Regulation Mechanism of ssDNA Aptamer in Nanozymes and Application of Nanozyme-Based Aptasensors in Food Safety
Abstract
:1. Introduction
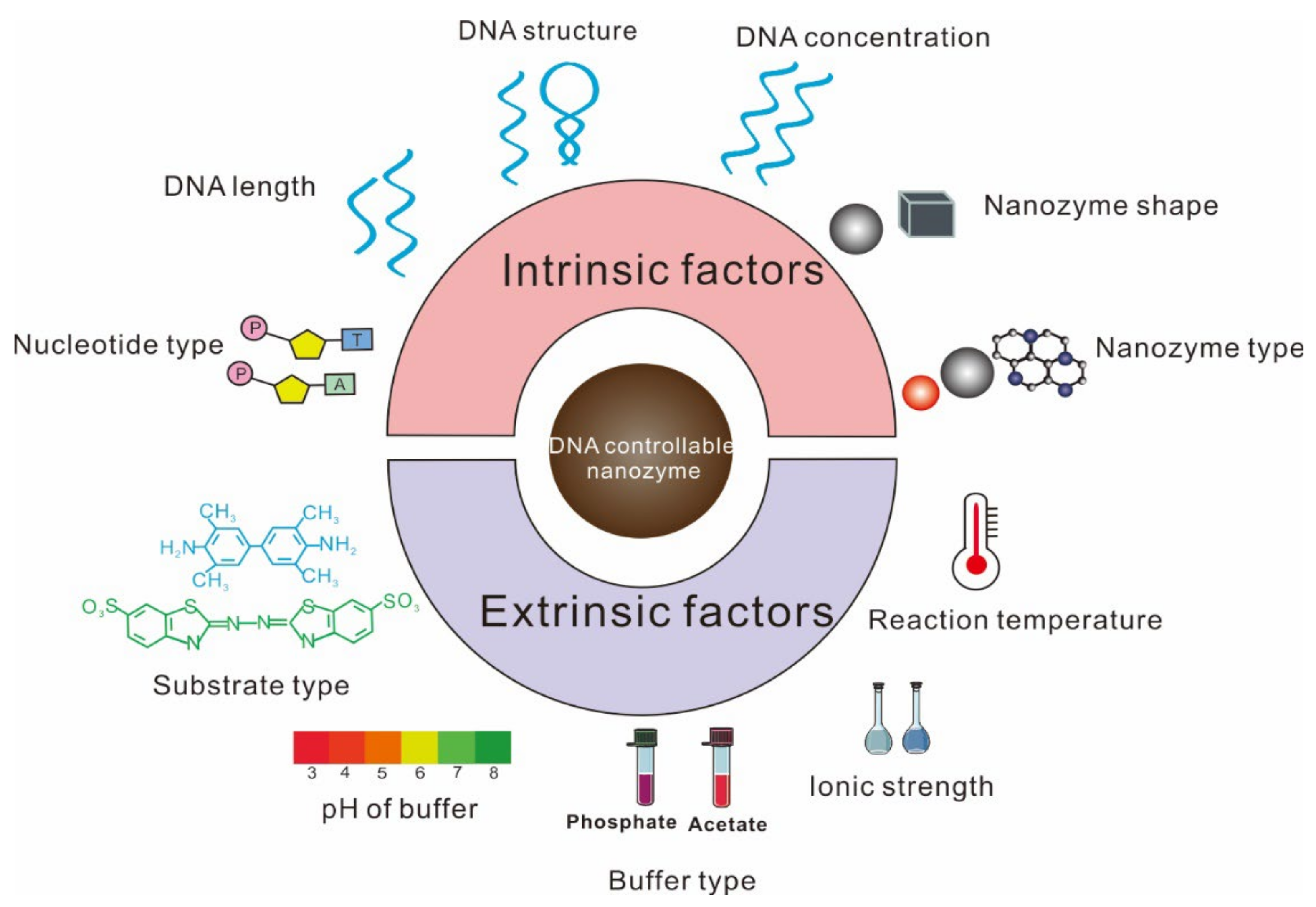
2. Factors Affecting the Regulation by ssDNA of Nanozyme Activity
2.1. Factors Affecting ssDNA Inhibition of Nanozyme Activity
2.2. Factors Affecting ssDNA to Improve Nanozyme Activity
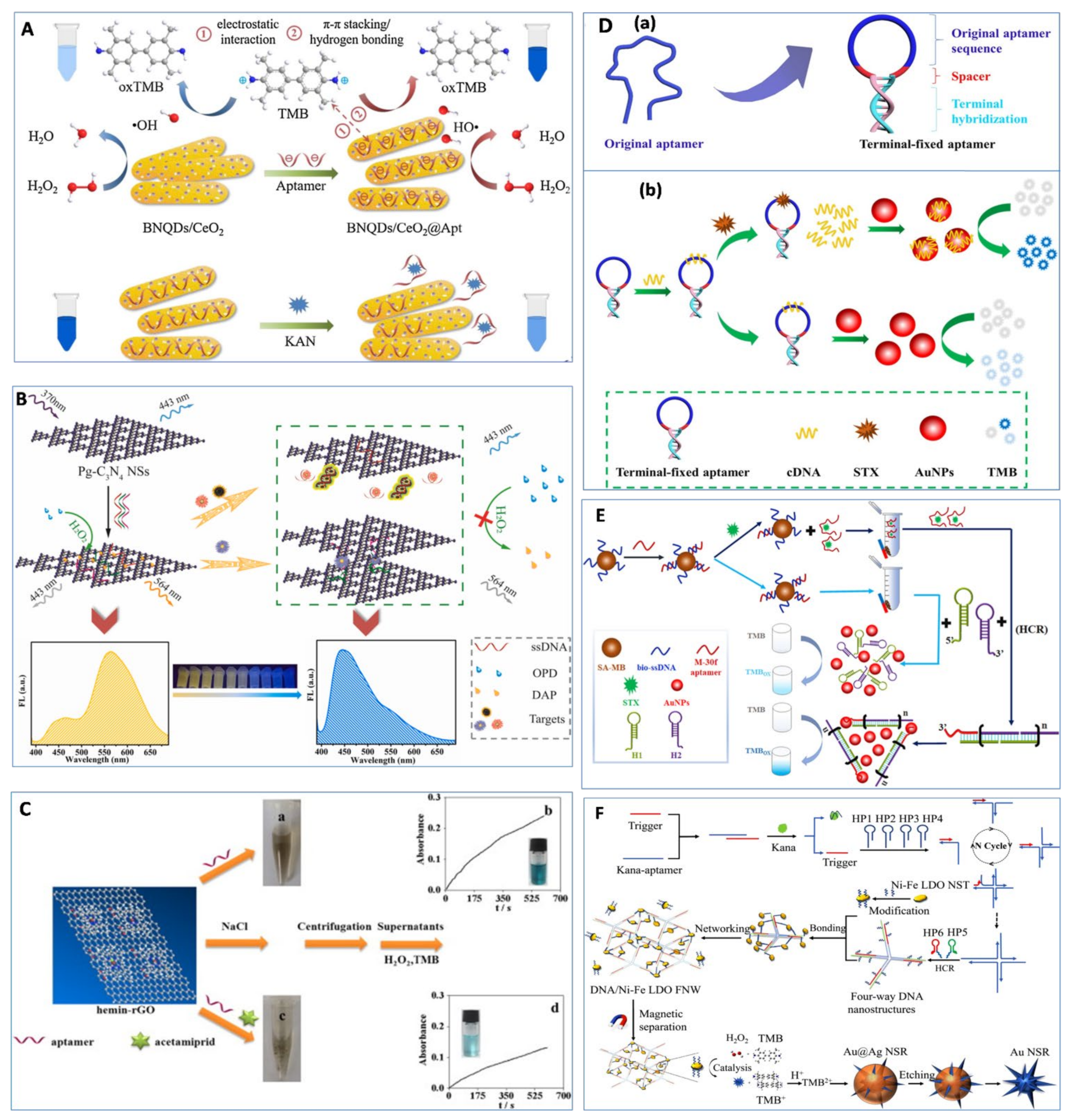
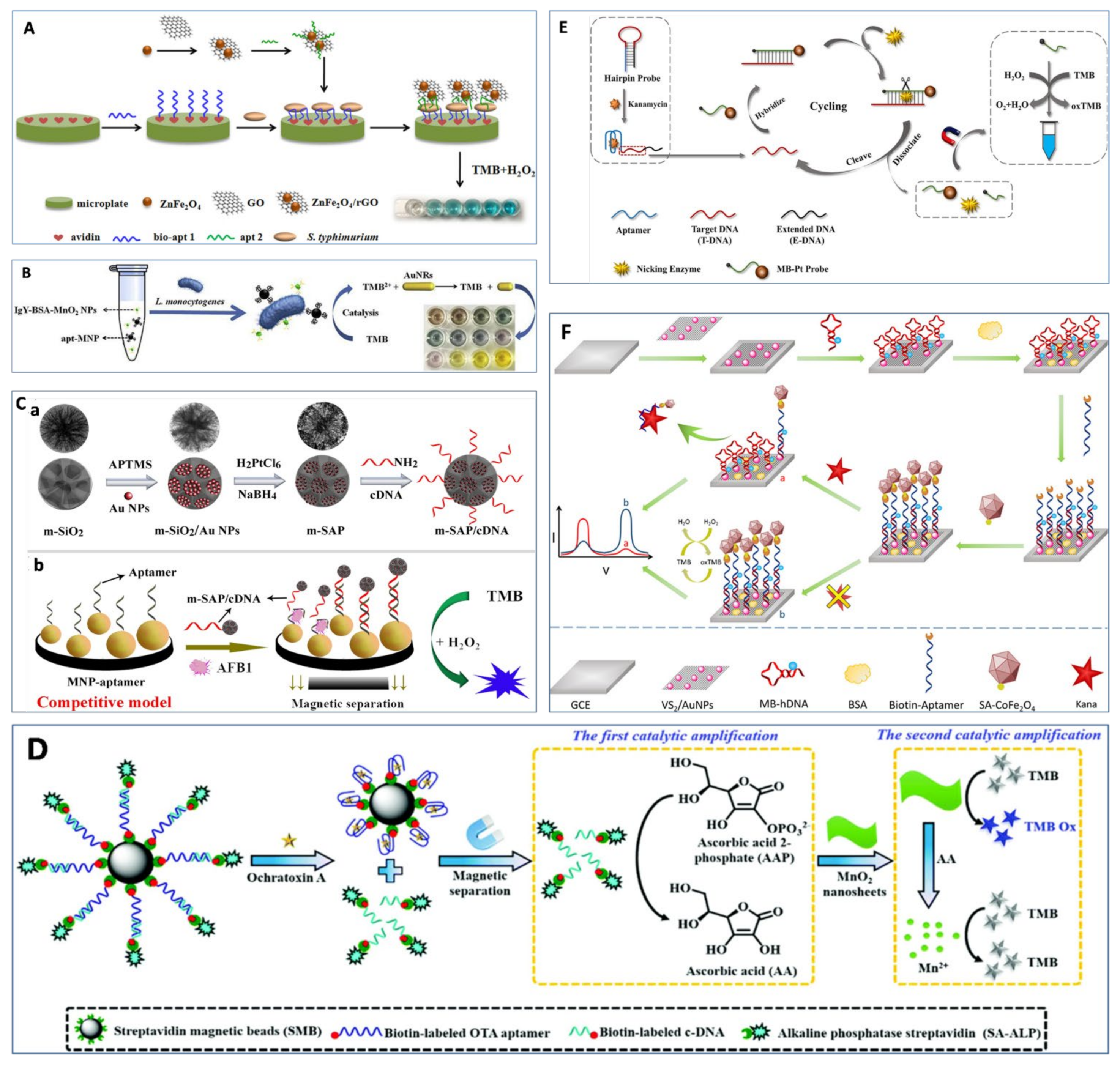
3. Nanozyme-Based Aptasensors
3.1. Nanozyme Activity Inhibited by ssDNA
3.2. Nanozyme Activity Enhanced by ssDNA
3.3. Nanozymes as Signal Tags
3.4. Other Methods Based on Nanozymes and Aptamers
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Han, Y.; Yang, W.; Luo, X.; He, X.; Zhao, H.; Tang, W.; Yue, T.; Li, Z. Carbon dots based ratiometric fluorescent sensing platform for food safety. Crit. Rev. Food Sci. 2020, 62, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Franco, J.L.; Logan, N.; Balasubramanian, P.; Kim, M.I.; Cao, C. Nanozymes in point-of-care diagnosis: An emerging futuristic approach for biosensing. Nano-Micro Lett. 2021, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Czescik, J.; Zamolo, S.; Darbre, T.; Rigo, R.; Sissi, C.; Pecina, A.; Riccardi, L.; De Vivo, M.; Mancin, F.; Scrimin, P. A gold nanoparticle nanonuclease relying on a Zn(II) mononuclear complex. Angew. Chem. Int. Ed. 2021, 60, 1423–1432. [Google Scholar] [CrossRef]
- Nandhakumar, P.; Kim, G.; Park, S.; Kim, S.; Kim, S.; Park, J.K.; Lee, N.S.; Yoon, Y.H.; Yang, H. Metal nanozyme with ester hydrolysis activity in the presence of ammonia-borane and its use in a sensitive immunosensor. Angew. Chem. Int. Ed. 2020, 59, 22419–22422. [Google Scholar] [CrossRef]
- Hu, X.; Huang, T.; Liao, H.; Hu, L.; Wang, M. The phosphatase-like activity of zirconium oxide nanoparticles and their application in near-infrared intracellular imaging. J. Mater. Chem. B 2020, 8, 4428–4433. [Google Scholar] [CrossRef]
- Li, B.; Chen, D.; Nie, M.; Wang, J.; Li, Y.; Yang, Y. Carbon dots/Cu2O composite with intrinsic high protease-Like activity for hydrolysis of proteins under physiological conditions. Part. Part. Syst. Char. 2018, 35, 1800277. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Jin, Y.L.; Cui, H.X.; Yan, X.Y.; Fan, K.L. Nanozyme-based catalytic theranostics. Rsc Adv. 2020, 10, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Yang, W.; Yang, X.; Zhu, L.; Chu, H.; Li, X.; Xu, W. Nanozymes: Activity origin, catalytic mechanism, and biological application. Coordin. Chem. Rev. 2021, 448, 214170. [Google Scholar] [CrossRef]
- Wang, W.Z.; Gunasekaran, S. Nanozymes-based biosensors for food quality and safety. Trends Anal. Chem. 2020, 126, 115841. [Google Scholar] [CrossRef]
- Wu, J.J.X.; Wang, X.Y.; Wang, Q.; Lou, Z.P.; Li, S.R.; Zhu, Y.Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Wang, P.; Min, D.; Chen, G.; Li, M.; Tong, L.; Cao, Y. Inorganic Nanozymes: Prospects for Disease Treatments and Detection Applications. Front. Chem. 2021, 9, 773285. [Google Scholar] [CrossRef]
- Navyatha, B.; Singh, S.; Nara, S. AuPeroxidase nanozymes promises and applications in biosensing. Biosens. Bioelectron. 2021, 175, 112882. [Google Scholar] [CrossRef]
- Ye, M.L.; Zhu, Y.; Lu, Y.; Gan, L.; Zhang, Y.; Zhao, Y.G. Magnetic nanomaterials with unique nanozymes-like characteristics for colorimetric sensors: A review. Talanta 2021, 230, 122299. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A review on metal- and metal oxide-based nanozymes: Properties, mechanisms, and applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- Jin, J.; Li, L.; Zhang, L.; Luan, Z.; Xin, S.; Song, K. Progress in the application of crbon dots-based nanozymes. Front. Chem. 2021, 9, 748044. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon nanozymes: Enzymatic properties, catalytic mechanism, and applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, S.; Tang, Y.; Zhang, X.; Bai, Y.; Pang, H. Advances in metal-organic framework-based nanozymes and their applications. Coordin. Chem. Rev. 2021, 449, 214216. [Google Scholar] [CrossRef]
- Jin, S.; Wu, C.; Ye, Z.; Ying, Y. Designed inorganic nanomaterials for intrinsic peroxidase mimics: A review. Sens. Actuator B Chem. 2019, 283, 18–34. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Tang, Z. Recent progress in the design of analytical methods based on nanozymes. J. Mater. Chem. B 2021, 9, 8174–8184. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, L.; Li, Y. Biosensors for rapid detection of Salmonella in food: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 149–197. [Google Scholar] [CrossRef]
- Tao, X.; Wang, X.; Liu, B.; Liu, J. Conjugation of antibodies and aptamers on nanozymes for developing biosensors. Biosens. Bioelectron. 2020, 168, 112537. [Google Scholar] [CrossRef]
- Yan, M.M.; Chen, G.; She, Y.X.; Ma, J.; Hong, S.H.; Shao, Y.; Abd El-Aty, A.M.; Wang, M.; Wang, S.S.; Wang, J. Sensitive and simple competitive biomimetic nanozyme-linked immunosorbent assay for colorimetric and surface-enhanced raman scattering sensing of triazophos. J. Agric. Food Chem. 2019, 67, 9658–9666. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Hayat, A.; Marty, J.L. Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. Trends Anal. Chem. 2018, 107, 60–77. [Google Scholar] [CrossRef]
- Wang, L.J.; Wang, R.H.; Wei, H.; Li, Y.B. Selection of aptamers against pathogenic bacteria and their diagnostics application. World J. Microb. Biot. 2018, 34, 149. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Y.; Xu, Y.; Yong, W.; Chu, X.G.; Wang, D.N. Aptamer and its potential applications for food safety. Crit. Rev. Food Sci. 2014, 54, 1548–1561. [Google Scholar] [CrossRef]
- Song, S.-H.; Gao, Z.-F.; Guo, X.; Chen, G.-H. Aptamer-based detection methodology studies in food safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Cheng, N.; Wang, X.; Huang, K.; Luo, Y. Recent advances in nucleic acid modulation for functional nanozyme. Catalysts 2021, 11, 638. [Google Scholar] [CrossRef]
- Huang, L.; Sun, D.-W.; Pu, H.; Wei, Q. Development of nanozymes for food quality and safety detection: Principles and recent applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1496–1513. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, D.; Zhou, X.; Yu, Y.; Liu, J.; Hu, N.; Wang, H.; Li, G.; Wu, Y. Recent progress in the construction of nanozyme-based biosensors and their applications to food safety assay. Trends Anal. Chem. 2019, 121, 115668. [Google Scholar] [CrossRef]
- Chang, Y.; Gao, S.; Liu, M.; Liu, J. Designing signal-on sensors by regulating nanozyme activity. Anal. Methods 2020, 12, 4708–4723. [Google Scholar] [CrossRef]
- Sharma, T.K.; Ramanathan, R.; Weerathunge, P.; Mohammadtaheri, M.; Daima, H.K.; Shuklaa, R.; Bansala, V. Aptamer-mediated ‘turn-off turn-on’ nanozyme activity of gold nanoparticles for kanamycin detection. Chem. Commun. 2014, 50, 15856–15859. [Google Scholar] [CrossRef] [Green Version]
- Pautler, R.; Kelly, E.Y.; Huang, P.J.; Cao, J.; Liu, B.; Liu, J. Attaching DNA to nanoceria: Regulating oxidase activity and fluorescence quenching. ACS Appl. Mater. Interfaces 2013, 5, 6820–6825. [Google Scholar] [CrossRef] [Green Version]
- Tarokh, A.; Pebdeni, A.B.; Othman, H.O.; Salehnia, F.; Hosseini, M. Sensitive colorimetric aptasensor based on g-C3N4@Cu2O composites for detection of Salmonella typhimurium in food and water. Microchim. Acta 2021, 188, 87. [Google Scholar] [CrossRef]
- Liu, S.; Yang, M.; Guo, W. Programmable and reversible regulation of catalytichemin@MOFs activities with DNA structures. Chem. Res. Chin. Univ. 2020, 36, 301–306. [Google Scholar] [CrossRef]
- Weerathunge, P.; Ramanathan, R.; Torok, V.A.; Hodgson, K.; Xu, Y.; Goodacre, R.; Behera, B.K.; Bansal, V. Ultrasensitive colorimetric detection of murine norovirus using nanozyme aptasensor. Anal. Chem. 2019, 91, 3270–3276. [Google Scholar] [CrossRef]
- Huang, L.J.; Chen, K.; Zhang, W.T.; Zhu, W.X.; Liu, X.N.; Wang, J.; Wang, R.; Hu, N.; Suo, Y.R.; Wang, J.L. ssDNA-tailorable oxidase-mimicking activity of spinel MnCo2O4 for sensitive biomolecular detection in food sample. Sens. Actuator B Chem. 2018, 269, 79–87. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Zhou, P.; Tao, H.; Wang, X.; Wu, Y. Oligonucleotide-induced regulation of the oxidase-mimicking activity of octahedral Mn3O4 nanoparticles for colorimetric detection of heavy metals. Microchim. Acta 2020, 187, 99. [Google Scholar] [CrossRef]
- Wang, C.; Tang, G.; Tan, H. Colorimetric determination of mercury(II) via the inhibition by ssDNA of the oxidase-like activity of a mixed valence state cerium-based metal-organic framework. Microchim. Acta 2018, 185, 475. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Song, C.; Kong, D.; Ruan, W.; Chang, Z.; Li, Y. Two luminescent metal-organic frameworks for the sensing of nitroaromatic explosives and DNA strands. J. Mater. Chem. A 2014, 2, 2213–2220. [Google Scholar] [CrossRef]
- Wang, L.; Liao, T.; Zhou, H.; Huang, Y.; Chen, P.; Yang, X.; Chen, X. Colorimetric method for Salmonella spp. detection based on peroxidase-like activity of Cu(II)-rGO nanoparticles and PCR. Anal. Biochem. 2021, 615, 114068. [Google Scholar] [CrossRef]
- Kim, M.I.; Park, K.S.; Park, H.G. Ultrafast colorimetric detection of nucleic acids based on the inhibition of the oxidase activity of cerium oxide nanoparticles. Chem. Commun. 2014, 50, 9577–9580. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J. Accelerating peroxidase mimicking nanozymes using DNA. Nanoscale 2015, 7, 13831–13835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Ding, W.; Liu, H.; Zhao, W.; Yao, Y.; Yao, C. Label-free colorimetric detection of deoxyribonuclease I activity based on the DNA-enhanced peroxidase-like activity of MIL-53(Fe). New J. Chem. 2019, 43, 12776–12784. [Google Scholar] [CrossRef]
- Lopez, A.; Zhang, Y.; Liu, J. Tuning DNA adsorption affinity and density on metal oxide and phosphate for improved arsenate detection. J. Coll. Interfaces Sci. 2017, 493, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.M.; Sina, A.A.I.; Carrascosa, L.G.; Shiddiky, M.J.A.; Trau, M. DNA-bare gold affinity interactions: Mechanism and applications in biosensing. Anal. Methods 2015, 7, 7042–7054. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Quan, X. Interface engineering catalytic graphene for smart colorimetric biosensing. ACS Nano 2012, 6, 3142–3151. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, F.; Liao, S.; Chen, M.; Zhu, Y.Q.; Liu, Q.; Chen, X. Single-stranded DNA modified protonated graphitic carbon nitride nanosheets: A versatile ratiometric fluorescence platform for multiplex detection of various targets. Talanta 2019, 197, 422–430. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Su, D.; Zhang, Y.; Lu, H.; Yan, X.; Bai, J.; Gao, Y.; Lu, G. The DNA controllable peroxidase mimetic activity of MoS2 nanosheets for constructing robust colorimetric biosensor. Nanoscale 2020, 12, 19420–19428. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Fan, Y.F.; Du, J.Y.; Song, Z.L.; Zhao, H.M. CNT-modified MIL-88 (NH2)-Fe for enhancing DNA-regulated peroxidase-like activity. J. Anal. Test. 2019, 3, 238–245. [Google Scholar] [CrossRef]
- Zeng, C.; Lu, N.; Wen, Y.; Liu, G.; Zhang, R.; Zhang, J.; Wang, F.; Liu, X.; Li, Q.; Tang, Z.; et al. Engineering nanozymes using DNA for catalytic regulation. ACS Appl. Mater. Inter. 2019, 11, 1790–1799. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, Z.; Zou, Y.; Zhang, J.; Xia, W.; Zhang, R.; He, Z.; Cai, X.; Lin, Y.; Duan, S.; et al. Engineering DNA-nanozyme interfaces for rapid detection of dental bacteria. ACS Appl. Mater. Inter. 2019, 11, 30640–30647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Adkins, G.B.; Shen, W.; Trinh, M.P.; Duan, L.; Jiang, J.; Zhong, W. Enhancement of the intrinsic peroxidase-like activity of graphitic carbon nitride nanosheets by ssDNAs and its application for detection of exosomes. Anal. Chem. 2017, 89, 12327–12333. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, L.; Wang, J.; Peng, B.; Ouyang, X.; Tan, J.; Yu, J.; Feng, H.; Tang, J. Enhanced peroxidase-like activity of boron nitride quantum dots anchored porous CeO2 nanorods by aptamer for highly sensitive colorimetric detection of kanamycin. Sens. Actuator B Chem. 2021, 330, 129318. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, Y.; Zhou, P.; Wang, C.; Tao, H.; Wu, Y. Colorimetric detection of kanamycin residue in foods based on the aptamer-enhanced peroxidase-mimicking activity of layered WS2 nanosheets. J. Agric. Food Chem. 2021, 69, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Hizir, M.S.; Top, M.; Balcioglu, M.; Rana, M.; Robertson, N.M.; Shen, F.; Sheng, J.; Yigit, M.V. Multiplexed activity of perAuxidase: DNA-capped AuNPs act as adjustable peroxidase. Anal. Chem. 2016, 88, 600–605. [Google Scholar] [CrossRef] [Green Version]
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.G.; Malinowska, E. Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8505–8513. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, H.; Lopez, A.; Su, H.; Liu, J. Promotion and inhibition of the oxidase-mimicking activity of nanoceria by phosphate, polyphosphate and DNA. ChemBioChem 2020, 21, 2178–2186. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, S.; Liu, J. Gold nanoparticles adsorb DNA and aptamer probes too strongly, and a comparison with graphene oxide for biosensing. Anal. Chem. 2019, 91, 14743–14750. [Google Scholar] [CrossRef]
- Park, J.Y.; Jeong, H.Y.; Kim, M.I.; Park, T.J. Colorimetric detection system for Salmonella Typhimurium based on peroxidase-like activity of magnetic nanoparticles with DNA aptamers. J. Nanomater. 2015, 2015, 527126. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, Z.; Hosseini, M.; Mohammadnejad, J.; Bakhshi, B.; Rezayan, A.H. Colorimetric aptasensor for Campylobacter jejuni cells by exploiting the peroxidase-like activity of Au@Pd nanoparticles. Microchim. Acta 2018, 185, 448. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, R.; Feng, S.; Xie, Y. Colorimetric zearalenone assay based on the use of an aptamer and of gold nanoparticles with peroxidase-like activity. Microchim. Acta 2018, 185, 535. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Zhang, C.; Fang, Z.; Bai, W.; Yan, M.; Zhu, C.; Chen, A. Aptamer based photometric assay for the antibiotic sulfadimethoxine based on the inhibition and reactivation of the peroxidase-like activity of gold nanoparticles. Microchim. Acta 2016, 184, 59–63. [Google Scholar] [CrossRef]
- Weerathunge, P.; Behera, B.K.; Zihara, S.; Singh, M.; Prasad, S.N.; Hashmi, S.; Mariathomas, P.R.D.; Bansal, V.; Ramanathan, R. Dynamic interactions between peroxidase-mimic silver NanoZymes and chlorpyrifos-specific aptamers enable highly-specific pesticide sensing in river water. Anal. Chim. Acta 2019, 1083, 157–165. [Google Scholar] [CrossRef]
- Li, J.; Yu, C.; Wu, Y.N.; Zhu, Y.; Xu, J.; Wang, Y.; Wang, H.; Guo, M.; Li, F. Novel sensing platform based on gold nanoparticle-aptamer and Fe-metal-organic framework for multiple antibiotic detection and signal amplification. Environ. Int. 2019, 125, 135–141. [Google Scholar] [CrossRef]
- Weerathunge, P.; Ramanathan, R.; Shukla, R.; Sharma, T.K.; Bansal, V. Aptamer-controlled reversible inhibition of gold nanozyme activity for pesticide sensing. Anal. Chem. 2014, 86, 11937–11941. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jurng, J. A simple colorimetric assay for the detection of metal ions based on the peroxidase-like activity of magnetic nanoparticles. Sens. Actuator B Chem. 2013, 176, 253–257. [Google Scholar] [CrossRef]
- Yuan, F.; Zhao, H.; Wang, X.; Quan, X. Determination of oxytetracycline by a graphene-gold nanoparticle-based colorimetric aptamer sensor. Anal. Lett. 2017, 50, 544–553. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Y.; Tao, H.; Chen, H.; Yang, W.; Qiu, S. Colorimetric detection of streptomycin in milk based on peroxidase-mimicking catalytic activity of gold nanoparticles. RSC Adv. 2017, 7, 38471–38478. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Wu, Y.; Tao, H.; Zhao, J.; Chen, H.; Qiu, S. Ultrasensitive and selective colorimetric detection of acetamiprid pesticide based on the enhanced peroxidase-like activity of gold nanoparticles. Anal. Methods 2017, 9, 5484–5493. [Google Scholar] [CrossRef]
- Qi, Y.; He, J.; Xiu, F.-R.; Yu, X.; Gao, X.; Li, Y.; Lu, Y.; Song, Z. A convenient chemiluminescence detection for bisphenol A in E-waste dismantling site based on surface charge change of cationic gold nanoparticles. Microchem. J 2019, 147, 789–796. [Google Scholar] [CrossRef]
- Qi, Y.; Xiu, F.-R.; Zheng, M.; Li, B. A simple and rapid chemiluminescence aptasensor for acetamiprid in contaminated samples: Sensitivity, selectivity and mechanism. Biosens. Bioelectron. 2016, 83, 243–249. [Google Scholar] [CrossRef]
- Xiu, F.R.; Lu, Y.W.; Qi, Y.Y.; Wang, Y.; He, J.H. Ultrasensitive and practical chemiluminescence sensing pesticide residue acetamiprid in agricultural products and environment: Combination of synergistically coupled co-amplifying signal and smart interface engineering. Talanta 2021, 235, 122811. [Google Scholar] [CrossRef]
- Zhu, S.J.; Tang, Y.; Shi, B.; Zou, W.Y.; Wang, X.L.; Wang, C.X.; Wu, Y.G. Oligonucleotide-mediated the oxidase-mimicking activity of Mn3O4 nanoparticles as a novel colorimetric aptasensor for ultrasensitive and selective detection of Staphylococcus aureus in food. Sens. Actuator B Chem. 2021, 349, 130809. [Google Scholar] [CrossRef]
- Das, R.; Dhiman, A.; Kapil, A.; Bansal, V.; Sharma, T.K. Aptamer-mediated colorimetric and electrochemical detection of Pseudomonas aeruginosa utilizing peroxidase-mimic activity of gold NanoZyme. Anal. Bioanal. Chem. 2019, 411, 1229–1238. [Google Scholar] [CrossRef]
- Das, R.; Chaterjee, B.; Kapil, A.; Sharma, T.K. Aptamer-NanoZyme mediated sensing platform for the rapid detection of Escherichia coli in fruit juice. Sens. Bio-Sens. Res. 2020, 27, 100313. [Google Scholar] [CrossRef]
- Wang, C.S.; Liu, C.; Luo, J.B.; Tian, Y.P.; Zhou, N.D. Direct electrochemical detection of kanamycin based on peroxidase-like activity of gold nanoparticles. Anal. Chim. Acta 2016, 936, 75–82. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Lavaee, P.; Emrani, A.S.; Ramezani, M.; Abnous, K. A novel colorimetric triple-helix molecular switch aptasensor based on peroxidase-like activity of gold nanoparticles for ultrasensitive detection of lead (II). RSC Adv. 2015, 5, 43508–43514. [Google Scholar] [CrossRef]
- Dehghani, Z.; Nguyen, T.; Golabi, M.; Hosseini, M.; Rezayan, A.H.; Mohammadnejad, J.; Wolff, A.; Vinayaka, A.C. Magnetic beads modified with Pt/Pd nanoparticle and aptamer as a catalytic nano-bioprobe in combination with loop mediated isothermal amplification for the on-site detection of Salmonella typhimurium in food and fecal samples. Food Control 2021, 121, 107664. [Google Scholar] [CrossRef]
- Lavaee, P.; Danesh, N.M.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Colorimetric aptamer based assay for the determination of fluoroquinolones by triggering the reduction-catalyzing activity of gold nanoparticles. Microchim. Acta 2017, 184, 2039–2045. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Emrani, A.S.; Abnous, K. Novel colorimetric aptasensor for zearalenone detection based on nontarget-induced aptamer walker, gold nanoparticles, and exonuclease-assisted recycling amplification. ACS Appl. Mater. Interfaces 2018, 10, 12504–12509. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Nameghi, M.A.; Zavvar, T.S.; Taghdisi, S.M. A novel colorimetric aptasensor for ultrasensitive detection of aflatoxin M-1 based on the combination of CRISPR-Cas12a, rolling circle amplification and catalytic activity of gold nanoparticles. Anal. Chim. Acta 2021, 1165, 338549. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, Y.; Huang, P.; Wu, F. Using target-specific aptamers to enhance the peroxidase-like activity of gold nanoclusters for colorimetric detection of tetracycline antibiotics. Talanta 2020, 208, 120342. [Google Scholar] [CrossRef]
- Tan, B.; Zhao, H.; Wu, W.; Liu, X.; Zhang, Y.; Quan, X. Fe3O4-AuNPs anchored 2D metal-organic framework nanosheets with DNA regulated switchable peroxidase-like activity. Nanoscale 2017, 9, 18699–18710. [Google Scholar] [CrossRef]
- Hu, C.; Xi, Q.; Ge, J.; Luo, F.; Tang, L.; Jiang, J.; Yu, R. Graphene-hemin hybrid nanosheets as a label-free colorimetric platform for DNA and small molecule assays. RSC Adv. 2014, 4, 64252–64257. [Google Scholar] [CrossRef]
- Hu, J.; Ni, P.; Dai, H.; Sun, Y.; Wang, Y.; Jiang, S.; Li, Z. Aptamer-based colorimetric biosensing of abrin using catalytic gold nanoparticles. Analyst 2015, 140, 3581–3586. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Hong, C.; Lin, Z.; Huang, Z. Rapid detection of malachite green in fish and water based on the peroxidase-like activity of Fe3O4NPs enhanced with aptamer. J. Food Compos. Anal. 2021, 104, 104162. [Google Scholar] [CrossRef]
- Bahadoran, A.; Jabarabadi, M.K.; Mahmood, Z.H.; Bokov, D.; Janani, B.J.; Fakhri, A. Quick and sensitive colorimetric detection of amino acid with functionalized-silver copper nanoparticles in the presence of cross linker. Spectrochim. Acta A 2021, 268, 120636. [Google Scholar] [CrossRef]
- Yang, Z.; Qian, J.; Yang, X.; Jiang, D.; Du, X.; Wang, K.; Mao, H.; Wang, K. A facile label-free colorimetric aptasensor for acetamiprid based on the peroxidase-like activity of hemin-functionalized reduced graphene oxide. Biosens. Bioelectron. 2015, 65, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhong, H.; Zheng, S.; Deng, P.; Li, N.; Yun, W.; Yang, L. A visual detection of bisphenol A based on peroxidase-like activity of hemin–graphene composites and aptamer. Anal. Methods 2018, 10, 2450–2455. [Google Scholar] [CrossRef]
- Chen, X.; Zhai, N.; Snyder, J.H.; Chen, Q.S.; Liu, P.P.; Jin, L.F.; Zheng, Q.X.; Lin, F.C.; Hu, J.M.; Zhou, H.N. Colorimetric detection of Hg2+ and Pb2+ based on peroxidase-like activity of graphene oxide-gold nanohybrids. Anal. Methods 2015, 7, 1951–1957. [Google Scholar] [CrossRef]
- Zhao, C.; Hong, C.; Lin, Z.; Chen, X.; Huang, Z. Detection of Malachite Green using a colorimetric aptasensor based on the inhibition of the peroxidase-like activity of gold nanoparticles by cetyltrimethylammonium ions. Microchim. Acta 2019, 186, 322. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, Y.; Yan, X.; Qi, X.; Wang, L.; Ma, R.; Wang, S.; Mao, X. Development of a terminal-fixed aptamer and a label-free colorimetric aptasensor for highly sensitive detection of saxitoxin. Sens. Actuator B Chem. 2021, 344, 130320. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, L.; Ma, R.; Wang, L.; Yan, X.; Qi, X.; Wang, S.; Mao, X. A competitive colorimetric aptasensor transduced by hybridization chain reaction-facilitated catalysis of AuNPs nanozyme for highly sensitive detection of saxitoxin. Anal. Chim. Acta 2021, 1173, 338710. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, T.Y.; Mao, W.; Zhong, Y.; Dai, S.S.; Zeng, X.M.; Liu, C.; Tang, S.; Qiao, F.; Shi, E.; et al. Three-dimensional DNA/Ni-Fe layered double oxide frame networks-induced “cusp-exposure” of Au@Ag nanostars for ultrasensitive determination of kanamycin. Sens. Actuator B Chem. 2021, 343, 130082. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Qiu, Y.; Li, J.; Wang, Z. Colorimetric aptasensor for the detection of Salmonella enterica serovar Typhimurium using ZnFe2O4-reduced graphene oxide nanostructures as an effective peroxidase mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef]
- Duan, N.; Yang, W.; Wu, S.; Zou, Y.; Wang, Z. A visual and sensitive detection of Escherichia coli based on aptamer and peroxidase-like mimics of copper-metal organic framework nanoparticles. Food Anal. Method. 2020, 13, 1433–1441. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, R.; Liu, W.; Liu, H.; Zhou, X.; Xing, D. Rapid and visual detection of Listeria monocytogenes based on nanoparticle cluster catalyzed signal amplification. Biosens. Bioelectron. 2016, 86, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Song, X.; Xu, K.; Chen, H.; Zhao, C.; Li, J. Colorimetric immunoassay for Listeria monocytogenes by using core gold nanoparticles, silver nanoclusters as oxidase mimetics, and aptamer-conjugated magnetic nanoparticles. Microchim. Acta 2018, 185, 360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Zhao, C.; Guo, X.; Song, X.; Zhao, W.; Liu, S.; Xu, K.; Li, J. A multicolorimetric assay for rapid detection of Listeria monocytogenes based on the etching of gold nanorods. Anal. Chim. Acta 2019, 1048, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, W.; Yang, L.; Tan, Y.; Xie, Q.; Yao, S. Copper-based metal-organic framework nanoparticles with peroxidase-like activity for sensitive colorimetric detection of Staphylococcus aureus. ACS Appl. Mater. Inter. 2017, 9, 24440–24445. [Google Scholar] [CrossRef]
- Yao, S.; Li, J.; Pang, B.; Wang, X.; Shi, Y.; Song, X.; Xu, K.; Wang, J.; Zhao, C. Colorimetric immunoassay for rapid detection of Staphylococcus aureus based on etching-enhanced peroxidase-like catalytic activity of gold nanoparticles. Microchim. Acta 2020, 187, 504. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, M.; Wang, Y.; Liu, J. Nanozyme and aptamer-based immunosorbent assay for aflatoxin B1. J. Hazard. Mater. 2020, 399, 123154. [Google Scholar] [CrossRef]
- Tian, F.Y.; Zho, J.; Jiao, B.N.; He, Y. Nanozyme-based cascade colorimetric aptasensor for amplified detection of ochratoxin A. Nanoscale 2019, 11, 9547–9555. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Z.; Li, B.; Du, J. Aptamer biorecognition-triggered hairpin switch and nicking enzyme assisted signal amplification for ultrasensitive colorimetric bioassay of kanamycin in milk. Food Chem. 2021, 339, 128059. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Y.; Wang, L.B.; Geng, Q.J.; Liu, D.X.; Duan, L.L.; Wang, Y.H.; Cui, J.S. Ratiometric dual signal-enhancing-based electrochemical biosensor for ultrasensitive kanamycin detection. ACS Appl. Mater. Interfaces 2020, 12, 52713–52720. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Li, Q.; Dou, L.; Liu, M.; Shao, S.; Zhu, J.; Shen, J.; Wang, Z.; Wen, K.; et al. Binding affinity-guided design of a highly sensitive noncompetitive immunoassay for small molecule detection. Food Chem. 2021, 351, 129270. [Google Scholar] [CrossRef]
- Sheng, Y.; Liang, J.; Xie, J. Indirect competitive determination of tetracycline residue in honey using an ultrasensitive gold-nanoparticle-linked aptamer assay. Molecules 2020, 25, 2144. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, L.; Tang, L.; Peng, B.; Huang, H.; Wang, J.; Yu, J.; Ouyang, X.; Tan, J. Ultrathin PtNi nanozyme based self-powered photoelectrochemical aptasensor for ultrasensitive chloramphenicol detection. Biosens. Bioelectron. 2019, 146, 111756. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qian, J.; Wang, K.; Yang, X.; Liu, Q.; Hao, N.; Wang, C.; Dong, X.; Huang, X. Colorimetric aptasensing of ochratoxin A using Au@Fe3O4 nanoparticles as signal indicator and magnetic separator. Biosens. Bioelectron. 2016, 77, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ren, C.C.; Wang, C.Q.; An, K.Q.; Cui, H.N.; Hao, N.; Wang, K. Gold nanoparticles mediated designing of versatile aptasensor for colorimetric/electrochemical dual-channel detection of aflatoxin B1. Biosens. Bioelectron. 2020, 166, 112443. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Wei, L.; Wu, S.; Duan, N.; Li, X.; Wang, Z. A colorimetric aptamer-based method for detection of cadmium using the enhanced peroxidase-like activity of Au-MoS2 nanocomposites. Anal. Biochem. 2020, 608, 113844. [Google Scholar] [CrossRef]
- Liu, Q.; He, Z.; Wang, H.; Feng, X.; Han, P. Magnetically controlled colorimetric aptasensor for chlorpyrifos based on copper-based metal-organic framework nanoparticles with peroxidase mimetic property. Microchim. Acta 2020, 187, 524. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, Y.; Duan, N.; Wang, Z. A colorimetric aptamer sensor based on the enhanced peroxidase activity of functionalized graphene/Fe3O4-AuNPs for detection of Lead (II) ions. Catalysts 2020, 10, 600. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, F.; Chi, H.; Zhao, G.; Zhang, Y.; Li, T.; Wei, Q. Electrochemical aptasensor based on gold modified thiol graphene as sensing platform and gold-palladium modified zirconium metal-organic frameworks nanozyme as signal enhancer for ultrasensitive detection of mercury ions. J. Colloid Interf. Sci. 2022, 606, 510–517. [Google Scholar] [CrossRef]
- Chen, G.; Jin, M.; Yan, M.; Cui, X.; Wang, Y.; Zheng, W.; Qin, G.; Zhang, Y.; Li, M.; Liao, Y.; et al. Colorimetric bio-barcode immunoassay for parathion based on amplification by using platinum nanoparticles acting as a nanozyme. Microchim. Acta 2019, 186, 339. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, S.; Song, X.; Xu, K.; Wang, J.; Li, J.; Zhao, C.; Jin, M. One-step colorimetric detection of Staphylococcus aureus based on target-induced shielding against the peroxidase mimicking activity of aptamer-functionalized gold-coated iron oxide nanocomposites. Talanta 2021, 232, 122448. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, R.; Jia, L. Enhancement of the peroxidase-like activity of aptamers modified gold nanoclusters by bacteria for colorimetric detection of Salmonella typhimurium. Talanta 2021, 221, 121476. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, S.; Wang, G.; Yun, Y.; Liu, G.; Zhang, W. Nanozyme applications: A glimpse of insight in food safety. Front. Bioeng. Biotechnol. 2021, 9, 727886. [Google Scholar] [CrossRef] [PubMed]

| S. No. | Contaminants | Nanozymes | Substrates | Nanozyme Reaction Condition (Buffer, Temperature, Reaction Time) | Sensitivity | Sample Matrix | Linear Range | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Kanamycin | AuNPs | TMB/H2O2 | - | 1.49 nM | - | 1 to 100 nM | [34] |
| 2 | S. typhimurium | Fe3O4 nanoparticles | TMB/H2O2 | 0.1 M acetate buffer (pH 4.0), 42 °C, 10 min | 7.5 × 105 CFU/mL | - | - | [62] |
| 3 | S. typhimurium | g-C3N4@Cu2O composites | TMB/H2O2 | -, -, 6 min | 15 CFU/mL | Milk | 15 to 1.5 × 105 CFU/mL | [36] |
| 4 | C. jejuni | Au@Pd nanoparticles | TMB/H2O2 | 20 mM phosphate buffer (pH 7.0), -, - | 100 CFU/mL | Milk | 10 to 106 CFU/mL | [63] |
| 5 | Zearalenone | AuNPs | TMB/H2O2 | -, 25 °C, 15 min | 10 ng/mL | Corn and corn oil | 10 to 250 ng/mL | [64] |
| 6 | Sulfadimethoxine | AuNPs | TMB/H2O2 | -, -, 10 min | 10 ng/mL | Milk | 0.01 to 1000 μg/mL | [65] |
| 7 | Chlorpyrifos | Tyrosine-capped silver nanoparticles | TMB/H2O2 | 37 °C, -, 2 min | 11.3 ppm | River water | 35 to 210 ppm | [66] |
| 8 | Chloramphenicol | Iron-based MOFs | TMB/H2O2 | 0.1 M NaAc–HAc buffer (pH 3.0), 40 °C, 20 min | 25 nM | Tap water | 50 to 200 nM | [67] |
| 9 | Acetamiprid | AuNPs | TMB/H2O2 | -, 37 °C, 10 min | 0.1 ppm | - | 2.5 to 25 ppm | [68] |
| 10 | Hg(II) | Fe3O4 nanoparticles | TMB/H2O2 | 0.2 M acetate buffer (pH 4.0), 25 °C, 10 min | 5 μM | - | 5 to 75 μM | [69] |
| 11 | Murine norovirus | AuNPs | TMB/H2O2 | -, -, 10 min | 30 viruses/mL | Human serum and shellfish homogenate | 200 to 10,000 viruses/mL | [38] |
| 12 | Oxytetracycline | Graphene–gold nanoparticle hybrid | TMB/H2O2 | Citrate buffer (pH 4.0), -, - | 0.91 nM | - | 0.17 to 0.5μM | [70] |
| 13 | Streptomycin | AuNPs | ABTS/H2O2 | 5 mM sodium acetate (pH 4.5), 30 °C, 10 min | 86 nM | Milk | 0.1 to 0.5 μM | [71] |
| 14 | Acetamiprid | AuNPs | ABTS/H2O2 | 3.5 mM NaAc–HAc buffer (pH 5.0), -, - | 1.02 μg/L | Wastewater and tomatoes | 10 to 160 ng/mL | [72] |
| 15 | Bisphenol A | Cationic AuNPs | Luminol/AgNO3 | -, -, - | 62 pg/mL | Soil of an electronic waste dismantling area | 0.1 to 40 ng/mL | [73] |
| 16 | Acetamiprid | AuNPs | Luminol/H2O2 | -, -, - | 62 pM | Wastewater, soil, and cucumber | 0.8 to 6.3 × 102 nM | [74] |
| 17 | Acetamiprid | GO/AuNPs | Luminol/H2O2 | -, -, - | 8.9 pM | Wastewater, soil samples, cucumber, and apple | 2.1 × 10−2 to 9 nM. | [75] |
| 18 | Hg(II) | MVC-MOF nanomaterials | TMB | 50 mM NaAc–HAc buffer (pH 4.0), room temperature, 30 min | 10.5 nM | Environmental water | 0.05 to 6 μM | [41] |
| 19 | Hg(II) | Octahedral Mn3O4 nanoparticles | TMB | 25 mM acetate buffer (pH 3.0), -, - | 3.8 μg/L | Tap water, river water, lake water, and waste water | 10 to 200 μg/L | [40] |
| Cd(II) | 2.4 μg/L | 5 to 100 μM | ||||||
| 20 | S. aureus | Octahedral Mn3O4 nanoparticles | TMB | 20 mM NaAc (pH 3.5), at room temperature, - | 3 CFU/mL | Milk and pork | 10 to 2 × 105 CFU/mL | [76] |
| 21 | OTA | MnCo2O4 submicrospheres | TMB | 0.1 M acetate buffer (pH 4.0), -, 10 min | 0.08 ng/mL | Maize | 0.1 to 10 ng/mL | [39] |
| 22 | P. aeruginosa | AuNPs | TMB/H2O2 | -, -, - | 60 CFU/mL | Water | 60.0 to 6.0 × 107 CFU/mL | [77] |
| 23 | E. coli | AuNPs | TMB/H2O2 | -, at room temperature, 2 min | 10 CFU/mL | Apple juice | 10 to 109 CFU/mL | [78] |
| 24 | Kanamycin | AuNPs | Thionine/H2O2 | 2 mM Hac–NaAc buffer (pH 4.0), 40 °C, 20 min | 0.06 nM | Honey | 0.1 to 60 nM | [79] |
| 25 | Pb(II) | AuNPs | TMB/H2O2 | -, 37 °C, 5 min | 602 pM in pure aqueous solution, 0.708 nM in tap water, 2.07 nM in rat serum. | Water and serum | 0.2 to 30 nM | [76] |
| 26 | S. typhimurium | Pt/Pd nanoparticles | TMB/H2O2 | 10 mM Tris buffer saline (pH 8.0), room temperature, 1 min | 10–15 CFU/mL in chicken meat, 3–10 CFU/mL in whole egg and chicken feces | Chicken meat, whole egg, and chicken feces | - | [81] |
| 27 | Ciprofloxacin | AuNPs | 4-nitrophenol/NaBH4 | -, -, 3 min | 1.2 nM in pure aqueous solution, 1.3 nM in milk, 2.6 nM in serum, and 3.2 nM in milk | Spiked water, serum, and milk | 4 to 500 nM | [82] |
| 28 | Zearalenone | AuNPs | 4-nitrophenol/NaBH4 | -, 23 °C, 7 min | 10 ng/L | Human serum sample | 20 to 80,000 ng/L | [83] |
| 29 | AFM1 | AuNPs | 4-nitrophenol/NaBH4 | -, room temperature, 6 min | 0.05 ng/L | Milk sample | 0.2 to 300 ng/L | [84] |
| S. No. | Contaminants | Nanozymes | Substrates | Nanozyme Reaction condition (Buffer, Temperature, Reaction Time) | Sensitivity | Sample Matrix | Linear Range | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Kanamycin | BNQDs/CeO2 nanorods | TMB/H2O2 | 0.2 M acetate buffer (pH 4.0), 30 °C, 10 min | 4.6 pM | Swine urine, milk, and wastewater | 0.01 to 100 nM | [56] |
| 2 | Tetracycline | AuNCs | TMB/H2O2 | 0.2 M NaAc–HAc buffer (pH 3.8), room temperature, 90 min | 46 nM (spectrophotometer), 0.5 μM (naked-eye detection) | Milk | 1 to 16 μM | [85] |
| 3 | Kanamycin | Layered WS2 nanosheets | TMB/H2O2 | 20 mM NaAc buffer (pH 4.0), -, 50 s | 0.06 µM | Milk, honey, and pork | 0.1 to 0.5 µM | [57] |
| 4 | Sulfadimethoxine | Cu(HBTC)-1/Fe3O4– AuNPs nanosheets | TMB/H2O2 | 0.2 M acetate buffer (pH 4.0), room temperature, 3 min | 1.70 μg/L | Tap water | 3.57 to 357.14 μg/L | [86] |
| 5 | Cocaine | Hemin-functionalized graphene nanosheets | ABTS/H2O2 | 50 mM NaH2PO4 (pH 3.6), -, immediate detection | 230 μM | - | 0.5 to 5 mM | [87] |
| 6 | Abrin | AuNPs | TMB/H2O2 | -, 25 °C, 10 min | 0.05 nM | Raw milk | 0.2 to 17.5 nM | [88] |
| 7 | Malachite green | Fe3O4 nanoparticles | TMB/H2O2 | 20 mM NaAc–HAc (pH 4.0), 37 °C, 10 min | 16.7 μg/kg | Fish and water | 0.06 to 2.38 μM | [89] |
| 8 | S. pneumoniae | Citric acid-functionalized silver/copper nanoparticles | TMB/H2O2 | -, 37 °C, 10 min | 65 CFU/mL | Milk and fruit juice | 102 to 108 CFU/mL | [90] |
| 9 | Hg(II) | Pg–C3N4 nanosheets | OPD/H2O2 | 10 mM NaAc–HAc buffer (pH 4.0), 35 °C, 3 min, | 0.01 nM | Real Xiangjiang River water from Changsha and tap water from laboratory | 0.05 to 100 nM | [50] |
| AFB1 | 0.01 pg/mL | Peanut, maize, and wheat | 0.1 to 60 pg/mL | |||||
| 10 | Acetamiprid | Hemin-rGO | TMB/H2O2 | 25 mM PBS (pH 5.0), 35 °C, 10 min | 40 nM | Wastewater | 0.1 to 10 μM | [91] |
| 11 | Bisphenol A | Hemin-rGO | TMB/H2O2 | -, -, - | 2 nM | Tap water | 5 to 100 nM | [92] |
| 12 | Hg(II) | Graphene oxide–gold nanohybrids | TMB/H2O2 | 33.3 mM sodium acetate buffer (pH 4.3), -, - | 300 nM | River water | 0 to 50 µM | [93] |
| Pb(II) | 500 nM | |||||||
| 13 | Malachite green | AuNPs | TMB/H2O2 | 10 mM NaAc–HAc (pH 4.0), -, 20 min | 1.8 nM | Fresh water and seawater | 10 to 500 nM | [94] |
| 14 | Saxitoxin | AuNPs | TMB/H2O2 | Acetate acid (pH 4.0), -, 15 min. | 142.3 pM | Seawater and scallop | 0.1457 to 37.30 nM | [95] |
| 15 | Saxitoxin | AuNPs | TMB/H2O2 | Acetate acid (pH 4.0), -, 25 °C, | 42.46 pM | Scallop | 78.13 to 2500 pM | [96] |
| 16 | Kanamycin | Ni–Fe LDO | TMB/H2O2 | 0.1 M NaAc–HAc (pH 4.0), -, 5 min | 3 aM | Milk | 0.01 fM to 0.1 nM. | [97] |
| S. No. | Contaminants | Nanozymes | Substrates | Nanozyme Reaction Condition (Buffer, Temperature, Reaction Time) | Sensitivity | Sample Matrix | Linear Range | References |
|---|---|---|---|---|---|---|---|---|
| 1 | S. typhimurium | ZnFe2O4-reduced graphene oxide nanostructures | TMB/H2O2 | NaAc (pH 3.5), -, 20 min | 11 CFU/mL | Milk | 11 to 1.10 × 105 CFU/mL | [98] |
| 2 | E. coli | Cu-MOF | TMB/H2O2 | 0.2 M acetate (pH 4.0), -, 10 min | 2 CFU/mL | Milk | 16 to 1.6 × 106 CFU/mL | [99] |
| 3 | L. monocytogenes | Fe3O4 nanoparticle cluster | TMB/H2O2 | -, -, - | 5.4 × 103 CFU/mL | Milk | 5.4 × 103 to 108 CFU/mL | [100] |
| 4 | L. monocytogenes | Silver nanoclusters | OPD | -, room temperature, 3 min | 10 CFU/mL | Pork | 10 to 106 CFU/mL | [101] |
| 5 | L. monocytogenes | MnO2 | TMB | -, -, - | 10 CFU/mL | Pork | 10 to 106 CFU/mL | [102] |
| 6 | S. aureus | Cu-MOF | TMB/H2O2 | -, 45 °C, 10 min | 20 CFU/mL | Milk | 50 to 10,000 CFU/mL | [103] |
| 7 | S. aureus | AuNPs | TMB/H2O2 | -, -, 5 min | 10 CFU/mL | Pork and milk | 10 to 106 CFU/mL | [104] |
| 8 | Tetracycline | AuNPs | TMB/H2O2 | Buffer (0.08 M Na2HPO4·12H2O, 0.1 M citric acid), -, 15 min | 2.7 pg/mL | Honey | 0.01 to 10 ng/mL | [110] |
| 9 | AFB1 | Mesoporous SiO2/Au–Pt | TMB/H2O2 | -, room temperature, 10 min | 5 pg/mL | Peanut | 0.01 to 1000 ng/mL | [105] |
| 10 | Chloramphenicol | PtNi nanowires | 4-chloro-1-naphthol/H2O2 | -, room temperature, 20 min | 26 fM | Pig urine, river water, and milk | 0.1 pM to 100 nM | [111] |
| 11 | OTA | Au@Fe3O4 nanoparticles | TMB/H2O2 | 0.2 M acetate buffer solution (pH 4.0), 40 °C, 15 min | 30 pg/mL | Cereal | 0.5 to 100 ng/mL | [112] |
| 12 | AFB1 | AuNPs | TMB/H2O2 | 0.2 M acetate buffer solution, 40 °C, 20 min | 0.43 pg/mL | Corn | 5 to 200 ng/mL | [113] |
| 13 | Cd(II) | Au–MoS2 nanocomposites | TMB/H2O2 | -, room temperature, 5 min | 0.7 ng/mL | White wine | 1 to 500 ng/mL | [114] |
| 14 | Chlorpyrifos | Cu-MOF | TMB/H2O2 | -, 40 °C water bath, 15 min | 4.4 ng/mL | Winter jujube, apple, cabbage, and cucumber | 0 to 1250 ng/mL | [115] |
| 15 | OTA | MnO2 nanosheets | TMB | 0.2 M NaAc–HAc (pH 4.5), room temperature, 5 min | 0.069 nM | Grape juice | 1.25 to 250 nM | [106] |
| 16 | Pb(II) | Graphene/Fe3O4–Au nanoparticles | TMB/H2O2 | -, room temperature, 5 min | 0.63 ng/mL | Tap water | 1 to 300 ng/mL | [116] |
| 17 | Hg(II) | AuPd@UiO-67 nanomaterial | H2O2 | -, -, - | 0.16 nM | Tap water and lake water | 1.0 to 103 nM | [117] |
| 18 | Kanamycin | Pt nanoparticles | TMB/H2O2 | -, 45 °C, 10 min | 0.2 pg/mL | Milk | 0.5 to 2 × 105 pg/mL | [107] |
| 19 | Kanamycin | CoFe2O4 nanoparticles | TMB/H2O2 | 0.01 M PBS, -, - | 0.5 pM | Milk | 1 pM to 1 μM | [108] |
| 20 | Parathion | Platinum nanoparticles | TMB/H2O2 | -, -, - | 2 pg/mL | Water, pear, cabbage, and rice | 0.01 to 50 ng/mL | [118] |
| 21 | S. aureus | Fe3O4–Au nanoparticles | TMB/H2O2 | 0.2 M acetate acid –sodium acetate buffer (pH 4.0), room temperature, 2 min | 10 CFU/mL by eye, 26 CFU/mL by spectrophotometer | Tap water, Nanhu Lake water, industrial wastewater, urine sample, and milk | 10 to 106 CFU/mL | [119] |
| 22 | S. typhimurium | AuNCs | TMB/H2O2 | 10 mM PBS (pH 5.5), -, 3 min | 1 CFU/mL | Eggshell and egg white | 10 to 106 CFU/mL | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhou, H.; Hu, H.; Wang, Q.; Chen, X. Regulation Mechanism of ssDNA Aptamer in Nanozymes and Application of Nanozyme-Based Aptasensors in Food Safety. Foods 2022, 11, 544. https://doi.org/10.3390/foods11040544
Wang L, Zhou H, Hu H, Wang Q, Chen X. Regulation Mechanism of ssDNA Aptamer in Nanozymes and Application of Nanozyme-Based Aptasensors in Food Safety. Foods. 2022; 11(4):544. https://doi.org/10.3390/foods11040544
Chicago/Turabian StyleWang, Lijun, Hong Zhou, Haixia Hu, Qin Wang, and Xianggui Chen. 2022. "Regulation Mechanism of ssDNA Aptamer in Nanozymes and Application of Nanozyme-Based Aptasensors in Food Safety" Foods 11, no. 4: 544. https://doi.org/10.3390/foods11040544
APA StyleWang, L., Zhou, H., Hu, H., Wang, Q., & Chen, X. (2022). Regulation Mechanism of ssDNA Aptamer in Nanozymes and Application of Nanozyme-Based Aptasensors in Food Safety. Foods, 11(4), 544. https://doi.org/10.3390/foods11040544







