Whey Proteins-Fortified Milk with Adjusted Casein to Whey Proteins Ratio Improved Muscle Strength and Endurance Exercise Capacity without Lean Mass Accretion in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Animals and Experimental Design
2.3. Growth Performance and Body Composition
2.4. Protein Digestibility
2.5. Plasma Amino Acids Concentration
2.6. Grip Strength Test
2.7. Weight-Loaded Swimming Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Growth Performance
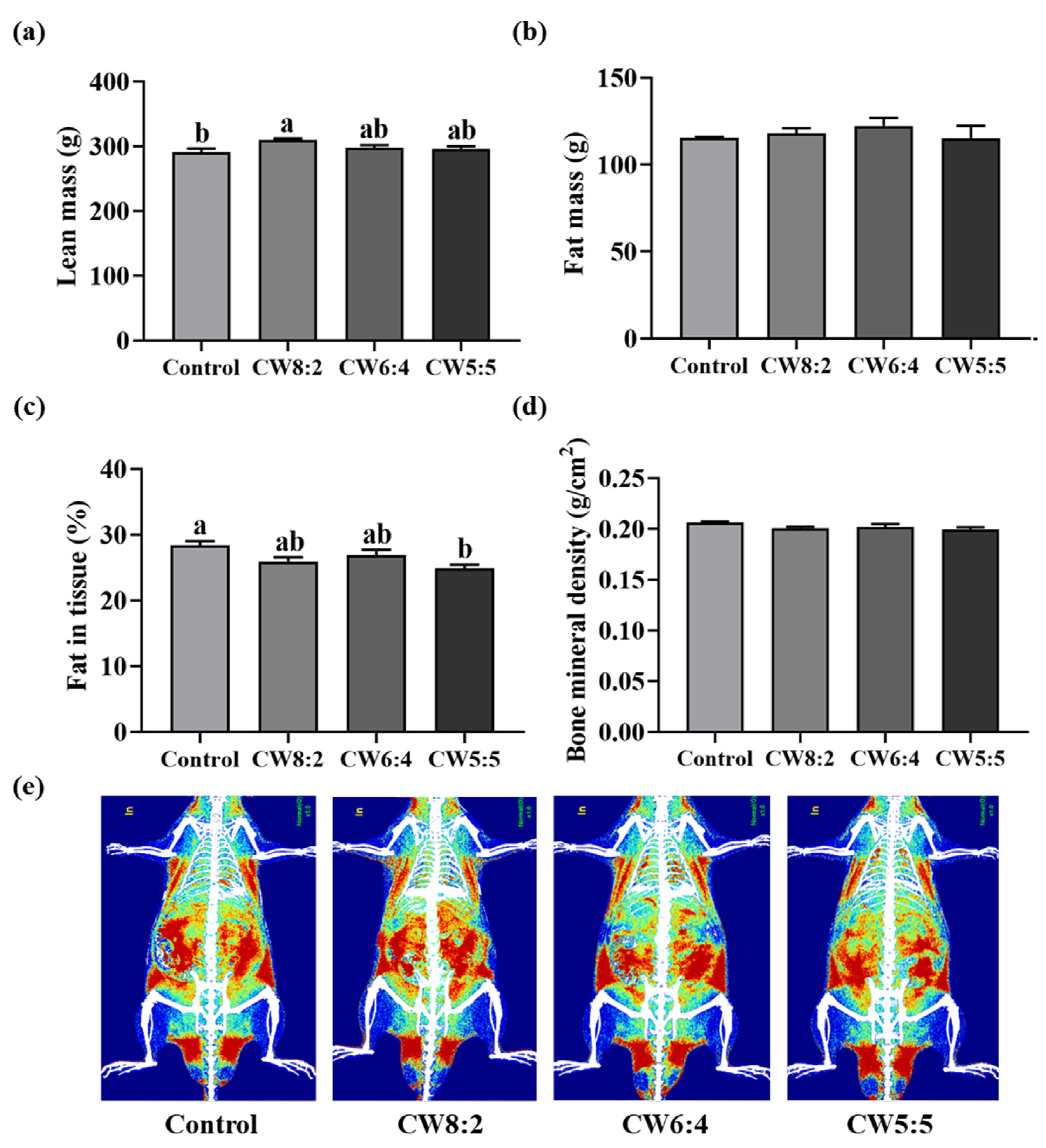
3.2. Body Composition
3.3. Protein Digestibility
3.4. Plasma Amino Acids Concentration
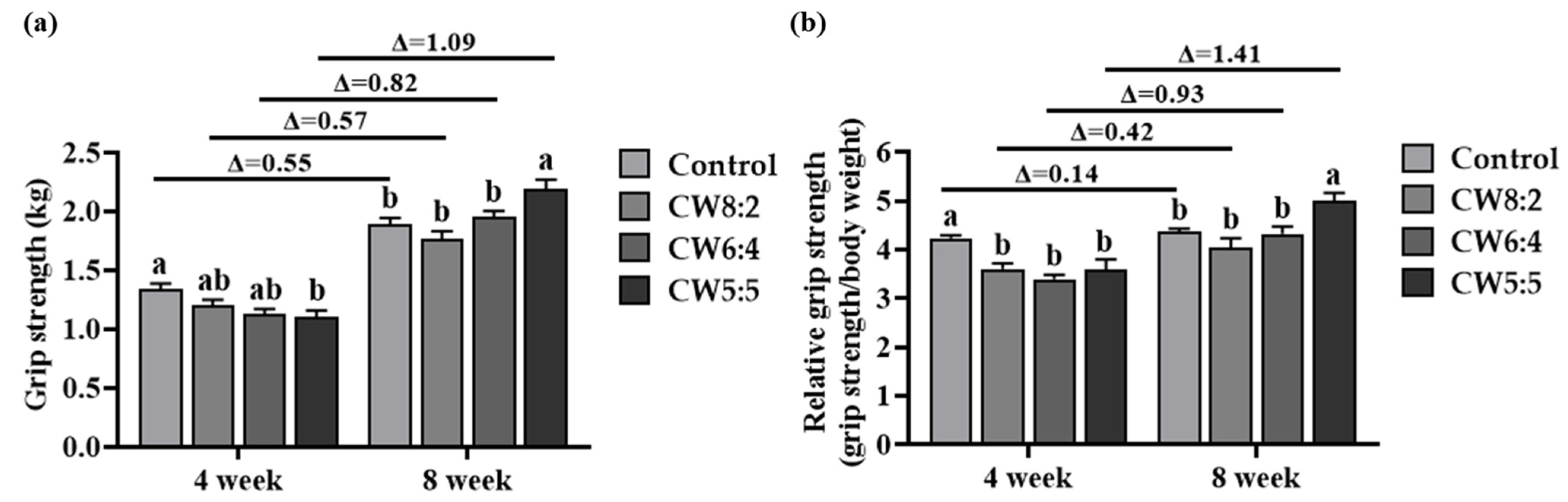
3.5. Grip Strength Test
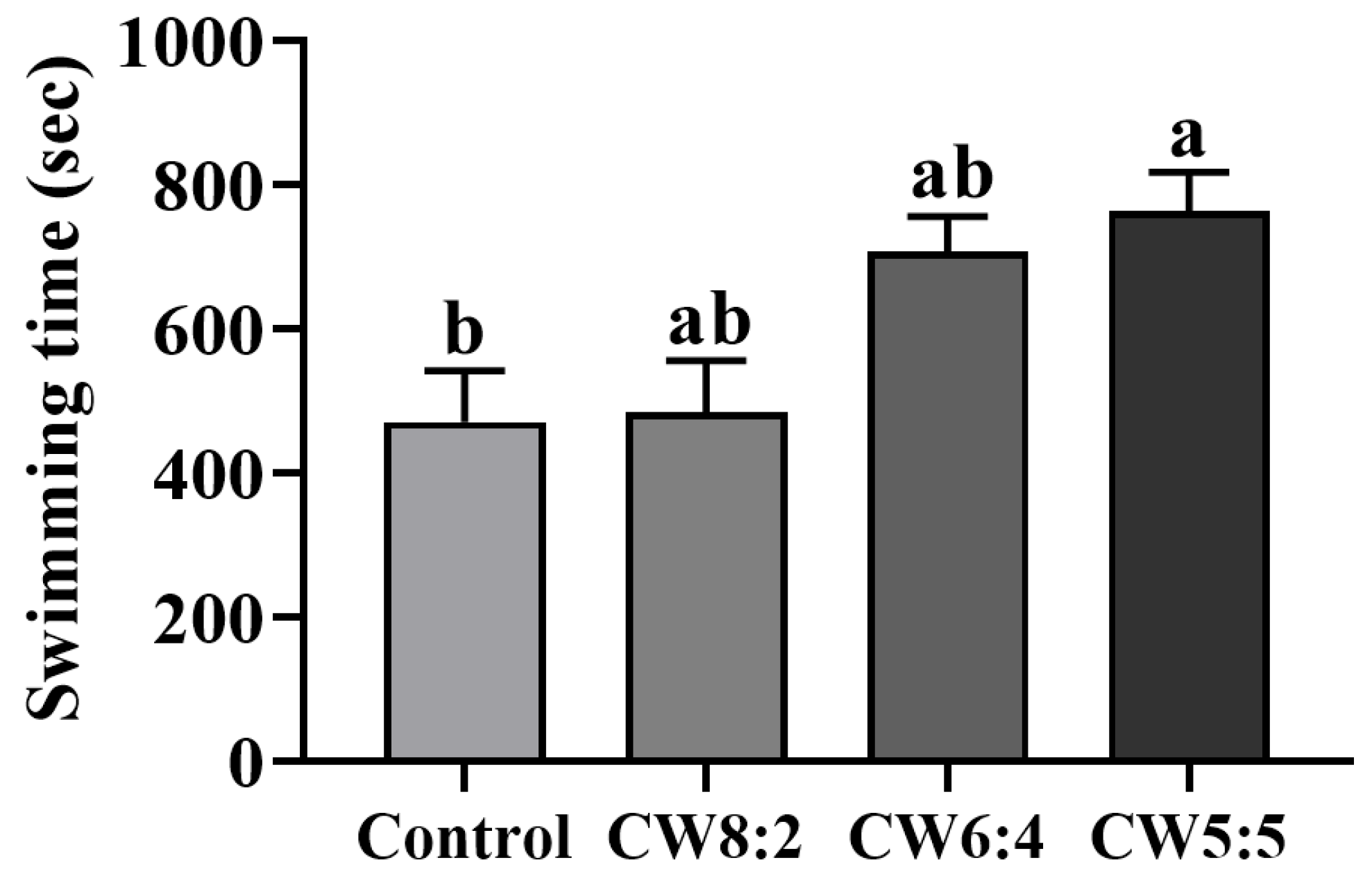
3.6. Weight-Loaded Swimming Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO. Dairy and Dairy Products; OECD-FAO Agricultural Outlook 2021–2030; FAO: Paris, France, 2021; pp. 178–189. [Google Scholar]
- Willett, W.C.; Ludwig, D.S. Milk and health. N. Engl. J. Med. 2020, 382, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, structure, and digestive dynamics of milk from different species—A review. Front. Nutr. 2020, 7, 577759–577775. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.; Gaudichon, C.; Tomé, D. Nutritional and physiological criteria in the assessment of milk protein quality for humans. J. Am. Coll. Nutr. 2000, 19, 191S–205S. [Google Scholar] [CrossRef]
- Master, P.B.Z.; Macedo, R.C.O. Effects of dietary supplementation in sport and exercise: A review of evidence on milk proteins and amino acids. Crit. Rev. Food Sci. Nutr. 2021, 61, 1225–1239. [Google Scholar] [CrossRef]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef]
- McKinnon, H.; Kruger, M.; Prosser, C.; Lowry, D. The effect of formulated goats’ milk on calcium bioavailability in male growing rats. J. Sci. Food Agric. 2010, 90, 112–116. [Google Scholar] [CrossRef]
- Budek, A.Z.; Bjornvad, C.R.; Mølgaard, C.; Bügel, S.; Vestergaard, M.; Pulkkinen, P.; Michaelsen, K.F.; Sangild, P.T. Effects of casein, whey and soy proteins on volumetric bone density and bone strength in immunocompromised piglets. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2007, 2, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, M.; Vasiljevic, T. Functional properties of whey proteins affected by heat treatment and hydrodynamic high-pressure shearing. J. Dairy Sci. 2009, 92, 1387–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Chen, N.; Ashaolu, T.J. Whey proteins and peptides in health-promoting functions—A review. Int. Dairy J. 2021, 126, 105269–105282. [Google Scholar] [CrossRef]
- Pennings, B.; Boirie, Y.; Senden, J.M.G.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J.C. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraguchi, F.K.; Pedrosa, M.L.; de Paula, H.; dos Santos, R.C.; Silva, M.E. Evaluation of biological and biochemical quality of whey protein. J. Med. Food 2010, 13, 1505–1509. [Google Scholar] [CrossRef]
- van Dijk, M.; Dijk, F.J.; Bunschoten, A.; van Dartel, D.A.; Van Norren, K.; Walrand, S.; Jourdan, M.; Verlaan, S.; Luiking, Y. Improved muscle function and quality after diet intervention with leucine-enriched whey and antioxidants in antioxidant deficient aged mice. Oncotarget 2016, 7, 17338–17355. [Google Scholar] [CrossRef] [Green Version]
- Wouters, A.G.; Rombouts, I.; Lagrain, B.; Delcour, J.A. Impact of casein and egg white proteins on the structure of wheat gluten-based protein-rich food. J. Sci. Food Agric. 2016, 96, 757–763. [Google Scholar] [CrossRef] [Green Version]
- Nastaj, M.; Sołowiej, B.G.; Gustaw, W.; Peréz-Huertas, S.; Mleko, S.; Wesołowska-Trojanowska, M. Physicochemical properties of High-Protein-Set Yoghurts obtained with the addition of whey protein preparations. Int. J. Dairy Technol. 2019, 72, 395–402. [Google Scholar] [CrossRef]
- Nastaj, M.; Mleko, S.; Terpiłowski, K.; Tomczyńska-Mleko, M. Effect of sucrose on physicochemical properties of high-protein meringues obtained from whey protein isolate. Appl. Sci. 2021, 11, 4764. [Google Scholar] [CrossRef]
- Jeong, E.W.; Park, G.R.; Kim, J.; Yun, S.-Y.; Imm, J.-Y.; Lee, H.G. Effect of Modified Casein to on Dispersion Stability, Protein Quality and Body Composition in Rats. Food Sci. Anim. Resour. 2021, 41, 855–868. [Google Scholar] [CrossRef]
- Lara-Villoslada, F.; Olivares, M.; Xaus, J. The balance between caseins and whey proteins in cow’s milk determines its allergenicity. J. Dairy Sci. 2005, 88, 1654–1660. [Google Scholar] [CrossRef]
- Wood, E.L.; Christian, D.G.; Arafat, M.; McColl, L.K.; Prosser, C.G.; Carpenter, E.A.; Levine, A.S.; Klockars, A.; Olszewski, P.K. Adjustment of Whey: Casein Ratio from 20: 80 to 60: 40 in Milk Formulation Affects Food Intake and Brainstem and Hypothalamic Neuronal Activation and Gene Expression in Laboratory Mice. Foods 2021, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis; AOAC: Arlington, VA, USA, 2005. [Google Scholar]
- Maclean, W.; Harnly, J.; Chen, J.; Chevassus-Agnes, S.; Gilani, G.; Livesey, G.; Warwick, P. Food energy–Methods of analysis and conversion factors. In Proceedings of Food and Agriculture Organization of the United Nations Technical Workshop Report; Agricultural Research Service, US Department of Agriculture: Beltsville, MD, USA, 2003; Volume 77, pp. 8–9. [Google Scholar]
- Dewey, K. Guiding Principles for Feeding Non-Breastfed Children 6–24 Months of Age; World Health Organization: Geneva, Switzerland, 2005; p. 20. [Google Scholar]
- Barr, S.I.; McCarron, D.A.; Heaney, R.P.; Dawson-Hughes, B.; Berga, S.L.; Stern, J.S.; Oparil, S. Effects of increased consumption of fluid milk on energy and nutrient intake, body weight, and cardiovascular risk factors in healthy older adults. J. Am. Diet. Assoc. 2000, 100, 810–817. [Google Scholar] [CrossRef]
- Berkey, C.S.; Rockett, H.R.H.; Willett, W.C.; Colditz, G.A. Milk, dairy fat, dietary calcium, and weight gain: A longitudinal study of adolescents. Arch. Pediatr. Adolesc. Med. 2005, 159, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouliot, Y.; Gauthier, S.F. Milk growth factors as health products: Some technological aspects. Int. Dairy J. 2006, 16, 1415–1420. [Google Scholar] [CrossRef]
- Yajima, T.; Kanno, T.; Katoku, Y.; Kuwata, T. Gut hypertrophy in response to the ratios of casein and whey protein in milk formulas in artificially reared rat pups. Neonatology 1998, 74, 314–322. [Google Scholar] [CrossRef]
- Abargouei, A.S.; Janghorbani, M.; Salehi-Marzijarani, M.; Esmaillzadeh, A. Effect of dairy consumption on weight and body composition in adults: A systematic review and meta-analysis of randomized controlled clinical trials. Int. J. Obes. 2012, 36, 1485–1493. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The role of milk-and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef]
- Burd, N.A.; Yang, Y.; Moore, D.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012, 108, 958–962. [Google Scholar] [CrossRef] [Green Version]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.J.; McGregor, R.A.; D’Souza, R.F.; Thorstensen, E.B.; Markworth, J.F.; Fanning, A.C.; Poppitt, S.D.; Cameron-Smith, D. Consumption of milk protein or whey protein results in a similar increase in muscle protein synthesis in middle aged men. Nutrients 2015, 7, 8685–8699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Fry, C.S.; Borack, M.S.; Cope, M.B.; Mukherjea, R.; et al. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J. Nutr. 2013, 143, 410–416. [Google Scholar] [CrossRef] [PubMed]
- McAllan, L.; Keane, D.; Schellekens, H.; Roche, H.M.; Korpela, R.; Cryan, J.F.; Nilaweera, K.N. Whey protein isolate counteracts the effects of a high-fat diet on energy intake and hypothalamic and adipose tissue expression of energy balance-related genes. Br. J. Nutr. 2013, 110, 2114–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojtahedi, M.C.; Thorpe, M.P.; Karampinos, D.C.; Johnson, C.L.; Layman, D.K.; Georgiadis, J.G.; Evans, E.M. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1218–1225. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Keenan, M.J.; Losso, J.N.; Raggio, A.M.; Shen, L.; McCutcheon, K.L.; Tulley, R.T.; Blackman, M.R.; Martin, R.J. Dietary whey protein decreases food intake and body fat in rats. Obesity 2011, 19, 1568–1573. [Google Scholar] [CrossRef] [Green Version]
- Phosanam, A.; Chandrapala, J.; Huppertz, T.; Adhikari, B.; Zisu, B. In vitro digestion of infant formula model systems: Influence of casein to whey protein ratio. Int. Diary J. 2021, 117, 105008–105015. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Torcello-Gómez, A.; Saha, S.; Mackie, A.R.; Wilde, P.J.; Brodkorb, A. Impact of caseins and whey proteins ratio and lipid content on in vitro digestion and ex vivo absorption. Food Chem. 2020, 319, 126514–126524. [Google Scholar] [CrossRef]
- Kanda, A.; Nakayama, K.; Sanbongi, C.; Nagata, M.; Ikegami, S.; Itoh, H. Effects of whey, caseinate, or milk protein ingestion on muscle protein synthesis after exercise. Nutrients 2016, 8, 339. [Google Scholar] [CrossRef] [Green Version]
- Morifuji, M.; Koga, J.; Kawanaka, K.; Higuchi, M. Branched-chain amino acid-containing dipeptides, identified from whey protein hydrolysates, stimulate glucose uptake rate in L6 myotubes and isolated skeletal muscles. J. Nutr. Sci. Vitaminol. 2009, 55, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Potter, S.M.; Kies, C.V. Influence of sulfur-amino acid content variation in plant vs animal protein on serum and tissue lipids in rats. Plant Foods Hum. Nutr. 1990, 40, 297–308. [Google Scholar] [CrossRef]
- Odell, O.J.; Wallis, G.A. The application of lactose in sports nutrition. Int. Dairy J. 2021, 116, 104970–104980. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J.C. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargreaves, M.; Finn, J.P.; Withers, R.T.; Scroop, G.C.; Mackay, M.; Snow, R.J.; Carey, M.F. Effect of muscle glycogen availability on maximal exercise performance. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Philp, A.; Hargreaves, M.; Baar, K. More than a store: Regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1343–E1351. [Google Scholar] [CrossRef] [Green Version]
- Ferguson-Stegall, L.; McCleave, E.L.; Ding, Z.; Doerner, P.G., III; Wang, B.; Liao, Y.-H.; Kammer, L.; Liu, Y.; Hwang, J.; Dessard, B.M.; et al. Postexercise carbohydrate–protein supplementation improves subsequent exercise performance and intracellular signaling for protein synthesis. J. Strength Cond. Res. 2011, 25, 1210–1224. [Google Scholar] [CrossRef]
- Grieger, J.A.; Nowson, C.A. Use of calcium, folate, and vitamin D3–fortified milk for 6 months improves nutritional status but not bone mass or turnover, in a group of Australian aged care residents. J. Nutr. Elder. 2009, 28, 236–254. [Google Scholar] [CrossRef]
- Chen, W.-C.; Huang, W.-C.; Chiu, C.-C.; Chang, Y.-K.; Huang, C.-C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Ishihara, K.; Tanaka, K.; Inoue, K.; Fushiki, T. An adjustable-current swimming pool for the evaluation of endurance capacity of mice. J. Appl. Physiol. 1996, 81, 1843–1849. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Yi, S.; Zhang, H.; Wang, J. Ingestion of soy–whey blended protein augments sports performance and ameliorates exercise-induced fatigue in a rat exercise model. Food Funct. 2017, 8, 670–679. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakamura, F.; Mizokawa, S.; Matsumura, A.; Nozaki, S.; Watanabe, Y. Establishment and assessment of a rat model of fatigue. Neurosci. Lett. 2003, 352, 159–162. [Google Scholar] [CrossRef]
- Bergström, J.; Hermansen, L.; Hultman, E.; Saltin, B. Diet, muscle glycogen and physical performance. Acta. Physiol. Scand. 1967, 71, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Morifuji, M.; Kanda, A.; Koga, J.; Kawanaka, K.; Higuchi, M. Preexercise ingestion of carbohydrate plus whey protein hydrolysates attenuates skeletal muscle glycogen depletion during exercise in rats. Nutrition 2011, 27, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Campos-Ferraz, P.L.; Bozza, T.; Nicastro, H.; Lancha, A.H., Jr. Distinct effects of leucine or a mixture of the branched-chain amino acids (leucine, isoleucine, and valine) supplementation on resistance to fatigue, and muscle and liver-glycogen degradation, in trained rats. Nutrition 2013, 29, 1388–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Item (Unit) | CW8:2 | CW6:4 | CW5:5 |
|---|---|---|---|
| Moisture (%) | 0.79 | 1.68 | 1.99 |
| Crude ash (%) | 6.45 | 6.26 | 6.15 |
| Crude protein (%) | 29.8 | 30.4 | 31.3 |
| Crude fat (%) | 17.8 | 16.7 | 16.6 |
| Carbohydrates (%) | 45.2 | 45.0 | 44.0 |
| Calories (kcal/100 g) | 459 | 450 | 450 |
| Control | CW8:2 | CW6:4 | CW5:5 | |
|---|---|---|---|---|
| Nitrogen intake (g/rat) | 3.28 ± 0.16 | 3.53 ± 0.16 | 3.55 ± 0.11 | 3.66 ± 0.07 |
| Fecal nitrogen (g/rat) | 0.17 ± 0.01 | 0.19 ± 0.01 | 0.20 ± 0.01 | 0.20± 0.02 |
| True digestibility (%) | 95.5 ± 0.19 | 95.1 ± 0.44 | 94.8 ± 0.28 | 95.2 ± 0.42 |
| Amino Acids (µmol/L) | Control | CW8:2 | CW6:4 | CW5:5 |
|---|---|---|---|---|
| Ile | 44.3 ± 0.7 b | 50.5 ± 2.8 b | 67.8 ± 0.4 a | 66.7 ± 2.7 a |
| Leu | 69.4 ± 2.8 b | 80.3 ± 3.8 b | 122 ± 2.7 a | 117 ± 2.2 a |
| Val | 90.0 ± 0.3 b | 105 ± 6.5 b | 141 ± 1.8 a | 132 ± 2.4 a |
| Met | 39.5 ± 1.8 b | 43.1 ± 0.1 ab | 47.2 ± 0.3 a | 47.4 ± 0.0 a |
| Phe | 34.9 ± 0.1 b | 45.6 ± 0.3 a | 51.0 ± 1.4 a | 46.9 ± 1.8 a |
| Thr | 216 ± 17 | 209 ± 19 | 202 ± 12 | 225 ± 21 |
| Lys | 170 ± 8.2 | 179 ± 17 | 227 ± 1.5 | 204 ± 17 |
| ∑BCAAs | 204 ± 3.1 b | 236 ± 13 b | 331 ± 4.4 a | 316 ± 7.2 a |
| ∑EAAs | 782 ± 8.5 b | 866 ± 0.8 ab | 1030 ± 50 a | 972 ± 25 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, E.W.; Park, G.R.; Kim, J.; Baek, Y.; Go, G.-w.; Lee, H.G. Whey Proteins-Fortified Milk with Adjusted Casein to Whey Proteins Ratio Improved Muscle Strength and Endurance Exercise Capacity without Lean Mass Accretion in Rats. Foods 2022, 11, 574. https://doi.org/10.3390/foods11040574
Jeong EW, Park GR, Kim J, Baek Y, Go G-w, Lee HG. Whey Proteins-Fortified Milk with Adjusted Casein to Whey Proteins Ratio Improved Muscle Strength and Endurance Exercise Capacity without Lean Mass Accretion in Rats. Foods. 2022; 11(4):574. https://doi.org/10.3390/foods11040574
Chicago/Turabian StyleJeong, Eun Woo, Gyu Ri Park, Jiyun Kim, Youjin Baek, Gwang-woong Go, and Hyeon Gyu Lee. 2022. "Whey Proteins-Fortified Milk with Adjusted Casein to Whey Proteins Ratio Improved Muscle Strength and Endurance Exercise Capacity without Lean Mass Accretion in Rats" Foods 11, no. 4: 574. https://doi.org/10.3390/foods11040574
APA StyleJeong, E. W., Park, G. R., Kim, J., Baek, Y., Go, G.-w., & Lee, H. G. (2022). Whey Proteins-Fortified Milk with Adjusted Casein to Whey Proteins Ratio Improved Muscle Strength and Endurance Exercise Capacity without Lean Mass Accretion in Rats. Foods, 11(4), 574. https://doi.org/10.3390/foods11040574







