, A.L. Proteolysis and physicochemical changes in milk on storage as affected by UHT treatment, plasmin activity and KIO3 addition. Int. Dairy J. 1999, 9, 581–591. [Google Scholar] [CrossRef]
- Gagnaire, V.; Piot, M.; Camier, B.; Vissers, J.P.C.; Jan, G.; Léonil, J. Survey of bacterial proteins released in cheese: A proteomic approach. Int. J. Food Microbiol. 2004, 94, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Pappa, E.C.; Robertson, J.A.; Rigby, N.M.; Mellon, F.; Kandarakis, I.; Mills, E.N.C. Application of proteomic techniques to protein and peptide profiling of Teleme cheese made from different types of milk. Int. Dairy J. 2008, 18, 605–614. [Google Scholar] [CrossRef]
- Molina, E.; Ramos, M.; Amigo, L. Characterisation of the casein fraction of Ibérico cheese by electrophoretic techniques. J. Sci. Food Agric. 2002, 82, 1240–1245. [Google Scholar] [CrossRef]
- Gaiaschi, A.; Beretta, B.; Poiesi, C.; Conti, A.; Giuffrida, M.G.; Galli, C.L.; Restani, P. Proteolysis of β-casein as a marker of Grana Padano cheese ripening. J. Dairy Sci. 2001, 84, 60–65. [Google Scholar] [CrossRef]
- Mehta-D’Souza, P. Detection of glycoproteins in polyacrylamide gels using pro-q emerald 300 dye, a fluorescent periodate schiff-base stain. Methods Mol. Biol. 2012, 869, 561–566. [Google Scholar] [CrossRef]
- Gwarda, R.Ł.; Dzido, T.H. Two-dimensional high-performance thin-layer chromatography of tryptic bovine albumin digest using normal- and reverse-phase systems with silanized silica stationary phase. J. Chromatogr. A 2013, 1312, 152–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Ma, H.; Chen, S. Isolation and Identification of Dipeptidyl Peptidase IV-Inhibitory Peptides from Trypsin/Chymotrypsin-Treated Goat Milk Casein Hydrolysates by 2D-TLC and LC-MS/MS. J. Agric. Food Chem. 2015, 63, 8819–8828. [Google Scholar] [CrossRef]
- Panchagnula, V.; Mikulskis, A.; Song, L.; Wang, Y.; Wang, M.; Knubovets, T.; Scrivener, E.; Golenko, E.; Krull, I.S.; Schulz, M.; et al. Phosphopeptide analysis by directly coupling two-dimensional planar electrochromatography/thin-layer chromatography with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Chromatogr. A 2007, 1155, 112–123. [Google Scholar] [CrossRef]
- Emory, J.F.; Walworth, M.J.; Van Berkel, G.J.; Schulz, M.; Minarik, S. Direct analysis of reversed-phase high-performance thin layer chromatography separated tryptic protein digests using a liquid microjunction surface sampling probe/electrospray ionization mass spectrometry system. Eur. J. Mass Spectrom. 2010, 16, 21–33. [Google Scholar] [CrossRef]
- Treblin, M.; von Oesen, T.; Class, L.-C.; Kuhnen, G.; Clawin-Rädecker, I.; Martin, D.; Fritsche, J.; Rohn, S. Two-dimensional high-performance thin-layer chromatography for the characterization of milk peptide properties and a prediction of the retention behavior—A proof-of-principle study. J. Chromatogr. A 2021, 1653, 462442. [Google Scholar] [CrossRef] [PubMed]
- Spöttel, J.; Brockelt, J.; Badekow, S.; Rohn, S. Immunological Analysis of Isothiocyanate-Modified α-Lactalbumin Using High-Performance Thin Layer Chromatography. Molecules 2021, 26, 1842. [Google Scholar] [CrossRef] [PubMed]
- Morschheuser, L.; Mink, K.; Horst, R.; Kallinich, C.; Rohn, S. Immunological analysis of food proteins using high-performance thin-layer chromatography-immunostaining. J. Chromatogr. A 2017, 1526, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Morschheuser, L.; Wessels, H.; Pille, C.; Fischer, J.; Hünniger, T.; Fischer, M.; Paschke-Kratzin, A.; Rohn, S. HPTLC-aptastaining—Innovative protein detection system for high-performance thin-layer chromatography. Sci. Rep. 2016, 6, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badekow, S.; Treblin, M.; Spöttel, J.; Rohn, S. Benzyl isothiocyanate-modified α-lactalbumin—Two-dimensional high-performance thin-layer chromatography for analyzing modified peptides. J. Chromatogr. B 2021, 1181, 122937. [Google Scholar] [CrossRef] [PubMed]
- Pasilis, S.P.; Kertesz, V.; Van Berkel, G.J.; Schulz, M.; Schorcht, S. Using HPTLC/DESI-MS for peptide identification in 1D separations of tryptic protein digests. Anal. Bioanal. Chem. 2008, 391, 317–324. [Google Scholar] [CrossRef]
- Biller, J.; Morschheuser, L.; Riedner, M.; Rohn, S. Development of optimized mobile phases for protein separation by high performance thin layer chromatography. J. Chromatogr. A 2015, 1415, 146–154. [Google Scholar] [CrossRef]
- Morschheuser, L.; Mükusch, S.; Riedner, M.; Seitz, H.; Rohn, S. High-performance thin-layer chromatography as a fast screening tool for phosphorylated peptides. J. Chromatogr. B 2016, 1008, 198–205. [Google Scholar] [CrossRef]
- Gwarda, R.; Tomczyszyn, A.; Misicka, A.; Dzido, T.H. Retention and separation efficiency of some synthetic oligopeptides in reversed-phase thin-layer chromatography. Acta Chromatogr. 2015, 27, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Morlock, G.; Schwack, W. Hyphenations in planar chromatography. J. Chromatogr. A 2010, 1217, 6600–6609. [Google Scholar] [CrossRef]
- Tscherch, K.; Biller, J.; Lehmann, M.; Rohn, S. One- and Two-dimensional High-performance Thin-layer Chromatography as an Alternative Analytical Tool for Investigating Polyphenol—Protein Interactions. Phytochem. Anal. 2013, 24, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Bhusan, R.; Mahesh, V.K.; Mallikharjun, P.V. Thin layer chromatography of peptides and proteins: A review. Biomed. Chromatogr. 1989, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Urbanova, I.; Svec, F. Monolithic polymer layer with gradient of hydrophobicity for separation of peptides using two-dimensional thin layer chromatography and MALDI-TOF-MS detection. J. Sep. Sci. 2011, 34, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Bakry, R.; Bonn, G.K.; Mair, D.; Svec, F. Monolithic porous polymer layer for the separation of peptides and proteins using thin-layer chromatography coupled with MALDI-TOF-MS. Anal. Chem. 2007, 79, 486–493. [Google Scholar] [CrossRef]
- Srivastava, M. High-Performance Thin-Layer Chromatography (HPTLC); Springer: Berlin, Germany, 2011; ISBN 9783642140242. [Google Scholar]
- Papasotiriou, D.G.; Markoutsa, S.; Gorka, J.; Schleiff, E.; Karas, M.; Meyer, B. MALDI analysis of proteins after extraction from dissolvable ethylene glycol diacrylate cross-linked polyacrylamide gels. Electrophoresis 2013, 34, 2484–2494. [Google Scholar] [CrossRef]
- Puranik, M.; Bhawsar, D.V.; Rathi, P.; Yeole, P.G. Simultaneous Determination of Ofloxacin and Ornidazole in Solid Dosage Form by RP-HPLC and HPTLC Techniques. Indian J. Pharm. Sci. 2010, 72, 513–517. [Google Scholar] [CrossRef] [Green Version]
- Loescher, C.M.; Morton, D.W.; Razic, S.; Agatonovic-Kustrin, S. High performance thin layer chromatography (HPTLC) and high performance liquid chromatography (HPLC) for the qualitative and quantitative analysis of Calendula officinalis—Advantages and limitations. J. Pharm. Biomed. Anal. 2014, 98, 52–59. [Google Scholar] [CrossRef]
- Düsterhöft, E.M.; Engels, W.; Huppertz, T. Gouda and Related Cheeses; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 1, ISBN 9780122636530. [Google Scholar]
- Hoffmann, W.; Luzzi, G.; Steffens, M.; Clawin-Rädecker, I.; Franz, C.M.A.P.; Fritsche, J. Salt reduction in film-ripened, semihard Edam cheese. Int. J. Dairy Technol. 2019, 70, 270–282. [Google Scholar] [CrossRef]
- Pellegrino, L.; Tirelli, A. A sensitive HPLC method to detect hen’s egg white lysozyme in milk and dairy products. Int. Dairy J. 2000, 10, 435–442. [Google Scholar] [CrossRef]
- Giansanti, P.; Tsiatsiani, L.; Low, T.Y.; Heck, A.J.R. Six alternative proteases for mass spectrometry-based proteomics beyond trypsin. Nat. Protoc. 2016, 11, 993–1006. [Google Scholar] [CrossRef]
- Kühn, C.; von Oesen, T.; Hanschen, F.S.; Rohn, S. Determination of isothiocyanate-protein conjugates in milk and curd after adding garden cress (Lepidium sativum L.). Food Res. Int. 2018, 108, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Krell, M.; Cvancar, L.; Poloczek, M.; Hanschen, F.S.; Rohn, S. Determination of Isothiocyanate-Protein Conjugates in a Vegetable-Enriched Bread. Foods 2021, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Seletchi, E.D.; Duliu, O.G. Image processing and data analysis in computed tomography. Rom. J. Phys. 2007, 52, 667–675. [Google Scholar]
- Aswad, D.W. Determination of d- and l-aspartate in amino acid mixtures by high-performance liquid chromatography after derivatization with a chiral adduct of o-phthaldialdehyde. Anal. Biochem. 1984, 137, 405–409. [Google Scholar] [CrossRef]
- Neurath, H.; Greenstein, J.P.; Putnam, F.W.; Erickson, J.O. The chemistry of protein denaturation. Chem. Rev. 1944, 34, 157–265. [Google Scholar] [CrossRef]
- Nord, F.F.; Bier, M.; Terminiello, L. On the mechanism of enzyme action. LXI. The self digestion of trypsin, calcium-trypsin and acetyltrypsin. Arch. Biochem. Biophys. 1956, 65, 120–131. [Google Scholar] [CrossRef]
- Alves, P.; Arnold, R.J.; Clemmer, D.E.; Li, Y.; Reilly, J.P.; Sheng, Q.; Tang, H.; Xun, Z.; Zeng, R.; Radivojac, P. Fast and accurate identification of semi-tryptic peptides in shotgun proteomics. Bioinformatics 2008, 24, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Rahali, V.; Chobert, J.M.; Haertlé, T.; Guéguen, J. Emulsification of chemical and enzymatic hydrolysates of β-lactoglobulin: Characterization of the peptides adsorbed at the interface. Nahr.—Food 2000, 44, 89–95. [Google Scholar] [CrossRef]
- Šlechtová, T.; Gilar, M.; Kalíková, K.; Tesařová, E. Insight into Trypsin Miscleavage: Comparison of Kinetic Constants of Problematic Peptide Sequences. Anal. Chem. 2015, 87, 7636–7643. [Google Scholar] [CrossRef]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.-C.; Yates, J.R. Protein Analysis by Shotgun/Bottom-up Proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef] [Green Version]
- Lottspeich, F.; Engels, J.W. Bioanalytics; John Wiley & Sons, Incorporated: Weinheim, Germany, 2018; ISBN 9783527694440. [Google Scholar]
- Williams, S.C.; Badley, R.A.; Davis, P.J.; Puijk, W.C.; Meloen, R.H. Identification of epitopes within beta lactoglobulin recognised by polyclonal antibodies using phage display and PEPSCAN. J. Immunol. Methods 1998, 213, 1–17. [Google Scholar] [CrossRef]
- Miller, K.; Meredith, C.; Selo, I.; Wal, J.M. Allergy to bovine β-lactoglobulin: Specificity of immunoglobulin E generated in the Brown Norway rat to tryptic and synthetic peptides. Clin. Exp. Allergy 1999, 29, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, K.M.; Chatchatee, P.; Bardina, L.; Beyer, K.; Sampson, H.A. Identification of IgE and IgG binding epitopes on β- and κ-casein in cow’s milk allergic patients. Int. Arch. Allergy Immunol. 2001, 126, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Niemi, M.; Jylhä, S.; Laukkanen, M.L.; Söderlund, H.; Mäkinen-Kiljunen, S.; Kallio, J.M.; Hakulinen, N.; Haahtela, T.; Takkinen, K.; Rouvinen, J. Molecular Interactions between a Recombinant IgE Antibody and the β-Lactoglobulin Allergen. Structure 2007, 15, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yuan, S.; He, S.; Gao, J.; Chen, H. Identification and characterization of the antigenic site (epitope) on bovine β-lactoglobulin: Common residues in linear and conformational epitopes. J. Sci. Food Agric. 2015, 95, 2916–2923. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.S.; Camirand, W.M.; Pavlath, A.E. Structures and Functionalities of Milk Proteins. Crit. Rev. Food Sci. Nutr. 1996, 36, 807–844. [Google Scholar] [CrossRef]
- Heino, A.; Uusi-rauva, J.; Outinen, M. Pre-treatment methods of Edam cheese milk. Effect on cheese yield and quality. LWT—Food Sci. Technol. 2010, 43, 640–646. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Le, T.T.; Deeth, H.C.; Larsen, L.B. Proteomics of major bovine milk proteins: Novel insights. Int. Dairy J. 2016, 67, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Dumpler, J.; Wohlschläger, H.; Kulozik, U. Dissociation and coagulation of caseins and whey proteins in concentrated skim milk heated by direct steam injection. Dairy Sci. Technol. 2017, 96, 807–826. [Google Scholar] [CrossRef]







| Sample Set | HH Milk [%] | n | Samples of Different Cheese Production Stages | |||
|---|---|---|---|---|---|---|
| Milk Samples | Whey Samples | Cheese Curd Samples | Ripening Length of Cheese Samples [Weeks] | |||
| I | 0 | 9 | - | - | - | 6 |
| 10 | 3 | - | - | - | 6 | |
| 20 | 3 | - | - | - | 6 | |
| 30 | 3 | CM (n = 1) | WM (n = 1) | BSB, ASB (n = 1) | 1–6 | |
| II | 0 | 1 | PM | - | - | 6, 13 |
| 10 | 1 | - | - | - | 6, 13 | |
| 20 | 1 | - | - | - | 6, 13 | |
| 30 | 1 | CM | WM | BSB, ASB | 3, 6, 13 | |
| Peptide | Rf Value | m/z (MS1) | Position | Amino Acid Sequence | Miscleavages | (M + H)+[u] | Mass Difference [u] | Adducts |
|---|---|---|---|---|---|---|---|---|
| I | 0.03 | 1663.69 | 141–154 | TPEVDDEALEKFDK | 1 | 1635.77 | 28 | dimethylation |
| II | 0.05 | 1537.55 | 141–151 | TPEVDDEALEK | 0 | 1245.58 | 292 (260 + 32) | fluorescamine + methanol |
| III | 0.09 | 1163.57 | 92–99 | TKIPAVFK | 1 | 903.57 | 260 | fluorescamine |
| IV | 0.25 | 1198.62 | 57–66 | VYVEELKPTP | semi-tryptic peptide | 1156.62 (of the b-fragment) | 42 | trimethylation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treblin, M.; von Oesen, T.; Lüneburg, J.; Clawin-Rädecker, I.; Martin, D.; Schrader, K.; Zink, R.; Hoffmann, W.; Fritsche, J.; Rohn, S. High-Performance Thin-Layer Chromatography-Immunostaining as a Technique for the Characterization of Whey Protein Enrichment in Edam Cheese. Foods 2022, 11, 534. https://doi.org/10.3390/foods11040534
Treblin M, von Oesen T, Lüneburg J, Clawin-Rädecker I, Martin D, Schrader K, Zink R, Hoffmann W, Fritsche J, Rohn S. High-Performance Thin-Layer Chromatography-Immunostaining as a Technique for the Characterization of Whey Protein Enrichment in Edam Cheese. Foods. 2022; 11(4):534. https://doi.org/10.3390/foods11040534
Chicago/Turabian StyleTreblin, Mascha, Tobias von Oesen, Jana Lüneburg, Ingrid Clawin-Rädecker, Dierk Martin, Katrin Schrader, Ralf Zink, Wolfgang Hoffmann, Jan Fritsche, and Sascha Rohn. 2022. "High-Performance Thin-Layer Chromatography-Immunostaining as a Technique for the Characterization of Whey Protein Enrichment in Edam Cheese" Foods 11, no. 4: 534. https://doi.org/10.3390/foods11040534







