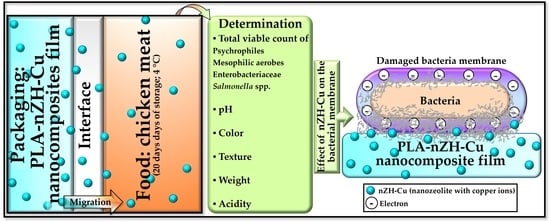
Evaluation of the PLA-nZH-Cu Nanocomposite Film on the Micro-Biological, Organoleptic and Physicochemical Qualities of Packed Chicken Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. nZH-Cu Nanocomposite Films
2.1.2. Chicken Breast Meat
2.1.3. Reagents and Equipment
2.2. Methods
2.2.1. Evaluation of the PLA-nZH-Cu Nanocomposite Film’s Role in the Microbiological Quality of Chicken Breast: Total Viable Count of Psychrophiles, Mesophilic Aerobes, Enterobacteriaceae, and Salmonella spp.
2.2.2. Evaluation of the PLA-nZH-Cu Nanocomposite Film’s Role in the Organoleptic Quality of Chicken Breast: Instrumental Color (Chroma, Hue, and E)
2.2.3. Evaluation of the PLA-nZH-Cu Nanocomposites Film’s Role in the Physicochemical Quality of Chicken Breast: Texture, Weight, pH, and Acidity
2.3. Statistical Analysis (ANOVA)
3. Results and Discussion
3.1. Evaluation of the PLA-nZH-Cu Nanocomposite Film’s Role in the Microbiological Quality of Chicken Breast: Total Viable Count of Psychrophilic, Mesophilic Aerobes, Enterobacteriaceae, and Salmonella spp.
3.2. Evaluation of the PLA-nZH-Cu Nanocomposite Film’s Role in the Organoleptic Quality of Chicken Breast: Instrumental Color (Chroma, Hue, and E)
3.3. Evaluation of the PLA-nZH-Cu Nanocomposite Film’s Role in the Physicochemical Quality of Chicken Breast: Texture, Weight, pH, and Acidity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barros Velázquez, J. Antimicrobial Food Packaging; Elsevier: San Diego, CA, USA, 2016; ISBN 9780128007235. [Google Scholar]
- White, D.G.; Shao, S.; Simjee, S.; Wagner, D.D.; McDermott, P.F. Antimicrobial Resistance in Foodborne Pathogens. Microbes Infect. 2002, 4, 405–412. [Google Scholar] [CrossRef]
- Cooperativa, C. Región Metropolitana Registra 60 Brotes de Salmonella Sólo Este Año. Available online: https://www.cooperativa.cl/noticias/pais/salud/region-metropolitana-registra-60-brotes-de-salmonella-solo-este-ano/2019-03-19/082614.html (accessed on 23 August 2019).
- Diario Concepcción, C. Salmonella. Available online: https://www.diarioconcepcion.cl/carta-al-director/2019/03/25/salmonella.html (accessed on 23 August 2019).
- Diario Soychile, C. Seremi de Salud Confirma Intoxicación de 18 Personas Por Salmonella En Iquique. Available online: https://www.soychile.cl/Iquique/Policial/2019/04/16/591263/Seremi-de-salud-confirma-intoxicacion-de-18-personas-por-Salmonella-en-Iquique.aspx (accessed on 23 August 2019).
- FAO, O.; de las N.U. para la A. y la A. Producción y Productos Avícolas. Available online: http://www.fao.org/poultry-production-products/production/es/ (accessed on 20 November 2020).
- Gill, C.O. Intrinsic Bacteria in Meat. J. Appl. Bacteriol. 1979, 47, 367–378. [Google Scholar] [CrossRef]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver Nanoparticles in Polymeric Matrices for Fresh Food Packaging. J. King Saud Univ.-Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Carter, A.J.; Adams, M.R.; Woodward, M.J.; La Ragione, R.M. Control Strategies for Salmonella Colonisation of Poultry: The Probiotic Perspective. Food Sci. Technol. Bull. Funct. Foods 2009, 5, 103–115. [Google Scholar] [CrossRef]
- Quintavalla, S.; Vicini, L. Antimicrobial Food Packaing in Meat Industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef]
- USDA La Etiqueta Del Pollo Dice “Fresco (A)”. Available online: https://www.fsis.usda.gov/wps/portal/informational/en-espanol/hojasinformativas/manejo-adecuado-de-alimentos/etiqueta-del-pollo/etiqueta-del-pollo (accessed on 10 October 2019).
- Automercado Recomendaciones Para El Manejo de Las Carnes En Manos Del Consumidor. Available online: https://automercadoesmilugar.com/recomendaciones-para-el-manejo-de-las-carnes-en-manos-del-consumidor/ (accessed on 10 October 2019).
- Montville, T.; Mtthews, K.; Kniel, K. Food Microbiology. An Introduction, 3rd ed.; ASM Press: Washington, DC, USA, 2012; ISBN 9781555816360. [Google Scholar]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology, 7th ed.; Springer: New York, NY, USA, 2006; ISBN 0-387-23413-6. [Google Scholar]
- Jorquera, D.; Galarce, N.; Borie, C. El Desafio de Controlar Las Enfermedades Transmitidas Por Alimentos: Bacteriófagos Como Una Nueva Herramienta Biotecnológica. Rev. Chil. Infectologiía 2015, 32, 678–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado de Melo, A.A.; Geraldine, R.M.; Silveira Araujo, M.F.; Lopes Torres, M.C.; Minafra e Rezende, C.S.; Fernandes, T.H.; Nonato De Oliveira, A. Microbiological Quality and Other Characteristics of Refrigerated Chicken Meat in Contact with Cellulose Acetate-Based Film Incorporated with Rosemary Essential Oil. Braz. J. Microbiol. 2012, 1419–1427. [Google Scholar] [CrossRef]
- Mead, G. Microbiological Quality of Poultry Meat: A Review. Braz. J. Poult. Sci. 2004, 6, 135–142. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Vázquez-Olmos, A.; Vega-Jiménez, A.; Paz-Díaz, B. Mecanosíntesis y Efecto Antimicrobiano de Óxidos Metálicos Nanoestructurados. Mundo Nano. Rev. Interdiscip. en Nanociencia y Nanotecnología 2018, 11, 29–44. [Google Scholar] [CrossRef]
- Amna, T.; Yang, J.; Ryu, K.S.; Hwang, I.H. Electrospun Antimicrobial Hybrid Mats: Innovative Packaging Material for Meat and Meat-Products. J. Food Sci. Technol. 2015, 52, 4600–4606. [Google Scholar] [CrossRef] [PubMed]
- Baeza Pinto, S. Situación de Las Enfermedades de Transmisión Alimentaria En Chile; Santiago, Chile, 2016. Available online: https://www.achipia.gob.cl/wp-content/uploads/2016/03/Silvia-Baeza-Minsal-Situacion-de-las-Enfermedades-de-Transmision-Alimentaria-en-Chile-1.pdf (accessed on 30 March 2017).
- Faúndez, G.; Troncoso, M.; Navarrete, P.; Figueroa, G. Antimicrobial Activity of Copper Surfaces against Suspensions of Salmonella Enterica and Campylobacter Jejuni. BMC Microbiol. 2004, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministerio de Salud, G. de Ch. Previene Las Enfermedades Transmitidas Por Los Alimentos. Available online: https://www.minsal.cl/minsal-llama-a-prevenir-enfermedades-transmitidas-por-alimentos-e-informa-sobre-el-uso-de-la-red-asistencial-en-periodo-estival/ (accessed on 30 March 2017).
- Vergara-Figueroa, J.; Alejandro-Martin, S.; Cerda-Leal, F.; Gacitúa, W. Dual Electrospinning of a Nanocomposites Biofilm: Potential Use as an Antimicrobial Barrier. Mater. Today Commun. 2020, 25, 101671. [Google Scholar] [CrossRef]
- Conte, A.; Longano, D.; Costa, C.; Ditaranto, N.; Ancona, A.; Cioffi, N.; Scrocco, C.; Sabbatini, L.; Contò, F.; Del Nobile, M.A. A Novel Preservation Technique Applied to Fiordilatte Cheese. Innov. Food Sci. Emerg. Technol. 2013, 19, 158–165. [Google Scholar] [CrossRef]
- Gavara, R.; López Carballo, G.; Hernández Munoz, P.; Catalá, R.; Muriel Galet, V.; Cerisuelo, J.; Domínguez, I. Practical Guide to Antimicrobial Active Packaging; First.; Smithers Pira Technology Ltd.: Shawbury, UK, 2015; ISBN 9781910242735. [Google Scholar]
- Romero-Bastida, C.A.; Zamudio-Flores, P.B.; Bello-Pérez, L.A. Antimicrobianos En Películas de Almidón Oxidado de Plátano: Efecto Sobre La Actividad Antibacteriana, Microestructura, Propiedades Mecánicas y de Barrera. Rev. Mex. Ing. Química 2011, 10, 445–453. [Google Scholar]
- Wang, Z.; Pan, Z.; Wang, J.; Zhao, R. A Novel Hierarchical Structured Poly(Lactic Acid)/Titania Fibrous Membrane with Excellent Antibacterial Activity and Air Filtration Performance. J. Nanomater. 2016, 2016, 17. [Google Scholar] [CrossRef]
- El-Wakil, A.E.A.A.; Moustafa, H.; Youssef, A.M. Antimicrobial Low-Density Polyethylene/Low-Density Polyethylene-Grafted Acrylic Acid Biocomposites Based on Rice Bran with Tea Tree Oil for Food Packaging Applications. J. Thermoplast. Compos. Mater. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Moustafa, H.; Karmalawi, A.M.; Youssef, A.M. Development of Dapsone-Capped TiO2 Hybrid Nanocomposites and Their Effects on the UV Radiation, Mechanical, Thermal Properties and Antibacterial Activity of PVA Bionanocomposites. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100482. [Google Scholar] [CrossRef]
- Moustafa, H.; Darwish, N.A.; Youssef, A.M. Rational Formulations of Sustainable Polyurethane/Chitin/Rosin Composites Reinforced with ZnO-Doped-SiO2 Nanoparticles for Green Packaging Applications. Food Chem. 2022, 371, 131193. [Google Scholar] [CrossRef]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT Bionanocomposites with Antimicrobial Natural Rosin for Green Packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef]
- Hall, I.J.; Paladino, E.; Szabó, P.; Brozio, S.; Hall, P.J.; Oseghale, C.I.; Passarelli, M.K.; Moug, S.J.; Black, R.A.; Wilson, C.G.; et al. Electrospun Collagen-Based Nanofibres: A Sustainable Material for Improved Antibiotic Utilisation in Tissue Engineering Applications. Int. J. Pharm. 2017, 531, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. Polylactic Acid: PLA Biopolymer Technology and Applications; Andrew, W., Ed.; Plastics Design Library, Elsevier: Waltham, MA, USA, 2012; ISBN 978-1-4377-4459-0. [Google Scholar]
- Abdulkhani, A.; Hosseinzadeh, J.; Ashori, A.; Dadashi, S.; Takzare, Z. Preparation and Characterization of Modified Cellulose Nanofibers Reinforced Polylactic Acid Nanocomposite. Polym. Test. 2014, 35, 73–79. [Google Scholar] [CrossRef]
- Dıblan, S.; Kaya, S. Antimicrobials Used in Active Packaging Films. Food Health 2018, 4, 63–79. [Google Scholar] [CrossRef]
- Hernández-Sánchez, H.; Gutiérrez-López, G. Food Nanoscience and Nanotechnology, 1st ed.; Hernández-Sánchez, H., Gutiérrez-López, G., Eds.; Springer: Pullman, WA, USA, 2015; ISBN 9783319135953. [Google Scholar]
- Kayaci, F.; Umu, O.C.O.; Tekinay, T.; Uyar, T. Antibacterial Electrospun Poly(Lactic Acid) (PLA) Nanofibrous Webs Incorporating Triclosan/Cyclodextrin Inclusion Complexes. J. Agric. Food Chem. 2013, 61, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.H.; Silva, J.A.; Navarro, F.; López-Dellamary, F.; Robledo, J.R. Síntesis de Nanocompuestos de Celulosa Para Aplicaciones Biomédicas En Base a Sus Propiedades Mecánicas. Rev. Iberoam. Polímeros 2014, 15, 275–285. [Google Scholar]
- Serna, L.; Albán, F. Ácido Poliláctico (PLA): Propiedades y Aplicaciones. Rev. Ing. y Compet. 2011, 5, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Anis, S.F.; Khalil, A.; Singaravel, G.P.; Hashaikeh, R. Fabrication of Micro and Nano-Zeolites through Electrospinning. In Proceedings of the Conference Paper, Sapporo, Japan, 7–10 July 2015; p. 3. [Google Scholar]
- Ifkovits, J.; Sundararaghavan, H.; Burdick, J. Electrospinning Fibrous Polymer Scaffolds for Tissue Engineering and Cell Culture. J. Vis. Exp. NIH 2010, 27, 590–609. [Google Scholar] [CrossRef]
- Luo, H.; Huang, Y.; Wang, D.; Shi, J. Coaxial-Electrospinning as a New Method to Study Confined Crystallization of Polymer. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 376–383. [Google Scholar] [CrossRef]
- Pandey, J.; Takagi, H.; Norio, A.; Kim, H.-J. Handbook of Polymer Nanocomposites. Processing, Performance and Application; Springer: Seoul, Korea, 2015; Volume C, ISBN 978-3-642-38648-0. [Google Scholar]
- Vongsetskul, T.; Chantarodsakun, T.; Wongsomboon, P.; Rangkupan, R.; Tangboriboonrat, P. Effect of Solvent and Processing Parameters on Electrospun Polyvinylpyrrolidone Ultra-Fine Fibers. Chiang Mai J. Sci. 2015, 42, 436–442. [Google Scholar]
- Wendorff, J.; Agarwal, S.; Greiner, A. Electrospinning. Materials, Processing, and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weingheim, Germany, 2012; ISBN 978-3-527-64773-6. [Google Scholar]
- Brody, A.; Strupinsky, E.; Kline, L. Active Packaging for Food Applications, 1st ed.; CRC Press: Washington, DC, USA, 2001; ISBN 9780367397289. [Google Scholar]
- Alswat, A.A.; Ahmad, M.B.; Hussein, M.Z.; Ibrahim, N.A.; Saleh, T.A. Copper Oxide Nanoparticles-Loaded Zeolite and Its Characteristics and Antibacterial Activities. J. Mater. Sci. Technol. 2017, 33, 889–896. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active Chicken Meat Packaging Based on Polylactide Films and Bimetallic Ag–Cu Nanoparticles and Essential Oil. J. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Figueroa, J.; Alejandro-Martín, S.; Pesenti, H.; Cerda, F.; Fernández-Pérez, A.; Gacitúa, W. Obtaining Nanoparticles of Chilean Natural Zeolite and Its Ion Exchange with Copper Salt (Cu2+) for Antibacterial Applications. Materials 2019, 12, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castañeda Serrano, M. del P.; Braña Varela, D.; Rosario Cortés, C.; Martínez Valdéz, W. Calidad Microbiológica de La Carne de Pollo; Facultad de Medicina y Zootecnia. Universidad Nacional Autónoma de México.: Ajuchitlán, México, 2013; Volume 1, ISBN 9786073700962. [Google Scholar]
- de Ch Minsal, G. Recomendaciones Para El Manejo de Alimentos En Situaciones de Emergencia, 2 March 2010, 1–9. Available online: https://www.minsal.cl/portal/url/item/814d800866cc95a1e04001011e01532b.pdf (accessed on 30 March 2017).
- Soler, M.; Mateos, M.; Safon, E.; Soler, P.; Garcés, C. Caracterización Del Color y Relación Con El PH de Pechuga de Pollo Durante El Procesado de Las Canales En Matadero. XLVIII Simp. Científico Avic. 2011, XLVIII, 4. [Google Scholar]
- Mathias-Rettig, K.; Ah-Hen, K. El Color En Los Alimentos Un Criterio de Calidad Medible. Agro Sur 2014, 42, 39–48. [Google Scholar] [CrossRef]
- X-Rite, I. Guía Para Entender La Comunicación Del Color. Inc. X-Rite 2002, 2, 26. [Google Scholar]
- Braña Varela, D.; Ramírez Rodríguez, E.; de la Rubio Lozano, M.S.; Sánchez Escarlante, A.; Torrescano Urrutia, G.; Arenas de Moreno, M.L.; Partida de la Peña, J.A.; Ponce de Alquiricia, E.; Ríos Rincón, F.G. Manual de Análisis de Calidad En Muestras de Carne; Centro Nacional de Investigación Disciplinaria en Fisiología y Mejoramiento Animal: Ajuchitlán, Mexico, 2011; ISBN 9786074256123. [Google Scholar]
- Graciano, A.; Peralta, E.; Soto-Valdez, H. Permeabilidad y Vida Útil de Los Alimentos. In AlimenPack; Hermosillo: Sonora, Mexico, 2006; pp. 15–19. [Google Scholar]
- Heredia, N.; Dávila-Aviña, J.E.; Solís Soto, L.; García, S. Productos Cárnicos: Principales Patógenos y Estrategias No Térmicas de Control. Nacameh 2014, 8, 20–42. [Google Scholar] [CrossRef]
- Ministerio de Salud, C. Norma General Técnica Sobre Inspección Médico Veterinaria de Aves de Corral y de Sus Carnes. Subsecr. Salud Pública 2010, 117, 1–48. [Google Scholar]






| Bacteria Group | Characteristics |
|---|---|
| Psychrophile | The optimal growth temperature of psychrophiles is low, and they can reproduce in refrigeration conditions (4 °C). They are the main bacteria responsible for the deterioration of refrigerated foods of animal origin. |
| Mesophile aerobes | Mesophile aerobes have intermediate thermal characteristics. Their presence shows the sanitary and hygienic quality in the elaboration of food. |
| Enterobacteriaceae | Such microorganisms are found in the intestine and feces of animals, birds, and man. As they do not resist high temperatures they survive under refrigeration and drying conditions. Their presence in food accounts for the lack of hygiene and rigorous handling of food due to cross contamination. |
| Salmonella spp. | Enterobacteriaceae usually contaminate raw meat, poultry, eggs, and dairy products. Salmonella spp. can sometimes cause serious infections in young children, the elderly, and people with weakened immune systems. |
| Microorganism | Treatment | Number of Days of Storage | ||
|---|---|---|---|---|
| 0 | 10 | 20 | ||
| Psychrophiles | Control | 2.36 ± 0.13 | 5.80 ± 0.04 a | 8.47 ± 0.10 a |
| PLA | - | 4.87 ± 0.02 b | 8.35 ± 0.13 b | |
| PLA-nZH (3%) | - | 4.82 ± 0.02 c | 7.90 ± 0.09 d | |
| PLA-nZH-Cu (1%) | - | 4.74 ± 0.08 d | 7.75 ± 0.21 e | |
| PLA-nZH-Cu (2%) | - | 4.91 ± 0.54 b | 8.27 ± 0.13 c | |
| PLA-nZH-Cu (3%) | - | 4.79 ± 0.01 c | 7.70 ± 0.02 d | |
| Mesophile aerobes | Control | 3.10 ± 0.13 | 4.54 ± 0.06 a | 9.05 ± 0.04 a |
| PLA | - | 4.34 ± 0.30 c | 8.70 ± 0.01 c | |
| PLA-nZH (3%) | - | 4.12 ± 0.06 d | 8.92 ± 0.12 b | |
| PLA-nZH-Cu (1%) | - | 4.27 ± 0.11 c | 8.87 ± 0.04 b | |
| PLA-nZH-Cu (2%) | - | 4.48 ± 0.12 b | 8.74 ± 0.09 c | |
| PLA-nZH-Cu (3%) | - | 3.83 ± 0.46 e | 8.98 ± 0.04 a | |
| Enterobacteriaceae | Control | 1.74 ± 0.22 | 2.66 ± 0.37 b | 7.96 ± 0.19 a |
| PLA | - | 2.64 ± 0.02 b | 7.78 ± 0.05 c | |
| PLA-nZH (3%) | - | 2.49 ± 0.33 c | 7.92 ± 0.04 a | |
| PLA-nZH-Cu (1%) | - | 2.85 ± 0.06 a | 7.87 ± 0.04 b | |
| PLA-nZH-Cu (2%) | - | 2.51 ± 0.18 c | 7.81 ± 0.34 c | |
| PLA-nZH-Cu (3%) | - | 2.14 ± 0.09 d | 7.52 ± 0.06 d | |
| Salmonella spp. | Control | Absence | Absence | Absence |
| PLA | - | Absence | Absence | |
| PLA-nZH (3%) | - | Absence | Absence | |
| PLA-nZH-Cu (1%) | - | Absence | Absence | |
| PLA-nZH-Cu (2%) | - | Absence | Absence | |
| PLA-nZH-Cu (3%) | - | Absence | Absence | |
| Number of Days of Storage | Treatment | Instrumental Color | ||
|---|---|---|---|---|
| Chroma | Hue | E | ||
| 0 | Control | 11.85 ± 2.87 | 1.39 ± 0.08 | 0.00 ± 0.00 |
| 10 | Control | 9.11 ± 1.44 a | 1.38 ± 0.01 a | 5.28 ± 3.52 b |
| PLA | 10.29 ± 1.33 a | 1.28 ± 0.10 a | 8.59 ± 6.91 a | |
| PLA-nZH (3%) | 8.66 ± 2.08 a | 1.18 ± 0.14 a | 9.54 ± 4.96 a | |
| PLA-nZH-Cu (1%) | 7.99 ± 4.57 b | 1.20 ± 0.38 a | 6.24 ± 0.48 b | |
| PLA-nZH-Cu (2%) | 9.82 ± 1.13 a | 1.38 ± 0.18 a | 7.98 ± 3.89 b | |
| PLA-nZH-Cu (3%) | 7.94 ± 2.90 b | 0.62 ± 0.03 b | 9.75 ± 2.21 a | |
| 20 | Control | 11.07 ± 2.77 a | 1.08 ± 0.05 a | 9.35 ± 3.96 b |
| PLA | 12.76 ± 2.42 a | 1.23 ± 0.17 a | 8.62 ± 3.71 b | |
| PLA-nZH (3%) | 11.23 ± 0.40 a | 1.21 ± 0.23 a | 9.45 ± 3.66 b | |
| PLA-nZH-Cu (1%) | 9.15 ± 0.88 b | 1.13 ± 0.10 a | 10.03 ± 7.47 b | |
| PLA-nZH-Cu (2%) | 9.81 ± 0.28 b | 1.38 ± 0.14 a | 8.37 ± 4.35 b | |
| PLA-nZH-Cu (3%) | 11.70 ± 1.23 a | 1.09 ± 0.13 a | 12.15 ± 5.89 a | |
| Number of Days of Storage | Treatment | Texture | Weight | Acidity and pH | |
|---|---|---|---|---|---|
| Firmness (N) | Lost (%) | Lactic Acid (%) | pH | ||
| 0 | Control | 8.53 ± 1.37 | 0.00 ± 0.00 | 0.13 ± 0.02 | 6.37 ± 0.32 |
| 10 | Control | 12.40 ± 3.04 b | 6.64 ± 1.96 b | 0.11 ± 0.02 a | 6.47 ± 0.33 a |
| PLA | 21.74 ± 14.77 a | 12.32 ± 1.31 a | 0.09 ± 0.04 a | 6.54 ± 0.07 a | |
| PLA-nZH (3%) | 12.82 ± 5.58 b | 10.06 ± 1.30 a | 0.11 ± 0.01 a | 6.43 ± 0.04 a | |
| PLA-nZH-Cu (1%) | 20.18 ± 9.74 a | 10.45 ± 2.48 a | 0.13 ± 0.00 a | 6.15 ± 0.05 a | |
| PLA-nZH-Cu (2%) | 13.79 ± 2.67 b | 7.39 ± 0.69 b | 0.13 ± 0.00 a | 6.15 ± 0.06 a | |
| PLA-nZH-Cu (3%) | 13.09 ± 0.98 b | 9.10 ± 0.54 a | 0.11 ± 0.01 a | 6.41 ± 0.38 a | |
| 20 | Control | 20.79 ± 1.55 b | 10.59 ± 3.89 b | 0.10 ± 0.02 a | 6.89 ± 0.08 a |
| PLA | 19.98 ± 5.76 b | 18.64 ± 1.17 a | 0.07 ± 0.01 a | 7.60 ± 0.08 a | |
| PLA-nZH (3%) | 25.06 ± 15.13 a | 14.37 ± 1.72 b | 0.07 ± 0.01 a | 7.59 ± 0.12 a | |
| PLA-nZH-Cu (1%) | 21.06 ± 5.49 b | 12.10 ± 0.78 b | 0.07 ± 0.02 a | 7.57 ± 0.29 a | |
| PLA-nZH-Cu (2%) | 19.14 ± 3.93 b | 11.61 ± 1.42 b | 0.08 ± 0.01 a | 7.21± 0.18 a | |
| PLA-nZH-Cu (3%) | 17.21 ± 2.69 b | 10.96 ± 1.38 b | 0.08 ± 0.01 a | 7.56 ± 0.35 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara-Figueroa, J.; Cerda-Leal, F.; Alejandro-Martín, S.; Gacitúa, W. Evaluation of the PLA-nZH-Cu Nanocomposite Film on the Micro-Biological, Organoleptic and Physicochemical Qualities of Packed Chicken Meat. Foods 2022, 11, 546. https://doi.org/10.3390/foods11040546
Vergara-Figueroa J, Cerda-Leal F, Alejandro-Martín S, Gacitúa W. Evaluation of the PLA-nZH-Cu Nanocomposite Film on the Micro-Biological, Organoleptic and Physicochemical Qualities of Packed Chicken Meat. Foods. 2022; 11(4):546. https://doi.org/10.3390/foods11040546
Chicago/Turabian StyleVergara-Figueroa, Judith, Fabiola Cerda-Leal, Serguei Alejandro-Martín, and William Gacitúa. 2022. "Evaluation of the PLA-nZH-Cu Nanocomposite Film on the Micro-Biological, Organoleptic and Physicochemical Qualities of Packed Chicken Meat" Foods 11, no. 4: 546. https://doi.org/10.3390/foods11040546
APA StyleVergara-Figueroa, J., Cerda-Leal, F., Alejandro-Martín, S., & Gacitúa, W. (2022). Evaluation of the PLA-nZH-Cu Nanocomposite Film on the Micro-Biological, Organoleptic and Physicochemical Qualities of Packed Chicken Meat. Foods, 11(4), 546. https://doi.org/10.3390/foods11040546