The Effect of High Voltage Electrical Discharge on the Physicochemical Properties and the Microbiological Safety of Rose Hip Nectars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterisation of Rose Hip Pulp
2.3. Preparation of Rose Hip Nectar Formulations and HVED Treatment
2.4. Analysis
2.4.1. Measuring of Electrical Conductivity (EC)
2.4.2. Ascorbic Acid Determination
2.4.3. Extraction and Determination Total Phenolic Compounds (TPC)
2.4.4. Extraction and Determination of Flavan-3-ols and Flavonols
2.4.5. Antioxidant Activity
2.4.6. Colour Measurement and Total Colour Change
2.5. Microbiological Analysis
2.6. Statistical Analyses
3. Results and Discussion
3.1. Characteristics of Rose Hip Pulp
3.2. Effect of HVED Treatment (Frequency and Time) on Physicochemical Properties of Rose Hip Nectar
3.3. Influence of HVED Treatments on Physicochemical Properties of Rose Hip Nectar Formulations
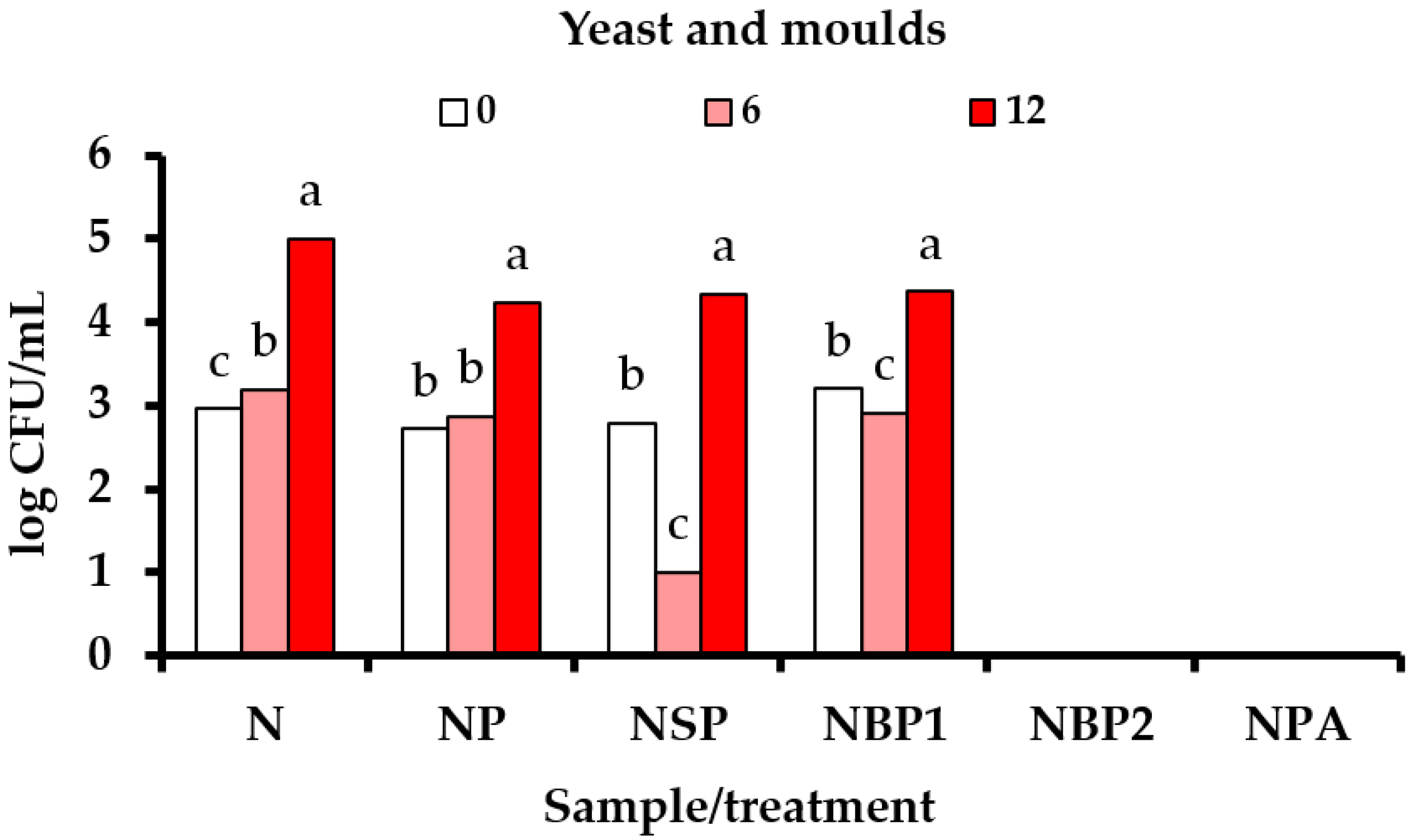
3.4. Influence of High Voltage Electrical Discharge (HVED) on Microbiological Quality of Rose Hip Nectar Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mármol, I.; Sánchez-De-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M. Therapeutic Applications of rose hips from different Rosa species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef] [PubMed]
- Tomljenović, N.; Jemrić, T.; Vuković, M. Variability in pomological traits of dog rose (Rosa canina L.) under the ecological conditions of the Republic of Croatia. Acta Agric. Serb. 2021, 26, 41–47. [Google Scholar] [CrossRef]
- Šindrak, Z.; Jemrić, T.; Baričević, L.; Dovedan, I.H.; Fruk, G. Kakvoća plodova sjemenjaka pasje ruže (Rosa canina L). J. Cent. Eur. Agric. 2012, 13, 321–330. [Google Scholar] [CrossRef][Green Version]
- Roman, I.; Stǎnilǎ, A.; Stǎnilǎ, S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013, 7, 73–82. [Google Scholar] [CrossRef]
- Igual, M.; Chiş, M.S.; Păucean, A.; Vodnar, D.C.; Muste, S.; Man, S.; Martínez-Monzó, J.; García-Segovia, P. Valorization of rose hip (Rosa canina) puree co-product in enriched corn extrudates. Foods 2021, 10, 2787. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frígola, A. High pressure treatment effect on physicochemical and nutritional properties of fluid foods during storage: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 307–322. [Google Scholar] [CrossRef]
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal treatments for fruit and vegetable juices and beverages: A literature overview. Compr. Rev. Food Sci. Food Saf. 2017, 16, 668–691. [Google Scholar] [CrossRef] [PubMed]
- Behera, G.; Rayaguru, K.; Nayak, P.K. Effect of microwave blanching on slice thickness and quality analysis of star fruit. Curr. Res. Nutr. Food Sci. 2017, 5, 274–281. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Senan, A.M.; Sultana, T.; Nasiru, M.M.; Shah, A.A.; Zhuang, H.; Jianhao, Z. Influence of combined effect of ultra-sonication and high-voltage cold plasma treatment on quality parameters of carrot juice. Foods 2019, 8, 593. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-Surface Modification of Biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Keener, K.M. Effects of cold plasma on food quality: A review. Foods 2018, 7, 4. [Google Scholar] [CrossRef]
- Yokoyama, T.; Miyazaki, S.; Akagi, H.; Ikawa, S.; Kitano, K. Kinetics of bacterial inactivation by peroxynitric acid in the presence of organic contaminants. Appl. Environ. Microbiol. 2021, 87, e01860-20. [Google Scholar] [CrossRef]
- Xu, L.; Garner, A.L.; Tao, B.; Keener, K.M. Microbial inactivation and quality changes in orange juice treated by high voltage atmospheric cold plasma. Food Bioproc. Technol. 2017, 10, 1778–1791. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Liao, J.; Jiang, A.; Xiu, Z.; Gaova, S.; Guan, Y.; Yang, X.; Feng, K.; Liu, C. Effect of atmospheric cold plasma treatment on antioxidant activities and reactive oxygen species production in postharvest blueberries during storage. Sci. Food Agric. 2020, 100, 5586–5595. [Google Scholar] [CrossRef] [PubMed]
- Ozen, E.; Singh, R.K. Atmospheric cold plasma treatment of fruit juices: A review. Trends Food Sci. Technol. 2020, 103, 144–151. [Google Scholar] [CrossRef]
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 2015, 32, 127–135. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Colonna, W.; Keener, K.M. Effect of high voltage atmospheric cold plasma on white grape juice quality. J. Sci. Food Agric. 2017, 97, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, J.; Muhammad, A.I.; Suo, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Application of a dielectric barrier discharge atmospheric cold plasma (dbd-acp) for esherichia coli inactivation in apple juice. J. Food Sci. 2018, 83, 401–408. [Google Scholar] [CrossRef]
- Starek, A.; Sagan, A.; Andrejko, D.; Chudzik, B.; Kobus, Z.; Kwiatkowski, M.; Terebun, P.; Pawłat, J. Possibility to extend the shelf life of NFC tomato juice using cold atmospheric pressure plasma. Sci. Rep. 2020, 10, 20959. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Režek Jambrak, A.; Milošević, S.; Dragović-Uzelac, V.; Zorić, Z.; Herceg, Z. The effect of gas phase plasma treatment on the anthocyanin and phenolic acid content of sour cherry marasca (Prunus cerasus var. Marasca) juice. LWT 2015, 62, 894–900. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Li, J.; Liu, S.; Zhang, H.; Bai, Y. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 2018, 254, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Surowsky, B.; Fröhling, A.; Gottschalk, N.; Schlüter, O.; Knorr, D. Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms. Int. J. Food Microbiol. 2014, 174, 63–71. [Google Scholar] [CrossRef]
- Montenegro, J.; Ruan, R.; Ma, H.; Chen, P. Inactivation of E. coli O157: H7 using a pulsed nonthermal plasma system. J. Food Sci. 2002, 67, 646–648. [Google Scholar] [CrossRef]
- Dasan, B.G.; Boyaci, I.H. Effect of cold atmospheric plasma on inactivation of Escherichia coli and physicochemical properties of apple, orange, tomato juices, and sour cherry nectar. Food Bioproc. Technol. 2018, 11, 334–343. [Google Scholar] [CrossRef]
- Tarabová, B.; Tampieri, F.; Maran, E.; Marotta, E.; Ostrihoňová, A.; Krewing, M.; Machala, Z. Chemical and antimicrobial effects of air non-thermal plasma processing of fresh apple juice with focus on safety aspects. Foods 2021, 10, 2055. [Google Scholar] [CrossRef]
- Shi, X.M.; Zhang, G.J.; Wu, X.L.; Li, Y.X.; Ma, Y.; Shao, X.J. Effect of low-temperature plasma on microorganism inactivation and quality of freshly squeezed orange juice. IEEE Plasma Sci. 2011, 39, 1591–1597. [Google Scholar] [CrossRef]
- Association Official Analytical Chemists. Official Methods of Analysis of AOAC International, 12th ed.; Association Official Analytical Chemists: Washington, DC, USA, 1980. [Google Scholar]
- Carr, M.H.; Haynes, D. The estimation of pectin as calcium pectate and the application of this method to the determination of the soluble pectin in apples. Biochem. J. 1922, 16, 60–69. [Google Scholar] [CrossRef]
- Ministarstvo Poljoprivrede (12/11/2021), Pravilnik o Voćnim Sokovima i Njima Sličnim Proizvodima Namijenjenim za Konzumaciju. Narodne Novine NN 48/2013. 2013. Available online: https://narodne-novine.nn.hr/eli/sluzbeni/2013/48/941 (accessed on 27 December 2021).
- Amerine, M.A.; Ough, C.S. Methods for Analysis of Musts and Wines; John Wiley & Sons: New York, NY, USA, 1988; pp. 377–382. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.B. Hydrophilic and lipophilic antioxidant activity in different leaves of three lettuce varieties. Int. J. Food Prop. 2005, 8, 521–528. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Cserhalmi, Z. Non-Thermal Pasteurization of Fruit Juice Using High Voltage Pulsed Electric Fields. In Handbook of Fruits and Fruit Processing; Hui, Y.H., Ed.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 95–114. [Google Scholar]
- HRN ISO 21527-2:2012; Microbiology of Food Chain-Part 2. HZN Glasilo: Zagreb, Croatia, 2012.
- HRN EN ISO 11290-1:2017; Microbiology of Food Chain-Part 1. HZN Glasilo: Zagreb, Croatia, 2017.
- HRN ISO 16649-2:2001; Microbiology of Food Chain-Part 2. HZN Glasilo: Zagreb, Croatia, 2001.
- HRN EN ISO 21528-2:2017; Microbiology of Food Chain-Part 2. HZN Glasilo: Zagreb, Croatia, 2017.
- HRN EN ISO 6579-1:2017/A1:2020; Microbiology of Food Chain-Amandment 1. HZN Glasilo: Zagreb, Croatia, 2020.
- HRN EN ISO 6579-1:2017; Microbiology of Food Chain-Part 1. HZN Glasilo: Zagreb, Croatia, 2017.
- HRN EN ISO 4833-1:2013; Microbiology of Food Change-Part 1. HZN Glasilo: Zagreb, Croatia, 2013.
- Tomljenović, N.; Jemrić, T.; Šimon, S.; Žulj Mihaljević, M.; Gaši, F.; Pejić, I. Genetic variability within and among generative dog rose (Rosa spp.) off springs. J. Cent. Eur. Agric. 2019, 20, 609–625. [Google Scholar] [CrossRef]
- Stanić, L. Bioaktivne Komponente Plodova Pasje Ruže. Master’s Thesis, University of Zagreb, Zagreb, Croatia, 2017. [Google Scholar]
- Demir, F.; Ozcan, M. Chemical and technological properties of rose (Rosa canina L.) fruits grown wild in Turkey. J. Food Eng. 2001, 47, 333–336. [Google Scholar] [CrossRef]
- Kazankaya, A.; Turkoglu, N.; Yilmaz, M.; Balta, M.F. Pomological description of Rosa canina selections from eastern Anatolia, Turkey. Int. J. Bot. 2004, 1, 100–102. [Google Scholar] [CrossRef]
- Brkić, A. Fizikalno-Kemijski Sastav i Antioksidativna Aktivnost Plodova i Kaše Šipka (Rosa canina L.). Bachelor’s Thesis, Josip Juraj Strossmayer University of Osijek, Osijek, Croatia, 2020. [Google Scholar]
- Güneş, M. Olgunlaşma süresince bazı kuşburnu meyvelerinde meydana gelen fiziksel ve kimya. JAFAG 2016, 33, 214–222. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.K.; Lee, C.Y. Relative Antioxidant and Cytoprotective Activities of Common Herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Bhave, A.; Schulzova, V.; Chmelarova, H.; Mrnka, L.; Hajslova, J. Assessment of rosehips based on the content of their biologically active compounds. J. Food Drug Anal. 2017, 25, 681–690. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total polyphenol content and antioxidant capacity of rosehips of some Rosa species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Illera, A.E.; Chaple, S.; Sanz, M.T.; Ng, S.; Lu, P.; Jones, J.; Carey, E.; Bourke, P. Effect of cold plasma on polyphenol oxidase inactivation in cloudy apple juice and on the quality parameters of the juice during storage. Food Chem. X 2019, 3, 100049. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (paw): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Grymonpré, D.R.; Sharma, A.K.; Finney, W.C.; Locke, B.R. The role of Fenton’s reaction in aqueous phase pulsed streamer corona reactors. Chem. Eng. Sci. 2001, 82, 189–207. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, W.; Zeng, X.; Zhang, Q.A.; Gao, G.; Song, S. Effects of cold plasma treatment on cherry quality during storage. Int. J. Food Sci. Technol. 2021, 27, 441–455. [Google Scholar] [CrossRef]
- Herceg, Z.; Kovačević, D.B.; Kljusurić, J.G.; Jambrak, A.R.; Zorić, Z.; Dragović-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Gajdoš Kljusurić, J.; Putnik, P.; Vukušić, T.; Herceg, Z.; Dragović-Uzelac, V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016, 212, 323–331. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.F.; Rodrigues, S.; Fernandes, F.A.N. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT 2017, 84, 457–463. [Google Scholar] [CrossRef]
- Sunka, P.; Babicky, V.; Clupek, M.; Fuciman, M.; Lukeš, P.; Simek, M.; Beneš, J.; Locke, B.; Majcherov, Z. Potential applications of pulse electrical discharges in water. Acta Phys. Slovaca 2004, 54, 135–145. [Google Scholar]
- Ministarstvo Poljoprivrede. Vodič za Mikrobiološke Kriterije za Hranu (Microbiological Guidebook for Food); Ministarstvo Poljoprivrede: Zagreb, Croatia, 2011.
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Misra, N.N.; Schlüter, O.; Cullen, P.J. Cold Plasma in Food and Agriculture; Elsevier: London, UK, 2020; pp. 253–273. [Google Scholar]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Lacombe, A.; Niemira, B.A.; Gurtler, J.B.; Fan, X.; Sites, J.; Boyd, G.; Chen, H. Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes. Food Microbiol. 2015, 46, 479–484. [Google Scholar] [CrossRef]
| Parameter | Content |
|---|---|
| total dry matter (TDM) | 31.48 ± 0.35% |
| soluble solid (SS) | 27.17 ± 0.40% |
| pH | 3.50 ± 0.02 |
| total acidity (expressed as citric acid) TA | 3.14 ± 0.04% |
| ascorbic acid | 500.74 ± 13.60 mg/100 g |
| total (TS) and reducing sugar (RS) | 6.47 ± 0.27%; 6.25 ± 0.0% |
| pectin compounds (expressed as pectate) PC | 11.79 ± 2.13% |
| total phenolic compounds (gallic acid equivalent) | 1.24 ± 0.006 g GAE/100 mL |
| antioxidant activity (ABTS) | 138.451 µmol TE/100 mL |
| antioxidant activity (DPPH) | 106.930 µmol TE/100 mL |
| Colour parameters L* | 30.02 ± 0.11 |
| C* | 34.17 ± 0.17 |
| °h | 46.50 ± 0.50 |
| F (Hz) | Sample/ Time (min) | pH | Electrical Conduct. (µS/cm) | Ascorbic Acid (mg/100 g) | TPC (g GAE/100 mL) | Flavan-3-ols (mg/kg) | Flavonols (mg/kg) | AA (µmol TE/100 mL) | Colour Parameter | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABTS | DPPH | L* | a* | ||||||||
| 0 | control | 3.70 ± 0 | 2223.0 ± 2.83 c | 97.57 ± 1.04 b | 0.212 ± 0.06 c | 487.76 ± 19.44 a | 22.96 ± 0.82 c | 28.62 ± 0.09 a | 15.85 ± 0.34 a | 37.43 ± 0.33 a | 10.93 ± 0.71 a |
| 50 | 10 | 3.60 ± 0 | 2256.0 ± 15.56 c | 104.11 ± 0.78 a | 0.234 ± 0.02 b | 513.16 ± 2.55 a | 31.01 ± 0.12 b | 22.89 ± 0.51 c | 12.70 ± 0.10 b | 34.02 ± 0.02 b | 8.16 ± 0.02 b |
| 50 | 15 | 3.60 ± 0 | 2427.5 ± 13.44 b | 100.41 ± 1.83 a,b | 0.187 ± 0.01 d | 494.03 ± 6.25 a | 33.69 ± 0.05 a | 25.29 ± 0.80 b | 13.35 ± 0.29 b | 33.73 ± 0.12 b | 8.00 ± 0.13 b |
| 50 | 20 | 3.60 ± 0 | 2506.5 ± 9.19 a | 106.51 ± 2.09 a | 0.278 ± 0.05 a | 521.72 ± 19.33 a | 30.31 ± 0.01 b | 25.35 ± 0.08 b | 13.28 ± 0.69 b | 34.13 ± 0.08 b | 8.13 ± 0.16 b |
| 0 | control | 3.70 ± 0 | 2223.0 ± 2.83 c | 97.57 ± 1.04 b | 0.212 ± 0.06 b | 487.76 ± 19.44 b | 22.96 ± 0.82 a | 28.62 ± 0.09 a | 15.85 ± 0.34 a | 37.43 ± 0.33 a | 10.93 ± 0.71 a |
| 100 | 10 | 3.50 ± 0 | 2513.0 ± 11.31 b | 105.03 ± 2.09 b | 0.237 ± 0.02 a | 481.71 ± 2.11 b | 32.19 ± 0.14 a | 26.08 ± 1.26 b | 15.17 ± 0.44 a,b | 34.19 ± 0.08 b | 8.21 ± 0.07 b |
| 100 | 15 | 3.50 ± 0 | 2549.5 ± 6.36 a,b | 99.48 ± 2.62 b | 0.217 ± 0.05 b | 539.09 ± 3.48 a | 29.62 ± 7.56 a | 26.97 ± 0.66 a,b | 13.49 ± 0.98 c | 34.21 ± 0.07 b | 8.29 ± 0.06 b |
| 100 | 20 | 3.50 ± 0 | 2585.0 ± 25.46 a | 113.72 ± 1.82 a | 0.236 ± 0.02 a | 550.90 ± 4.97 a | 32.23 ± 0.18 a | 25.07 ± 0.55 b | 14.09 ± 0.06 b,c | 34.19 ± 0.06 b | 8.25 ± 0.24 b |
| Sample |
Electrical Conduct.
(µS/cm) | Ascorbic Acid (mg/100 g) |
TPC
(g GAE/100 mL) |
Flavan-3-ols
(mg/kg) |
Flavonols
(mg/kg) |
AA
(µmol TE/100 mL) | |
|---|---|---|---|---|---|---|---|
| ABTS | DPPH | ||||||
| N | 2223.0 ± 2.00 f | 96.30 ± 0.75 b | 0.189 ± 0.04 b | 491.273 ± 24.52 a | 37.285 ± 0.02 a | 40.930 ± 1.14 a | 17.917 ± 0.74 a |
| NP | 2651.7 ± 9.50 b | 111.69 ± 3.14 a | 0.186 ± 0.01 b | 487.757 ± 19.44 a,b | 22.961 ± 0.82 d | 29.703 ± 1.48 c | 15.57 ± 0.32 b |
| NSP | 2790.3 ± 11.5 a | 96.71 ± 1.31 b | 0.223 ± 0.02 a | 371.607 ± 4.43 c | 29.187 ± 0.09 b | 26.049 ± 1.03 d | 14.863 ± 0.36 b |
| NBP 1 | 2600.0 ± 2.00 c | 105.96 ± 1.31 a | 0.163 ± 0.01 c | 430.119 ± 3.39 b,c | 30.261 ± 0.20 b | 27.330 ± 0.84 c,d | 14.745 ± 0.55 b |
| NBP 2 | 2339.7 ± 1.15 e | 77.15 ± 2.80 c | 0.169 ± 0.08 c | 479.875 ± 14.81 a,b | 26.245 ± 1.22 c | 33.066 ± 0.43 b | 17.273 ± 1.03 a |
| NPA | 2508.7 ± 0.58 d | 44.20 ± 1.83 d | 0.128 ± 0.03 d | 401.884 ± 13.57 c | 17.398 ± 0.00 e | 14.621 ± 0.89 e | 8.969 ± 0.25 c |
| Sample | Colour Parameter | ΔE | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C* | °h | ||
| N | 36.23 ± 0.06 a | 6.76 ± 0.16 b | 14.67 ± 0.05 a | 16.40 ± 0.61 a | 65.30 ± 0.70 a | n.a. |
| NP | 33.85 ± 0.13 c | 8.08 ± 0.05 a | 12.24 ± 0.21 c,d | 14.66 ± 0.19 b,c | 56.40 ± 0.30 d | 3.65 |
| NSP | 33.53 ± 0.01 d | 8.04± 0.06 a | 11.84 ± 0.06 d | 14.27 ± 0.16 c | 55.97 ± 0.06 d | 4.11 |
| NBP 1 | 34.33 ± 0.03 b | 8.11 ± 0.15 a | 12.95 ± 0.29 b | 15.14 ± 0.10 b | 57.80 ± 0.10 c | 2.90 |
| NBP 2 | 36.33 ± 0.11 a | 6.94 ± 0.23 b | 14.64 ± 0.17 a | 16.32 ± 0.13 a | 64.20 ± 0.40 a | 0.21 |
| NPA | 33.75 ± 0.07 c | 6.67 ± 0.07 b | 12.38 ± 0.16 b,c | 14.10 ± 0.13 c | 61.20 ± 0.36 b | 4.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiban, N.N.; Šimović, M.; Polović, M.; Šarić, A.; Tomac, I.; Matić, P.; Jakobek, L. The Effect of High Voltage Electrical Discharge on the Physicochemical Properties and the Microbiological Safety of Rose Hip Nectars. Foods 2022, 11, 651. https://doi.org/10.3390/foods11050651
Tiban NN, Šimović M, Polović M, Šarić A, Tomac I, Matić P, Jakobek L. The Effect of High Voltage Electrical Discharge on the Physicochemical Properties and the Microbiological Safety of Rose Hip Nectars. Foods. 2022; 11(5):651. https://doi.org/10.3390/foods11050651
Chicago/Turabian StyleTiban, Nela Nedić, Mirela Šimović, Martina Polović, Antonija Šarić, Ivana Tomac, Petra Matić, and Lidija Jakobek. 2022. "The Effect of High Voltage Electrical Discharge on the Physicochemical Properties and the Microbiological Safety of Rose Hip Nectars" Foods 11, no. 5: 651. https://doi.org/10.3390/foods11050651
APA StyleTiban, N. N., Šimović, M., Polović, M., Šarić, A., Tomac, I., Matić, P., & Jakobek, L. (2022). The Effect of High Voltage Electrical Discharge on the Physicochemical Properties and the Microbiological Safety of Rose Hip Nectars. Foods, 11(5), 651. https://doi.org/10.3390/foods11050651






