Probiotic Effects and Metabolic Products of Enterococcus faecalis LD33 with Respiration Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Growth Conditions
2.2. Metabolic Products Determination
2.3. Antibacterial Activity Assay
2.4. Survival in Acid and Bile Salt
2.5. Adhesion Capability Assay
2.6. Inhibition of HT-29 Human Colon Cancer Cells
2.7. Statistical Analysis
3. Results and Discussion
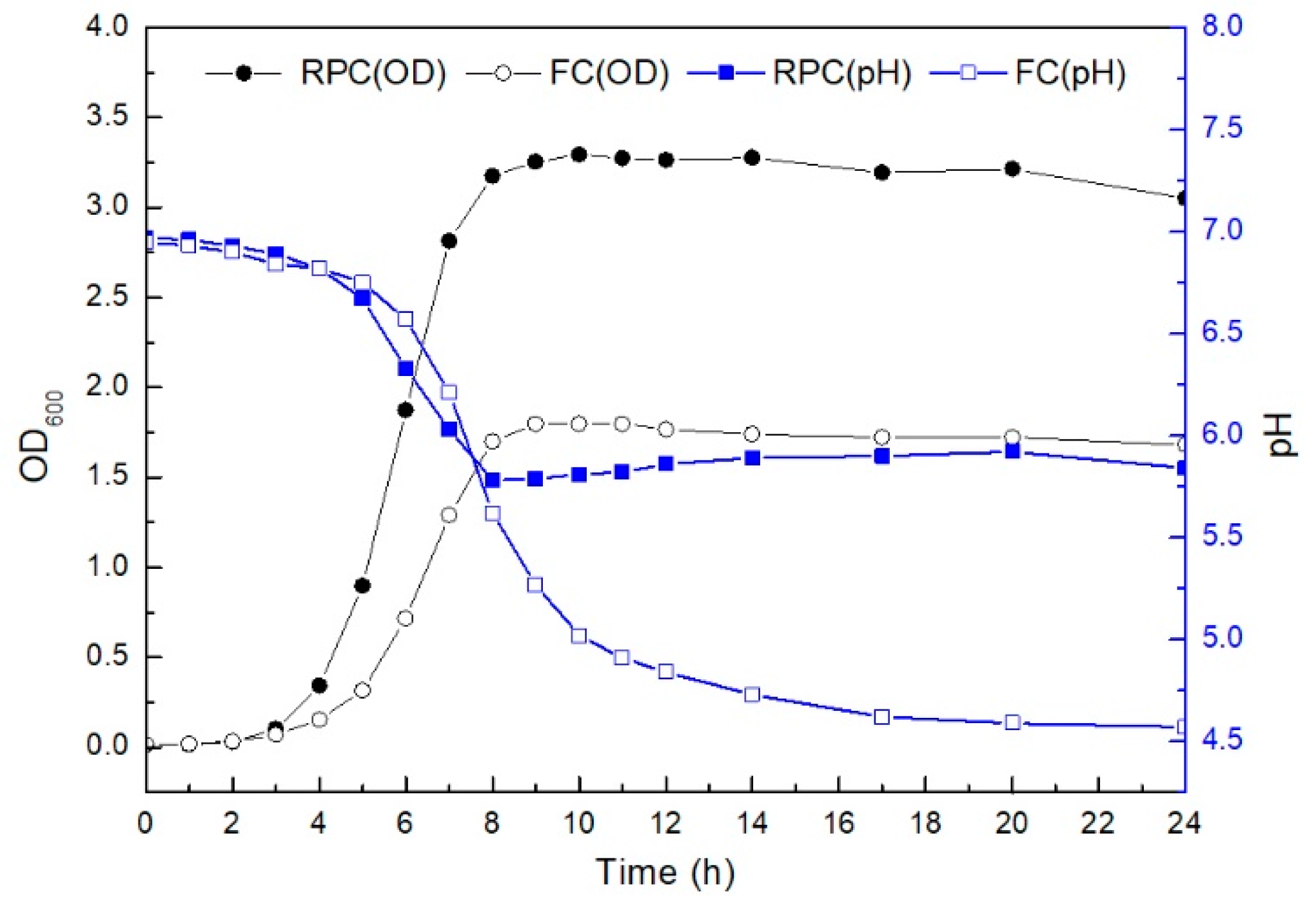
3.1. Respiration-Permissive Condition Leads to Biphasic Growth
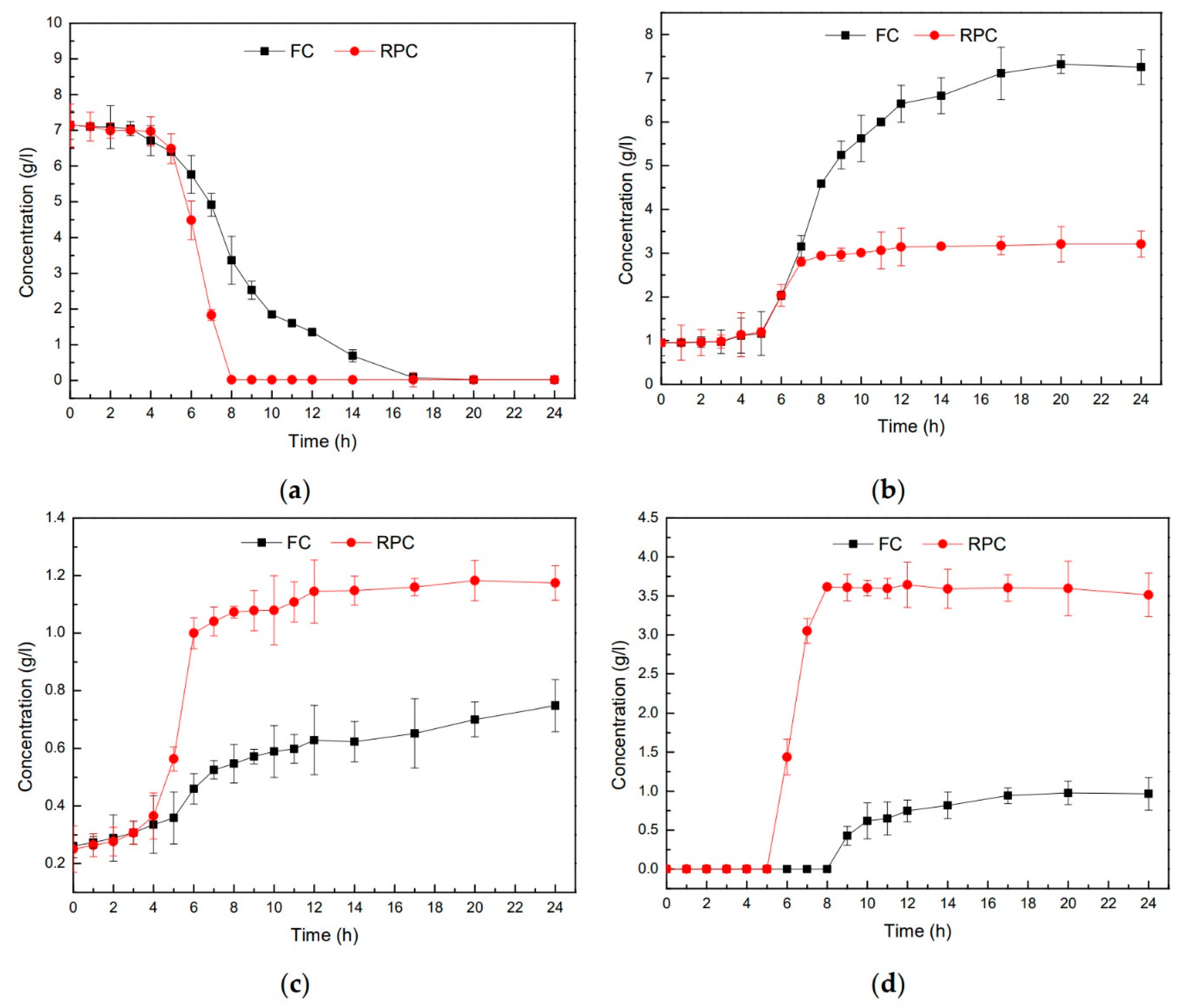
3.2. Metabolites Altered by the Respiration-Permissive Condition
3.3. Antibacterial Effect of E. faecalis LD33 on the Pathogen during the Growth Modes
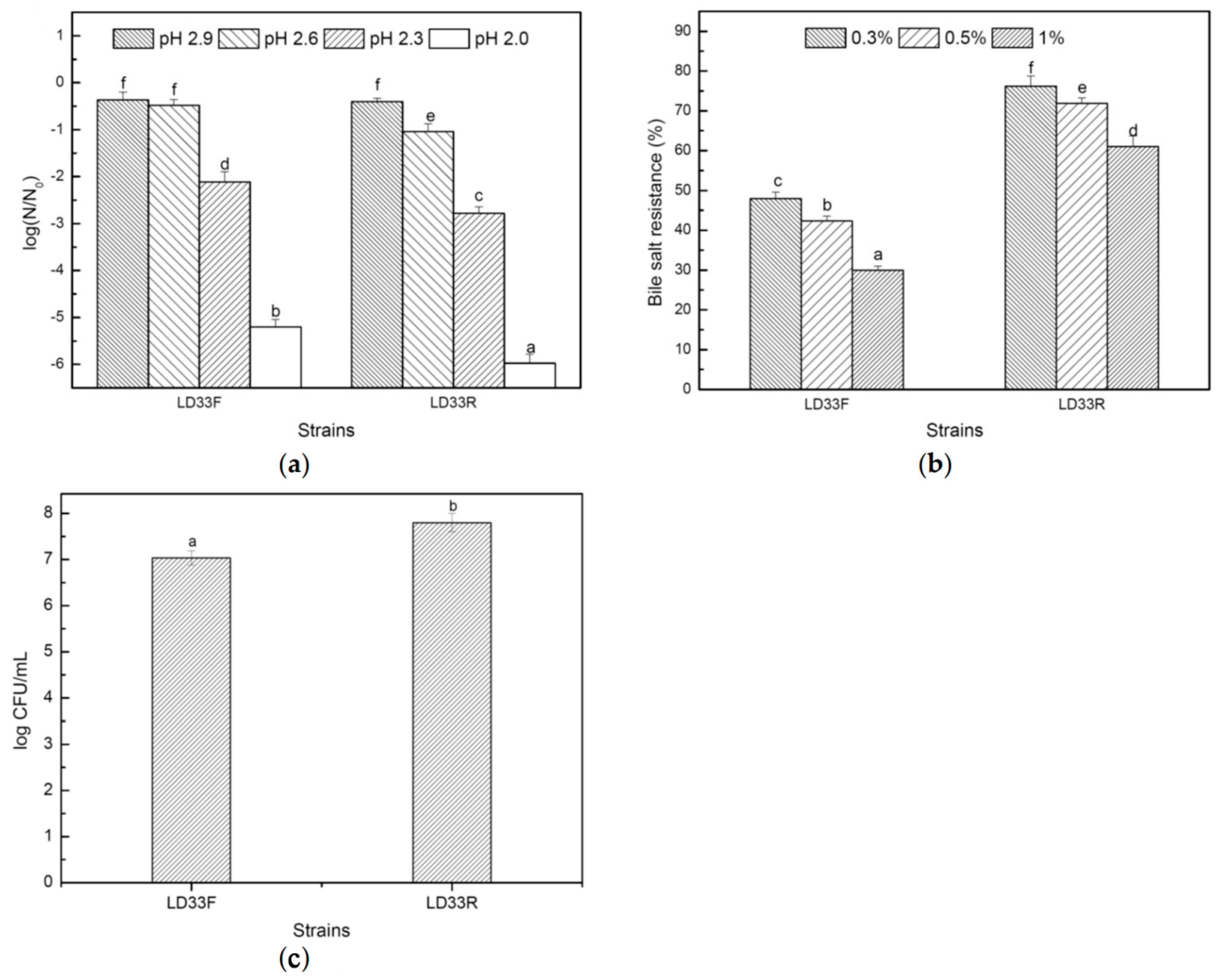
3.4. Tolerance of Acid and bile Salt and the Adherence Capability
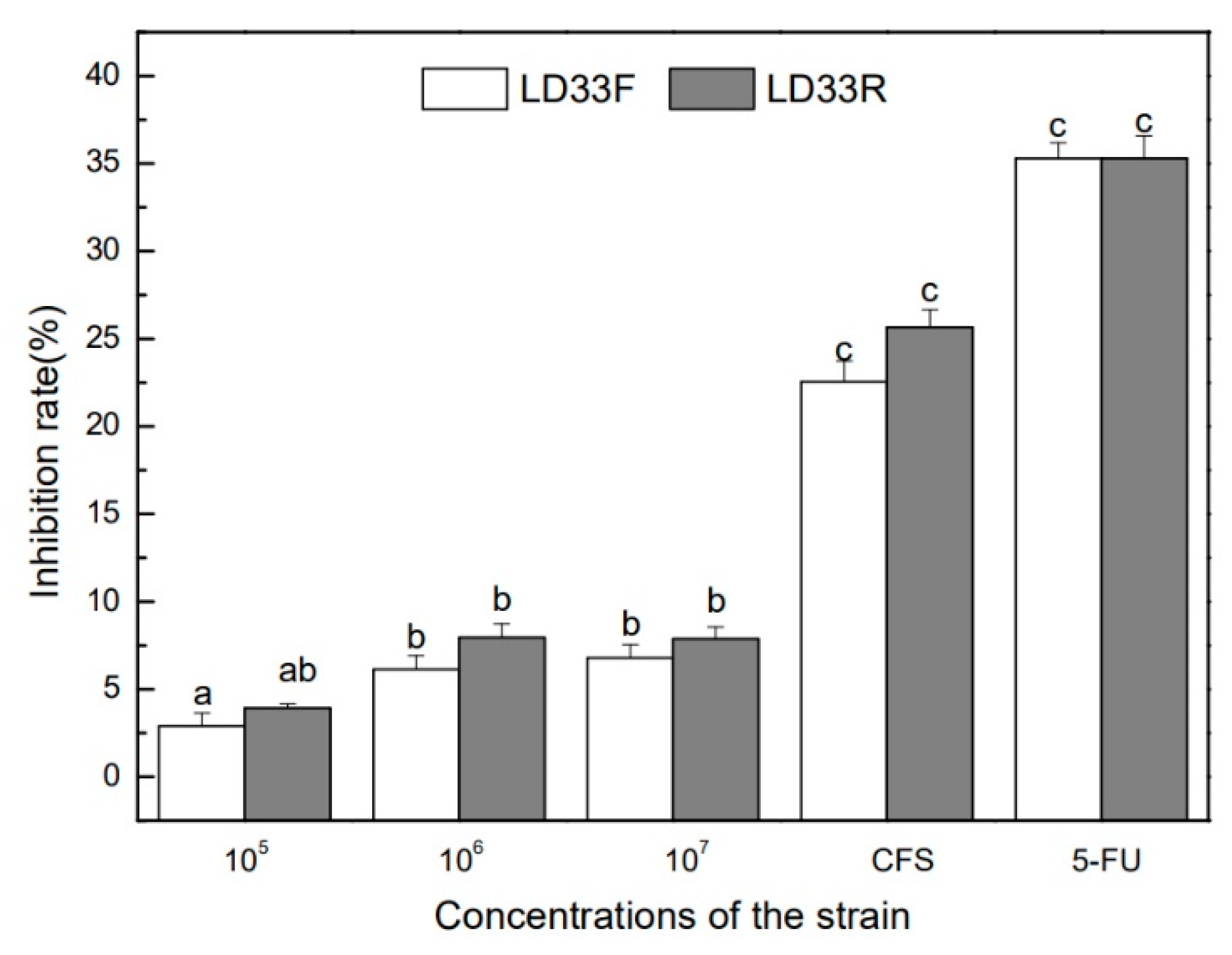
3.5. Growth Inhibition of HT-29 Human Colon Cancer Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lechardeur, D.; Cesselin, B.; Fernandez, A.; Lamberet, G.; Garrigues, C.; Pedersen, M.; Gaudu, P.; Gruss, A. Using heme as an energy boost for lactic acid bacteria. Curr. Opin. Biotechnol. 2011, 22, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.W.; Pedersen, M.B.; Hammer, K.; Jensen, P.R. Lactate dehydrogenase has no control on lactate production but has a strong negative control on formate production in Lactococcuslactis. JBIC J. Biol. Inorg. Chem. 2001, 268, 6379–6389. [Google Scholar] [CrossRef]
- Kawai, M.; Harada, R.; Yoda, N.; Yamasaki-Yashiki, S.; Fukusaki, E.; Katakura, Y. Suppression of lactate production by using sucrose as a carbon source in lactic acid bacteria. J. Biosci. Bioeng. 2020, 129, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Caplice, E. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef]
- Mun, S.; Seo, Y.; Chan, G.H.C. Characterization of the psychrotrophic lactic acid bacterium, Leuconostocgelidum subsp. aenigmaticum LS4 isolated from kimchi based on comparative analyses of its genomic and phenotypic properties. aenigmaticum LS4 Isolated from Kimchi Based on Comparative Analyses of Its Genomic and Phenotypic Properties. Foods 2021, 10, 1889. [Google Scholar]
- Zhang, H.; Xu, J.; Chen, Q.; Wang, H.; Kong, B. Physiological, Morphological and Antioxidant Responses of Pediococcuspentosaceus R1 and Lactobacillusfermentum R6 Isolated from Harbin Dry Sausages to Oxidative Stress. Foods 2021, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Konings, W.N. The cell membrane and the struggle for life of lactic acid bacteria. AntonieVan Leeuwenhoek 2002, 82, 3–27. [Google Scholar] [CrossRef]
- Duwat, P.; Sourice, S.; Cesselin, B.; Lamberet, G.; Vido, K.; Gaudu, P.; Le Loir, Y.; Violet, F.; Loubière, P.; Gruss, A. Respiration Capacity of the Fermenting Bacterium Lactococcuslactis and Its Positive Effects on Growth and Survival. J. Bacteriol. 2001, 183, 4509–4516. [Google Scholar] [CrossRef] [Green Version]
- Gaudu, P.; Vido, K.; Cesselin, B.; Kulakauskas, S.; Tremblay, J.; Rezaïki, L.; Lamberet, G.; Sourice, S.; Duwat, P.; Gruss, A. Respiration capacity and consequences in Lactococcuslactis. AntonieVan Leeuwenhoek 2002, 82, 263–269. [Google Scholar] [CrossRef]
- Winstedt, L.; Frankenberg, L.; Hederstedt, L.; von Wachenfeldt, C. Enterococcus faecalis V583 Contains a Cytochrome bd-Type Respiratory Oxidase. J. Bacteriol. 2000, 182, 3863–3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sijpesteijn, A.K. Induction of cytochrome formation and stimulation of oxidative dissimilation by hemin in Streptococcus lactis and Leuconostocmesenteroides. AntonieVan Leeuwenhoek 1970, 36, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; van der Veen, S.; Nakajima, H.; Abee, T. Effect of respiration and manganese on oxidative stress resistance of Lactobacillus plantarum WCFS1. Microbiology 2012, 158, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.B.; Gaudu, P.; Lechardeur, D.; Petit, M.-A.; Gruss, A. Aerobic Respiration Metabolism in Lactic Acid Bacteria and Uses in Biotechnology. Annu. Rev. Food Sci. Technol. 2012, 3, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Watthanasakphuban, N.; Virginia, L.; Haltrich, D.; Peterbauer, C. Analysis and Reconstitution of the Menaquinone Biosynthesis Pathway in Lactiplantibacillusplantarum and Lentilactibacillusbuchneri. Microorganisms 2021, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Zotta, T.; Parente, E.; Ricciardi, A. Aerobic metabolism in the genus Lactobacillus: Impact on stress response and potential applications in the food industry. J. Appl. Microbiol. 2017, 122, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Razvi, A.; Zhang, Z.; Lan, C.Q. Effects of glucose and nitrogen source concentration on batch fermentation kinetics of Lactococcuslactis under hemin-stimulated respirative condition. Biotechnol. Prog. 2008, 24, 852–858. [Google Scholar] [CrossRef]
- Cesselin, B.; Garrigues, C.; Pedersen, M.B.; Roussel, C.; Gruss, A.; Gaudu, P. Task Distribution between Acetate and Acetoin Pathways to Prolong Growth in Lactococcuslactis under Respiration Conditions. Appl. Environ. Microbiol. 2018, 84, e01005-18. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, A.; Zotta, T.; Ianniello, R.G.; Boscaino, F.; Matera, A.; Parente, E. Effect of Respiratory Growth on the Metabolite Production and Stress Robustness of Lactobacillus casei N87 Cultivated in Cheese Whey Permeate Medium. Front. Microbiol. 2019, 10, 851. [Google Scholar] [CrossRef]
- Zotta, T.; Ricciardi, A.; Ianniello, R.G.; Storti, L.V.; Glibota, N.A.; Parente, E. Aerobic and respirative growth of heterofermentative lactic acid bacteria: A screening study. Food Microbiol. 2018, 76, 117–127. [Google Scholar] [CrossRef]
- Tachon, S.; Brandsma, J.B.; Yvon, M. NoxE NADH Oxidase and the Electron Transport Chain Are Responsible for the Ability of Lactococcuslactis to Decrease the Redox Potential of Milk. Appl. Environ. Microbiol. 2010, 76, 1311–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johanson, A.; Goel, A.; Olsson, L.; Franzén, C.J. Respiratory Physiology of Lactococcuslactis in Chemostat Cultures and Its Effect on Cellular Robustness in Frozen and Freeze-Dried Starter Cultures. Appl. Environ. Microbiol. 2020, 86, e02785-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siciliano, R.A.; Pannella, G.; Lippolis, R.; Ricciardi, A.; Mazzeo, M.F.; Zotta, T. Impact of aerobic and respirative life-style on Lactobacillus casei N87 proteome. Int. J. Food Microbiol. 2019, 298, 51–62. [Google Scholar] [CrossRef]
- Wunderlich, P.; Braun, L.; Fumagalli, I.; D’Apuzzo, V.; Heim, F.; Karly, M.; Lodi, R.; Politta, G.; Vonbank, F.; Zeltner, L. Double-Blind Report on the Efficacy of Lactic Acid-Producing Enterococcus SF68 in the Prevention of Antibiotic-Associated Diarrhoea and in the Treatment of Acute Diarrhoea. J. Int. Med Res. 1989, 17, 333–338. [Google Scholar] [CrossRef]
- Chang, C.-I.; Liu, W.-Y. An evaluation of two probiotic bacterial strains, Enterococcus faecium SF68 and Bacillus toyoi, for reducing edwardsiellosis in cultured European eel, Anguilla anguilla L. J. Fish Dis. 2002, 25, 311–315. [Google Scholar] [CrossRef]
- Veir, J.K.; Knorr, R.; Cavadini, C.; Sherrill, S.J.; Benyacoub, J.; Satyaraj, E.; Lappin, M.R. Effect of supplementation with Enterococcus faecium (SF68) on immune functions in cats. Veter-Ther. Res. Appl. Veter-Med. 2007, 8, 229–238. [Google Scholar]
- Buydens, P.; Debeuckelaere, S. Efficacy of SF 68 in the Treatment of Acute Diarrhea a Placebo-Controlled Trial. Scand. J. Gastroenterol. 1996, 31, 887–891. [Google Scholar] [CrossRef]
- Benyacoub, J.; Pérez, P.F.; Rochat, F.; Saudan, K.Y.; Reuteler, G.; Antille, N.; Humen, M.; De Antoni, G.L.; Cavadini, C.; Blum, S.; et al. Enterococcus faecium SF68 Enhances the Immune Response to Giardia intestinalis in Mice. J. Nutr. 2005, 135, 1171–1176. [Google Scholar] [CrossRef] [Green Version]
- Franz, C.M.; Huch, M.; Abriouel, H.; Holzapfel, W.; Galvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Andersen, H.W.; Solem, C.; Hammer, K.; Jensen, P.R. Twofold Reduction of Phosphofructokinase Activity in Lactococcuslactis Results in Strong Decreases in Growth Rate and in Glycolytic Flux. J. Bacteriol. 2001, 183, 3458–3467. [Google Scholar] [CrossRef] [Green Version]
- Starrenburg, M.J.C.; Hugenholtz, J. Citrate Fermentation by Lactococcus and Leuconostoc spp. Appl. Environ. Microbiol. 1991, 57, 3535–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammor, S.; Tauveron, G.; Dufour, E.; Chevallier, I. Antibacterial activity of lactic acid bacteria against spoilage and pathogenic bacteria isolated from the same meat small-scale facility: 1—Screening and characterization of the antibacterial compounds. Food Control 2006, 17, 454–461. [Google Scholar] [CrossRef]
- Thirabunyanon, M.; Boonprasom, P.; Niamsup, P. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol. Lett. 2008, 31, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Moraes, P.; Perin, L.; Todorov, S.; Silva, A.; Franco, B.; Nero, L. Bacteriocinogenic and virulence potential of Enterococcus isolates obtained from raw milk and cheese. J. Appl. Microbiol. 2012, 113, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; van der Veen, S.; Abee, T. Impact of Respiration on Resistance of Lactobacillus plantarum WCFS1 to Acid Stress. Appl. Environ. Microbiol. 2012, 78, 4062–4064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Succi, M.; Tremonte, P.; Reale, A.; Sorrentino, E.; Grazia, L.; Pacifico, S.; Coppola, R. Bile salt and acid tolerance of Lactobacillusrhamnosus strains isolated from Parmigiano Reggiano cheese. FEMS Microbiol. Lett. 2005, 244, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauviere, G.; Coconnier, M.-H.; Kerneis, S.; Fourniat, J.; Servin, A.L. Adhesion of human Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J. Gen. Microbiol. 1992, 138, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, L.W.; Du, M.; Yi, H.; Guo, C.; Tuo, Y.; Han, X.; Li, J.; Zhang, L.; Yang, L. Antimicrobial activity against Shigellasonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol. Res. 2011, 167, 27–31. [Google Scholar] [CrossRef]
- Haza, A.I.; Zabala, A.; Morales, P. Protective effect and cytokine production of a Lactobacillus plantarum strain isolated from ewes’ milk cheese. Int. Dairy J. 2004, 14, 29–38. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Garrigues, C.; Tuphile, K.; Brun, C.; Vido, K.; Bennedsen, M.; Møllgaard, H.; Gaudu, P.; Gruss, A. Impact of Aeration and Heme-Activated Respiration on Lactococcuslactis Gene Expression: Identification of a Heme-Responsive Operon. J. Bacteriol. 2008, 190, 4903–4911. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, T.; Takahashi, M.; Suzuki, H. Acetoin Fermentation by Citrate-Positive Lactococcuslactis subsp. lactis 3022 Grown Aerobically in the Presence of Hemin or Cu2+. Appl. Environ. Microbiol. 1990, 56, 2644–2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pangsomboon, K.; Kaewnopparat, S.; Pitakpornpreecha, T.; Srichana, T. Antibacterial activity of a bacteriocin from Lactobacillusparacasei HL32 against Porphyromonasgingivalis. Arch. Oral Biol. 2006, 51, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Yi, H.; Shi, J.; Xue, C.; Li, H.; Jiao, Y.; Shigwedha, N.; Du, M.; Han, X. Qualitative detection of class IIabacteriocinogenic lactic acid bacteria from traditional Chinese fermented food using a YGNGV-motif-based assay. J. Microbiol. Methods 2014, 100, 121–127. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, L.; Han, X.; Du, M.; Zhang, Y.; Li, J.; Sun, K.; Hou, Y. Isolation and applied potential of lactic acid bacteria from Chinese traditional fermented food in specific ecological localities. Food Sci. Biotechnol. 2011, 20, 1685–1690. [Google Scholar] [CrossRef]
- Valledor, S.J.D.; Dioso, C.M.; Bucheli, J.E.V.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Kim, B.; Holzapfel, W.H.; Todorov, S.D. Characterization and safety evaluation of two beneficial, enterocin-producing Enterococcus faecium strains isolated from kimchi, a Korean fermented cabbage. Food Microbiol. 2022, 102, 103886. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K.; Alsaloom, A.N. Characterization of Lactic Bacteria Isolated from Raw Milk and Their Antibacterial Activity against Bacteria as the Cause of Clinical Bovine Mastitis. J. Food Qual. 2021, 2021, 6466645. [Google Scholar] [CrossRef]
- Terzić-Vidojević, A.; Veljović, K.; Tolinački, M.; Živković, M.; Lukić, J.; Lozo, J.; Fira, D.; Jovčić, B.; Strahinić, I.; Begović, J.; et al. Diversity of non-starter lactic acid bacteria in autochthonous dairy products from Western Balkan Countries—Technological and probiotic properties. Food Res. Int. 2020, 136, 109494. [Google Scholar] [CrossRef]
- Ma, J.; Xu, C.; Yu, H.; Feng, Z.; Yu, W.; Gu, L.; Liu, Z.; Chen, L.; Jiang, Z.; Hou, J. Electro-encapsulation of probiotics in gum Arabic-pullulan blend nanofibres using electrospinning technology. Food Hydrocoll. 2021, 111, 106381. [Google Scholar] [CrossRef]
- De Matos, F.E., Jr.; da Silva, M.P.; Kasemodel, M.G.C.; Santos, T.T.; Burns, P.; Reinheimer, J.; Vinderola, G.; Favaro-Trindade, C.S. Evaluation of the viability and the preservation of the functionality of microencapsulated Lactobacillusparacasei BGP1 and Lactobacillusrhamnosus 64 in lipid particles coated by polymer electrostatic interaction. J. Funct. Foods 2019, 54, 98–108. [Google Scholar] [CrossRef]
- Ester, B.; Noelia, B.; Laura, C.-J.; Francesca, P.; Cristina, B.; Rosalba, L.; Marco, D.R. Probiotic survival and in vitro digestion of L. salivarius spp. salivarius encapsulated by high homogenization pressures and incorporated into a fruit matrix. LWT 2019, 111, 883–888. [Google Scholar] [CrossRef]
- Tuo, Y.; Zhang, L.; Yi, H.; Zhang, Y.; Zhang, W.; Han, X.; Du, M.; Jiao, Y.; Wang, S. Short communication: Antiproliferative effect of wild Lactobacillus strains isolated from fermented foods on HT-29 cells. J. Dairy Sci. 2010, 93, 2362–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-M.; Zhang, L.-W.; Fan, R.-B.; Han, X.; Yi, H.-X.; Zhang, L.-L.; Xue, C.-H.; Li, H.-B.; Zhang, Y.-H.; Shigwedha, N. Induction of HT-29 cells apoptosis by lactobacilli isolated from fermented products. Res. Microbiol. 2014, 165, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Bong, Y.; Kim, H. Fermentation characteristic and increased health functionality of kimchi by kimchi lactic acid bacteria starters (644.14). FASEB J. 2014, 28, 644.14. [Google Scholar] [CrossRef]
- Chang, J.; Shim, Y.; Cha, S.; Chee, K. Probiotic characteristics of lactic acid bacteria isolated from kimchi. J. Appl. Microbiol. 2010, 109, 220–230. [Google Scholar] [CrossRef]
- Özel, B.; Şimşek, Ö.; Akçelik, M.; Saris, P.E.J. Innovative approaches to nisin production. Appl. Microbiol. Biotechnol. 2018, 102, 6299–6307. [Google Scholar] [CrossRef] [Green Version]
- Buss, G.P.; Wilson, C.M. Exploring the cytotoxic mechanisms of Pediocin PA-1 towards HeLa and HT29 cells by comparison to known bacteriocins: Microcin E492, enterocin heterodimer and Divercin V41. PLoS ONE 2021, 16, e0251951. [Google Scholar] [CrossRef]
| Diameter of the Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|
| L. monocytogenes | S. aureus | E. coli | Salm. Typhimurium | Sh. Sonnei | |
| LD33 R | 18.26 ± 0.47 d | 16.58 ± 0.53 c | _ | 14.21 ± 0.27 ab | 17.81 ± 0.49 d |
| LD33 F | 14.55 ± 0.25 ab | 15.53 ± 0.32 b | _ | 15.01 ± 0.63 b | 18.57 ± 0.66 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Yang, H.; Shigwedha, N.; Zhang, S.; Liu, F.; Zhang, L. Probiotic Effects and Metabolic Products of Enterococcus faecalis LD33 with Respiration Capacity. Foods 2022, 11, 606. https://doi.org/10.3390/foods11040606
Jiao Y, Yang H, Shigwedha N, Zhang S, Liu F, Zhang L. Probiotic Effects and Metabolic Products of Enterococcus faecalis LD33 with Respiration Capacity. Foods. 2022; 11(4):606. https://doi.org/10.3390/foods11040606
Chicago/Turabian StyleJiao, Yuehua, Han Yang, Nditange Shigwedha, Shuang Zhang, Fei Liu, and Lanwei Zhang. 2022. "Probiotic Effects and Metabolic Products of Enterococcus faecalis LD33 with Respiration Capacity" Foods 11, no. 4: 606. https://doi.org/10.3390/foods11040606
APA StyleJiao, Y., Yang, H., Shigwedha, N., Zhang, S., Liu, F., & Zhang, L. (2022). Probiotic Effects and Metabolic Products of Enterococcus faecalis LD33 with Respiration Capacity. Foods, 11(4), 606. https://doi.org/10.3390/foods11040606








