Rheological Characteristics of Model Gluten-Free Dough with Plantago Seeds and Husk Incorporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixolab Tests
2.3. Uniaxial Deformation
2.4. Bread Preparation and Evaluation
2.5. Statistical Analysis
3. Results and Discussion
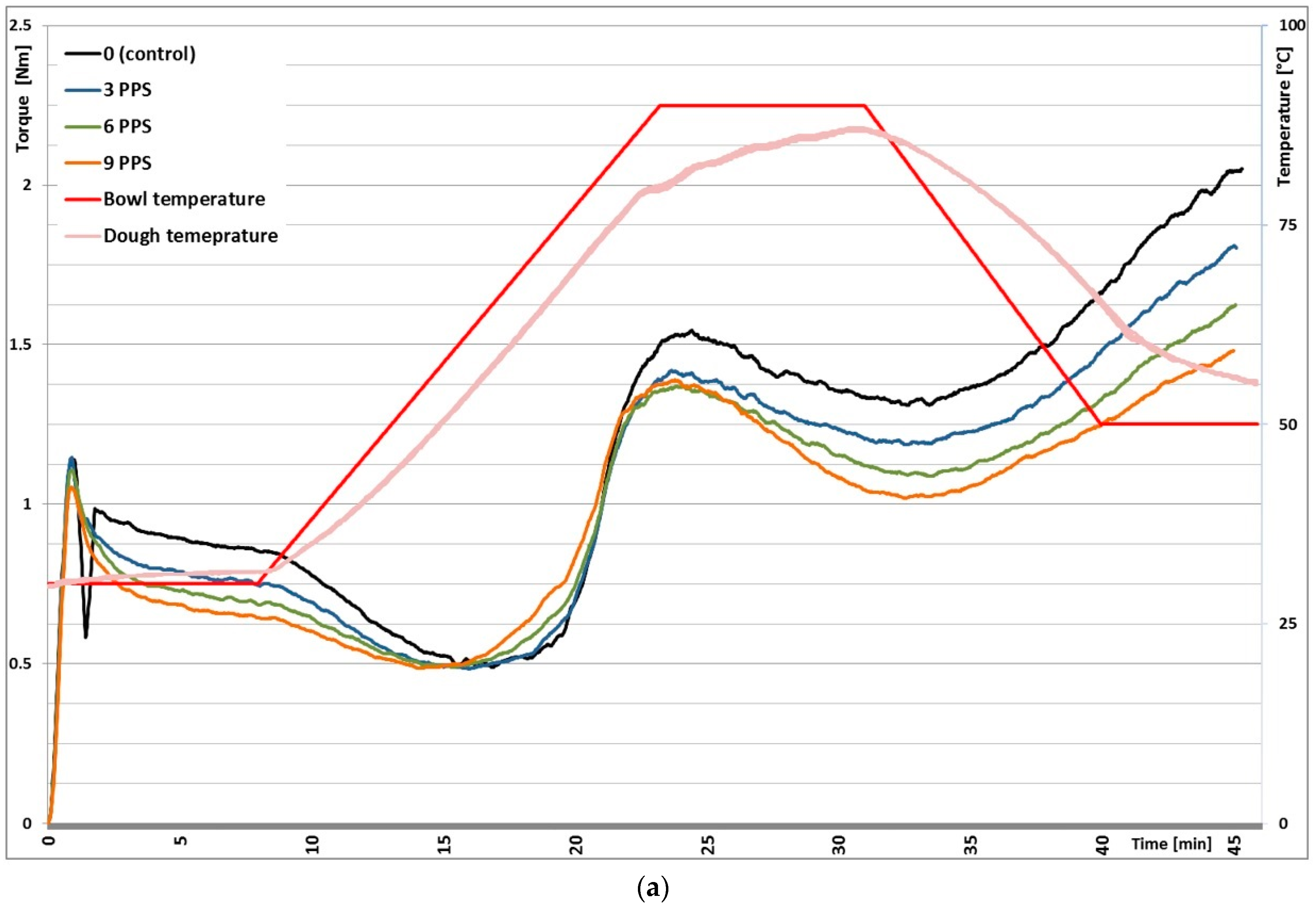
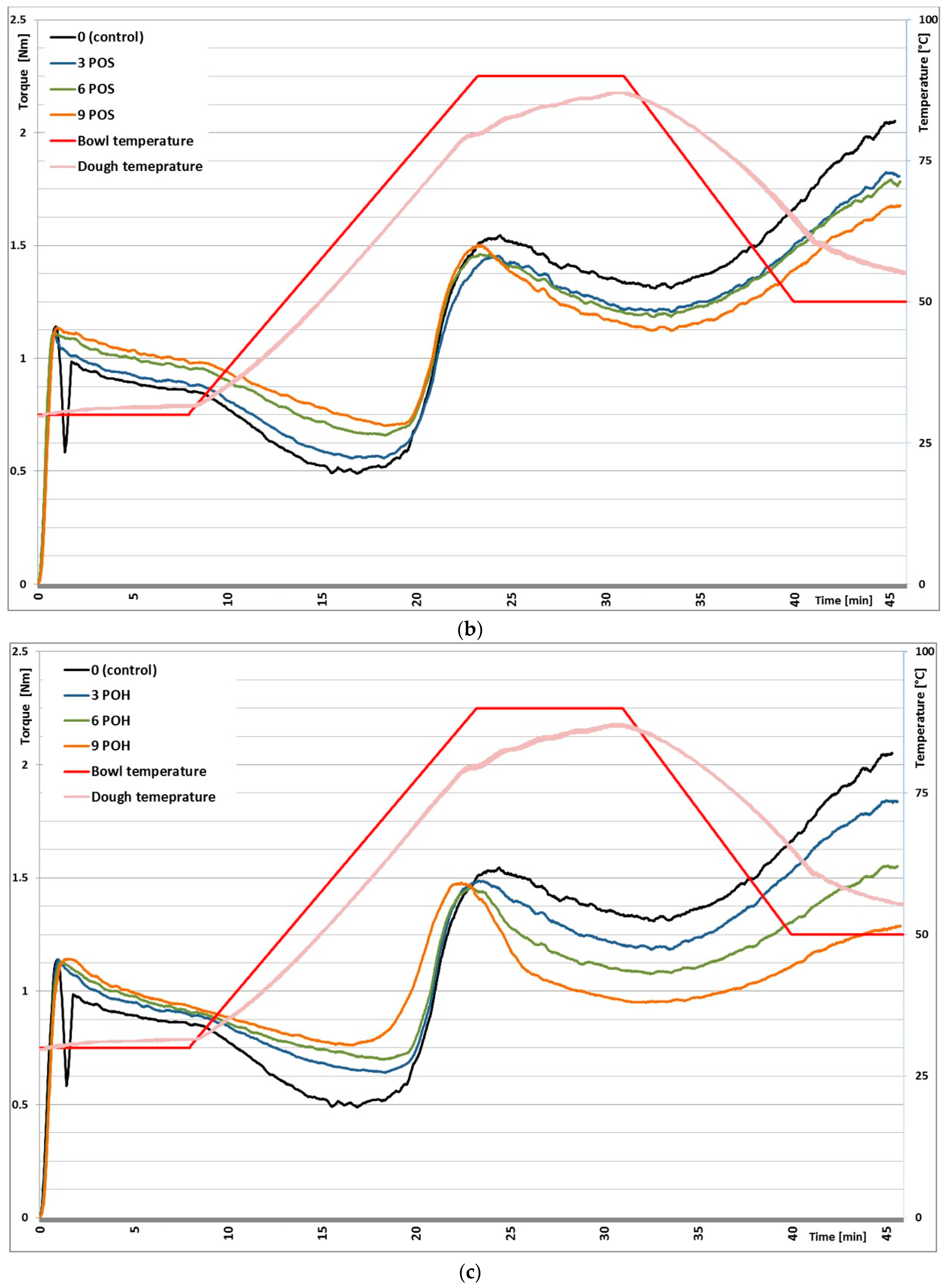
3.1. Mixolab Rheological Profiles
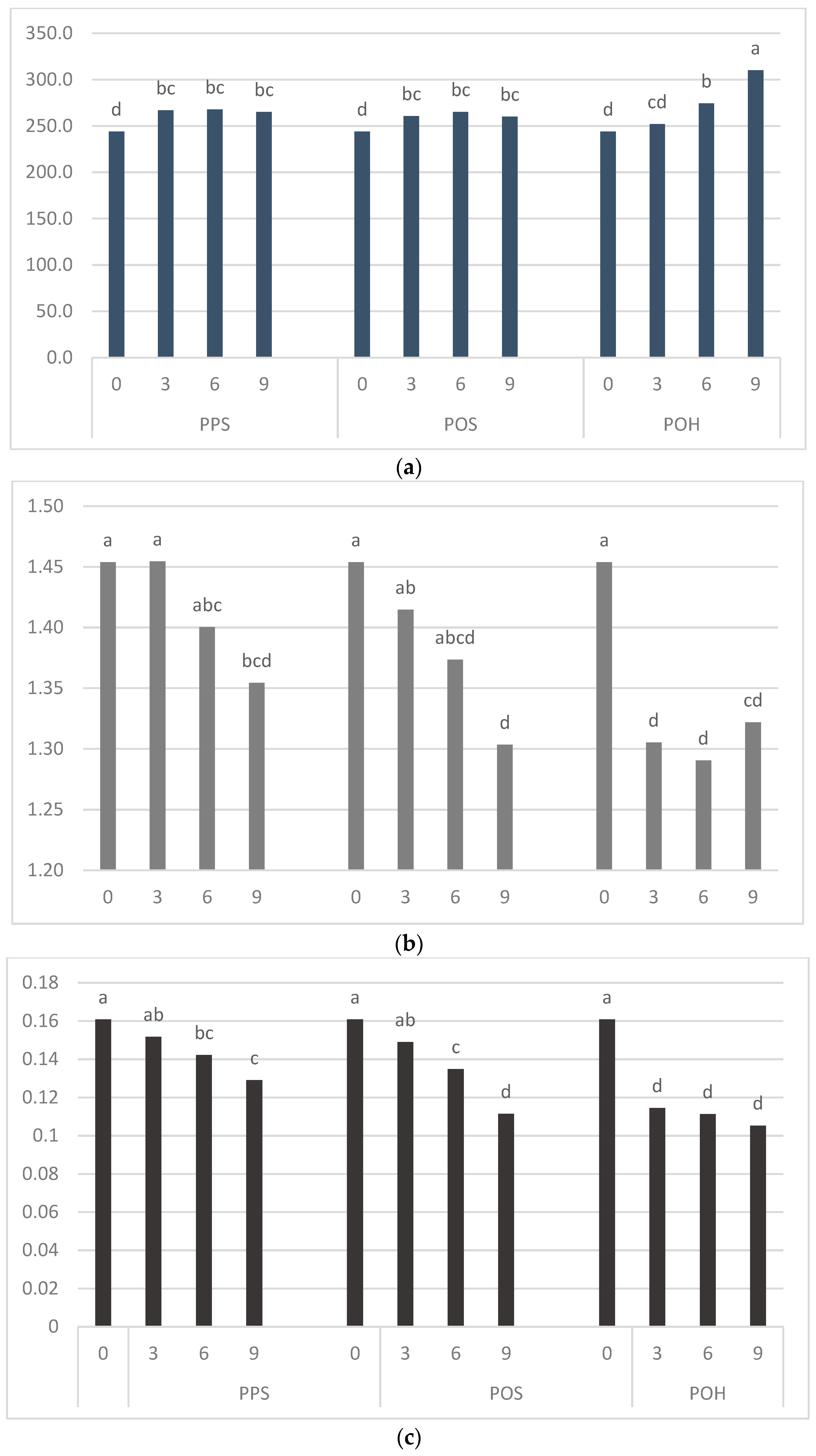
3.2. Uniaxial Deformation
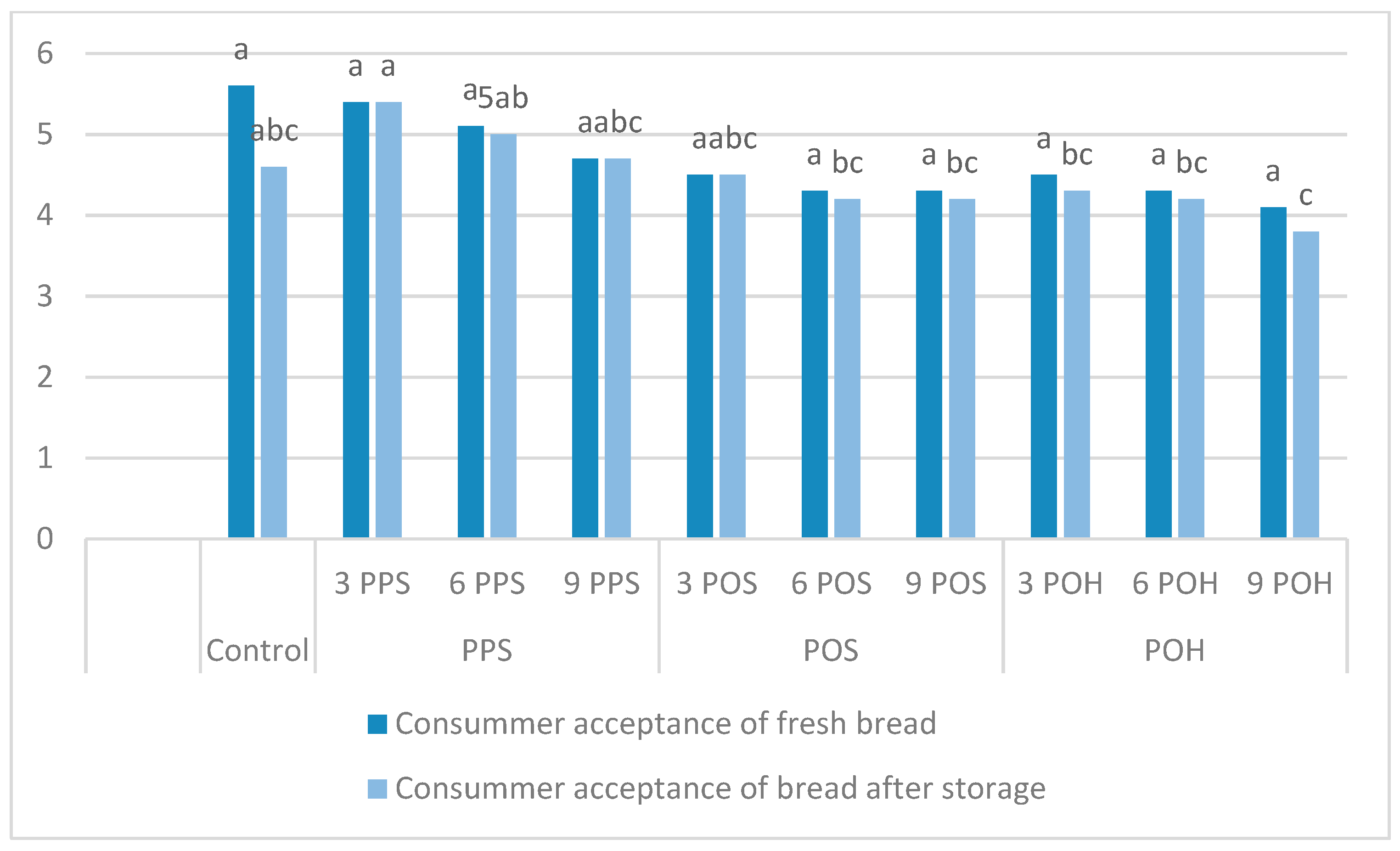
3.3. Bread Quality
3.4. Texture Profile Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, M.; Morteza-Semnani, K.; Ansoroudi, F.; Fallah, S.; Amin, G. Evaluation of binding properties of Plantago psyllium seed mucilage. Acta Pharm. 2010, 60, 339–348. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Wang, Q.; Young, J.C. Fractionation and physicochemical characterization of psyllium gum. Carbohydr. Polym. 2008, 73, 35–43. [Google Scholar] [CrossRef]
- Gupta; Sen, C.; Jeyarani, T.; Rajiv, J. Rheology, fatty acid profile and quality characteristics of nutrient enriched pizza base. J. Food Sci. Technol. 2015, 52, 2926–2933. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sierra, M.; Farmafibra Group; García, J.; Fernández, N.; Diez, M.; Calle, A. Therapeutic effects of psyllium in type 2 diabetic patients. Eur. J. Clin. Nutr. 2002, 56, 830–842. [Google Scholar] [CrossRef]
- Fischer, M.H.; Yu, N.; Gray, G.R.; Ralph, J.; Anderson, L.; Marlett, J.A. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohydr. Res. 2004, 339, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Muhammad, G.; Jantan, I.; Bukhari, S.N.A. Psyllium Arabinoxylan: A Versatile Biomaterial for Potential Medicinal and Pharmaceutical Applications. Polym. Rev. 2015, 56, 1–30. [Google Scholar] [CrossRef]
- Niu, Y.; Xie, Z.; Zhang, H.; Sheng, Y.; Yu, L. Effects of Structural Modifications on Physicochemical and Bile Acid-Binding Properties of Psyllium. J. Agric. Food Chem. 2013, 61, 596–601. [Google Scholar] [CrossRef]
- Farahnaky, A.; Askari, H.; Majzoobi, M.; Mesbahi, G. The impact of concentration, temperature and pH on dynamic rheology of psyllium gels. J. Food Eng. 2010, 100, 294–301. [Google Scholar] [CrossRef]
- Franco, E.A.N.; Sanches-Silva, A.; dos Santos, R.R.; de Melo, N.R. Psyllium (Plantago ovata Forsk): From evidence of health benefits to its food application. Trends Food Sci. Technol. 2020, 96, 166–175. [Google Scholar] [CrossRef]
- Fratelli, C.; Muniz, D.G.; Santos, F.G.; Capriles, V.D. Modelling the effects of psyllium and water in gluten-free bread: An approach to improve the bread quality and glycemic response. J. Funct. Foods 2018, 42, 339–345. [Google Scholar] [CrossRef]
- Khan, A.; Khalid, W.; Safdar, S.; Usman, M.; Shakeel, M.; Jamal, N.; Jha, R.; Baig, M.; Shehzadi, S.; Khalid, M.Z.; et al. Nutritional and Therapeutic Benefits of Psyllium Husk (Plantago Ovata). ASMI 2021, 4, 43–50. [Google Scholar]
- Kamaljit, K.; Amarjeet, K.; Pal, S.T. Analysis of Ingredients, Functionality, Formulation Optimization and Shelf Life Evaluation of High Fiber Bread. Am. J. Food Technol. 2011, 6, 306–313. [Google Scholar] [CrossRef][Green Version]
- Sim, S.Y.; Aziah, A.A.N.; Cheng, L.H. Quality and functionality of Chinese steamed bread and dough added with selected non-starch polysaccharides. J. Food Sci. Technol. 2015, 52, 303–310. [Google Scholar] [CrossRef]
- AproduI; Iuliana; BanuI, I. Influence of dietary fiber, water, and glucose oxidase on rheological and baking properties of maize based gluten-free bread. Food Sci. Biotechnol. 2015, 24, 1301–1307. [Google Scholar] [CrossRef]
- Cappa, C.; Lucisano, M.; Mariotti, M. Influence of Psyllium, sugar beet fibre and water on gluten-free dough properties and bread quality. Carbohydr. Polym. 2013, 98, 1657–1666. [Google Scholar] [CrossRef]
- Mancebo, C.M.; Miguel, M.; Ángel, S.; Martínez, M.M.; Gómez, M. Optimisation of rheological properties of gluten-free doughs with HPMC, psyllium and different levels of water. J. Cereal Sci. 2015, 61, 8–15. [Google Scholar] [CrossRef]
- Mariotti, M.; Lucisano, M.; Pagani, M.A.; Ng, P.K. The role of corn starch, amaranth flour, pea isolate, and Psyllium flour on the rheological properties and the ultrastructure of gluten-free doughs. Food Res. Int. 2009, 42, 963–975. [Google Scholar] [CrossRef]
- Zandonadi, R.P.; Botelho, R.B.A.; Araújo, W.M.C. Psyllium as a Substitute for Gluten in Bread. J. Am. Diet. Assoc. 2009, 109, 1781–1784. [Google Scholar] [CrossRef]
- Fratelli, C.; Santos, F.; Muniz, D.; Habu, S.; Braga, A.; Capriles, V. Psyllium Improves the Quality and Shelf Life of Gluten-Free Bread. Foods 2021, 10, 954. [Google Scholar] [CrossRef]
- Ngemakwe, P.N.; Le Roes-Hill, M.; Jideani, V. Advances in gluten-free bread technology. Food Sci. Technol. Int. 2015, 21, 256–276. [Google Scholar] [CrossRef]
- Torbica, A.; Hadnađev, M.; Dapčević, T. Rheological, textural and sensory properties of gluten-free bread formulations based on rice and buckwheat flour. Food Hydrocoll. 2010, 24, 626–632. [Google Scholar] [CrossRef]
- Gujral, H.S.; Rosell, C.M. Improvement of the breadmaking quality of rice flour by glucose oxidase. Food Res. Int. 2004, 37, 75–81. [Google Scholar] [CrossRef]
- Dobraszczyk, B.J.; Morgenstern, M.P. Rheology and the breadmaking process. J. Cereal Sci. 2003, 38, 229–245. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Aldughpassi, A.D.; Sidhu, J.S.; Al-Foudari, M.Y.; Al-Othman, A.R. Effect of psyllium husk addition on the instrumental texture and consumer acceptability of high-fiber wheat pan bread and buns. Ann. Agric. Sci. 2021, 66, 75–80. [Google Scholar] [CrossRef]
- Filipčev, B.; Pojić, M.; Šimurina, O.; Mišan, A.; Mandić, A. Psyllium as an improver in gluten-free breads: Effect on volume, crumb texture, moisture binding and staling kinetics. LWT 2021, 151, 112156. [Google Scholar] [CrossRef]
- Santos, F.G.; Fratelli, C.; Alencar, N.M.M.; Capriles, V.D. Modelling the effects of psyllium and water on dough parameters using Mixolab® and their relationship with physical properties and acceptability of gluten-free bread. Res. Soc. Dev. 2020, 9, 1–12. [Google Scholar] [CrossRef]
- Santos, F.G.; Aguiar, E.V.; Braga, A.R.C.; Alencar, N.M.; Rosell, C.M.; Capriles, V.D. An integrated instrumental and sensory approach to describe the effects of chickpea flour, psyllium, and their combination at reducing gluten-free bread staling. Food Packag. Shelf Life 2021, 28, 100659. [Google Scholar] [CrossRef]
- Santos, F.G.; Aguiar, E.V.; Rosell, C.M.; Capriles, V.D. Potential of chickpea and psyllium in gluten-free breadmaking: Assessing bread’s quality, sensory acceptability, and glycemic and satiety indexes. Food Hydrocoll. 2021, 113, 106487. [Google Scholar] [CrossRef]
- AACCI Method 54-21.02. Rheological Behavior of Flour by Farinograph: Constant Flour Weight Procedure. AACC International Approved Methods of Analysis, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2009.
- AACCI Method 54-60.01. Determination of Rheological Behavior as a Function of Mixing and Temperature Increase in Wheat Flour and Whole Wheat Meal by Mixolab. AACC International. Approved Methods of Analysis, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2009.
- Harati, H.; Bekes, F.; Howell, K.; Noonan, S.; Florides, C.; Beasley, J.; Diepeveen, D.; Appels, R. Signatures for torque variation in wheat dough structure are affected by enzymatic treatments and heating. Food Chem. 2020, 316, 126357. [Google Scholar] [CrossRef]
- Dunnewind, B.; Sliwinski, E.; Grolle, K.; Vliet, T. THE KIEFFER DOUGH AND GLUTEN EXTENSIBILITY RIG - AN EXPERIMENTAL EVALUATION. J. Texture Stud. 2003, 34, 537–560. [Google Scholar] [CrossRef]
- Ferrero, C. Hydrocolloids in wheat breadmaking: A concise review. Food Hydrocoll. 2017, 68, 15–22. [Google Scholar] [CrossRef]
- Rosell, C.M.; Collar, C.; Haros, M. Assessment of hydrocolloid effects on the thermo-mechanical properties of wheat using the Mixolab. Food Hydrocoll. 2007, 21, 452–462. [Google Scholar] [CrossRef]
- Dobraszczyk, B. The physics of baking: Rheological and polymer molecular structure–function relationships in breadmaking. J. Non-Newtonian Fluid Mech. 2004, 124, 61–69. [Google Scholar] [CrossRef]
| Rice Flour | Plantago psyllium Seeds | Plantago ovata Seeds | Plantago ovata Husk | |
|---|---|---|---|---|
| Energy value | 1484 kJ/349 kcal | 1133 kJ/275 kcal | 1133 kJ/275 kcal | 749 kJ/178 kcal |
| Fat | 0.6 g | 7.2 g | 7.3 g | 0.0 g |
| Saccharides | 79.0 g | 63.1 g | 64.0 | 77.4 g |
| Protein | 7.0 g | 14.4 g | 14.0 g | 0.4 g |
| Sample | Rice Flour | Plantago Addition | Sucrose | Yeast | Salt |
|---|---|---|---|---|---|
| Control | 100 | 0 | 1.86 | 1.80 | 1.5 |
| 3 PPS | 97 | 3 | 1.86 | 1.80 | 1.5 |
| 6 PPS | 94 | 6 | 1.86 | 1.80 | 1.5 |
| 9 PPS | 91 | 9 | 1.86 | 1.80 | 1.5 |
| 3 POS | 97 | 3 | 1.86 | 1.80 | 1.5 |
| 6 POS | 94 | 6 | 1.86 | 1.80 | 1.5 |
| 9 POS | 91 | 9 | 1.86 | 1.80 | 1.5 |
| 3 POH | 97 | 3 | 1.86 | 1.80 | 1.5 |
| 6 POH | 94 | 6 | 1.86 | 1.80 | 1.5 |
| 9 POH | 91 | 9 | 1.86 | 1.80 | 1.5 |
| Blend | Hydration (%) | Dough Development Time (min) | Dough Stability (min) | Dough Softening (FU) | Mixing Tolerance Index (FU) | |
|---|---|---|---|---|---|---|
| Control | 61.2 ± 0.49 g | 2.30 ± 0.14 g | 1.45 ± 0.07 b | 95.0 ± 2.66 c | 128.4 ± 0.74 d | |
| PPS | 3% | 70.3 ± 1.34 f | 2.45 ± 0.07 fg | 1.45 ± 0.07 b | 103.5 ± 0.71 b | 156.5 ± 1.88 c |
| 6% | 73.7 ± 1.44 d | 2.55 ± 0.07 fg | 1.45 ± 0.07 b | 116.0 ± 1.41 a | 164.4 ± 2.13 b | |
| 9% | 74.9 ± 1.46 cd | 2.55 ± 0.21 fg | 1.45 ± 0.07 b | 116.5 ± 2.12 a | 174.2 ± 0.63 a | |
| POS | 3% | 70.8 ± 0.71 ef | 3.55 ± 0.07 e | 1.55 ± 0.07 b | 88.0 ± 1.41 de | 109.4 ± 0.52 f |
| 6% | 74.6 ± 0.64 cd | 4.25 ± 0.07 d | 1.55 ± 0.07 b | 87.0 ± 1.41 e | 97.9 ± 1.13 g | |
| 9% | 75.8 ± 0.62 c | 5.10 ± 0.14 c | 1.60 ± 0.14 b | 87.0 ± 1.41 e | 91.0 ± 0.90 h | |
| POH | 3% | 71.9 ± 0.85 de | 2.65 ± 0.07 f | 1.55 ± 0.07 b | 94.5 ± 2.12 cd | 120.1 ± 0.26 e |
| 6% | 84.5 ± 1.41 b | 6.55 ± 0.07 b | 1.65 ± 0.21 b | 93.5 ± 1.41 cde | 109.1 ± 0.17 f | |
| 9% | 98.1 ± 1.89 a | 8.80 ± 0.14 a | 2.50 ± 0.28 a | 88.5 ± 0.71 cde | 98.6 ± 0.63 g | |
| Blend | C2 (N·m) | C3 (N·m) | C4 (N·m) | C5 (N·m) | C5–C4 (N·m) | |
|---|---|---|---|---|---|---|
| Control | 0.578 ± 0.007 d | 1.613 ± 0.028 a | 1.367 ± 0.019 a | 2.008 ± 0.006 a | 0.642 ± 0.013 a | |
| PPS | 3% | 0.482 ± 0.003 e | 1.390 ± 0.042 d | 1.204 ± 0.025 b | 1.678 ± 0.016 c | 0.475 ± 0.040 b |
| 6% | 0.485 ± 0.006 e | 1.360 ± 0.016 d | 1.090 ± 0.003 de | 1.518 ± 0.008 e | 0.428 ± 0.011 bc | |
| 9% | 0.483 ± 0.004 e | 1.396 ± 0.010 d | 1.020 ± 0.003 e | 1.376 ± 0.002 g | 0.356 ± 0.003 cd | |
| POS | 3% | 0.583 ± 0.036 d | 1.447 ± 0.010 c | 1.220 ± 0.020 b | 1.723 ± 0.004 b | 0.503 ± 0.016 b |
| 6% | 0.655 ± 0.006 c | 1.472 ± 0.014 bc | 1.188 ± 0.007 bc | 1.674 ± 0.010 c | 0.486 ± 0.017 b | |
| 9% | 0.700 ± 0.002 b | 1.514 ± 0.015 b | 1.110 ± 0.017 cd | 1.568 ± 0.013 d | 0.458 ± 0.004 b | |
| POH | 3% | 0.655 ± 0.021 c | 1.484 ± 0.006 bc | 1.116 ± 0.025 cd | 1.716 ± 0.008 b | 0.601 ± 0.044 a |
| 6% | 0.693 ± 0.008 b | 1.479 ± 0.030 bc | 1.094 ± 0.025 de | 1.455 ± 0.011 f | 0.361 ± 0.013 cd | |
| 9% | 0.772 ± 0.017 a | 1.483 ± 0.004 bc | 0.977 ± 0.039 f | 1.277 ± 0.016 h | 0.300 ± 0.040 d | |
| Blend | Resistance to Extension (N) | Dough Energy (N·mm) | Dough Extensibility (mm) | |
|---|---|---|---|---|
| Control | 0.123 ± 0.007 f | 0.863 ± 0.016 d | 10.806 ± 1.291 a | |
| PPS | 3% | 0.136 ± 0.016 ef | 0.885 ± 0.014 d | 9.099 ± 1.268 a |
| 6% | 0.183 ± 0.018 cde | 0.869 ± 0.013 d | 10.664 ± 0.479 a | |
| 9% | 0.230 ± 0.026 c | 0.976 ± 0.021 d | 13.930 ± 1.274 a | |
| POS | 3% | 0.165 ± 0.016 def | 1.140 ± 0.018 d | 11.543 ± 1.152 a |
| 6% | 0.224 ± 0.012 c | 1.539 ± 0.025 c | 11.795 ± 1.284 a | |
| 9% | 0.318 ± 0.036 b | 1.964 ± 0.022 b | 11.130 ± 0.726 a | |
| POH | 3% | 0.214 ± 0.014 cd | 1.452 ± 0.016 c | 12.273 ± 1.488 a |
| 6% | 0.342 ± 0.016 b | 2.049 ± 0.028 b | 11.617 ± 1.097 a | |
| 9% | 0.480 ± 0.026 a | 2.905 ± 0.026 a | 12.020 ± 1.489 a | |
| Blend | Hardness (N) | Cohesiveness (%) | Springiness (%) | Chewiness (-) | Resilience (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Stored | Fresh | Stored | Fresh | Stored | Fresh | Stored | Fresh | Stored | ||
| Control | 26.73 ± 2.21 a | 23.85 ± 2.30 ab | 0.73 ± 0.04 b | 0.68 ± 0.04 bc | 0.69 ± 0.03 b | 0.68 ± 0.04 b | 13.45 ± 1.12 a | 10.94 ± 0.79 abcd | 0.44 ± 0.03 a | 0.39 ± 0.04 ab | |
| PPS | 3% | 22.00 ± 2.78 ab | 14.65 ± 1.37 d | 0.72 ± 0.02 b | 0.72 ± 0.08 abc | 0.73 ± 0.03 ab | 0.76 ± 0.05 b | 11.34 ± 1.09 ab | 8.04 ± 0.43 d | 0.40 ± 0.01 a | 0.41 ± 0.06 ab |
| 6% | 16.95 ± 2.58 bcd | 17.88 ± 0.15 cd | 0.76 ± 0.02 ab | 0.74 ± 0.04 ab | 0.80 ± 0.07 ab | 0.74 ± 0.09 b | 10.46 ± 1.55 abc | 9.99 ± 1.04 abcd | 0.45 ± 0.02 a | 0.44 ± 0.02 a | |
| 9% | 13.89 ± 1.18 cd | 20.51 ± 1.28 bc | 0.75 ± 0.03 ab | 0.68 ± 0.02 bc | 0.80 ± 0.08 ab | 0.72 ± 0.05 b | 8.43 ± 1.05 bcd | 13.41 ± 1.61 a | 0.44 ± 0.01 a | 0.37 ± 0.03 b | |
| POS | 3% | 11.62 ± 1.65 de | 17.48 ± 1.75 cd | 0.75 ± 0.05 ab | 0.73 ± 0.04 abc | 0.70 ± 0.09 b | 0.66 ± 0.08 b | 6.14 ± 1.10 cd | 8.41 ± 0.83 cd | 0.47 ± 0.05 a | 0.43 ± 0.03 a |
| 6% | 14.31 ± 1.86 cd | 17.59 ± 1.39 cd | 0.77 ± 0.06 ab | 0.73 ± 0.03 abc | 0.72 ± 0.05 ab | 0.73 ± 0.07 b | 7.97 ± 0.84 cd | 9.34 ± 0.79 bcd | 0.46 ± 0.04 a | 0.42 ± 0.01 ab | |
| 9% | 19.81 ± 0.16 bc | 23.44 ± 1.72 ab | 0.79 ± 0.02 ab | 0.72 ± 0.00 abc | 0.73 ± 0.04 ab | 0.78 ± 0.05 b | 11.46 ± 1.04 ab | 13.18 ± 0.66 a | 0.45 ± 0.03 a | 0.40 ± 0.01 ab | |
| POH | 3% | 21.87 ± 2.71 ab | 24.75 ± 1.90 a | 0.79 ± 0.03 ab | 0.66 ± 0.02 c | 0.77 ± 0.08 ab | 0.77 ± 0.04 b | 13.09 ± 1.25 ab | 12.68 ± 1.38 ab | 0.47 ± 0.02 a | 0.37 ± 0.04 ab |
| 6% | 15.26 ± 2.20 bcd | 19.26 ± 1.44 c | 0.79 ± 0.02 ab | 0.75 ± 0.03 ab | 0.82 ± 0.10 ab | 0.82 ± 0.09 ab | 9.93 ± 0.65 abc | 11.74 ± 1.45 abc | 0.45 ± 0.01 a | 0.42 ± 0.01 ab | |
| 9% | 5.89 ± 0.39 e | 9.00 ± 0.26 e | 0.81 ± 0.01 a | 0.77 ± 0.02 a | 0.88 ± 0.11 a | 0.86 ± 0.09 ab | 4.28 ± 0.81 d | 5.98 ± 0.43 e | 0.43 ± 0.04 a | 0.40 ± 0.03 ab | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pejcz, E.; Burešová, I. Rheological Characteristics of Model Gluten-Free Dough with Plantago Seeds and Husk Incorporation. Foods 2022, 11, 536. https://doi.org/10.3390/foods11040536
Pejcz E, Burešová I. Rheological Characteristics of Model Gluten-Free Dough with Plantago Seeds and Husk Incorporation. Foods. 2022; 11(4):536. https://doi.org/10.3390/foods11040536
Chicago/Turabian StylePejcz, Ewa, and Iva Burešová. 2022. "Rheological Characteristics of Model Gluten-Free Dough with Plantago Seeds and Husk Incorporation" Foods 11, no. 4: 536. https://doi.org/10.3390/foods11040536
APA StylePejcz, E., & Burešová, I. (2022). Rheological Characteristics of Model Gluten-Free Dough with Plantago Seeds and Husk Incorporation. Foods, 11(4), 536. https://doi.org/10.3390/foods11040536






