Interaction between Fish Skin Gelatin and Pea Protein at Air-Water Interface after Ultrasound Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
Standardization of the FG and PPI Solutions
2.2. Ultrasound Treatment
2.3. pH and Temperature Values
2.4. Hydrodynamic Diameter (Dh)
2.5. ζ-Potential Measurements
2.6. SEC-MALS
2.7. In Bulk Rheological Analysis
2.8. Foaming Properties
2.9. Bubble Size with Turbiscan Tower
2.10. Confocal Laser Scanning Microscopy (CLSM)
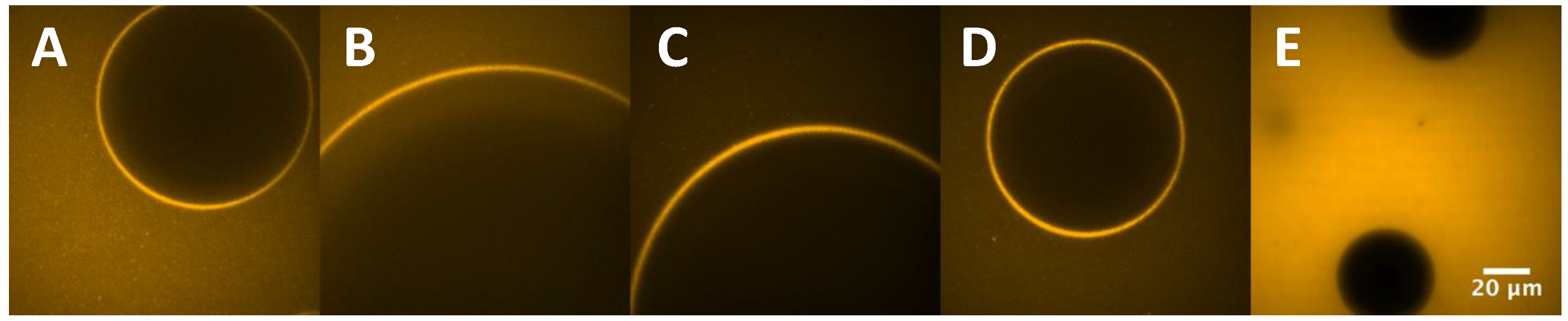
2.11. Interfacial Properties
2.12. Statistical Analysis
3. Results and Discussion
3.1. pH Values
3.2. Hydrodynamic Diameter (Dh) and ζ-Potential
3.3. SEC-MALS
3.4. Rheology
3.5. Foaming Properties
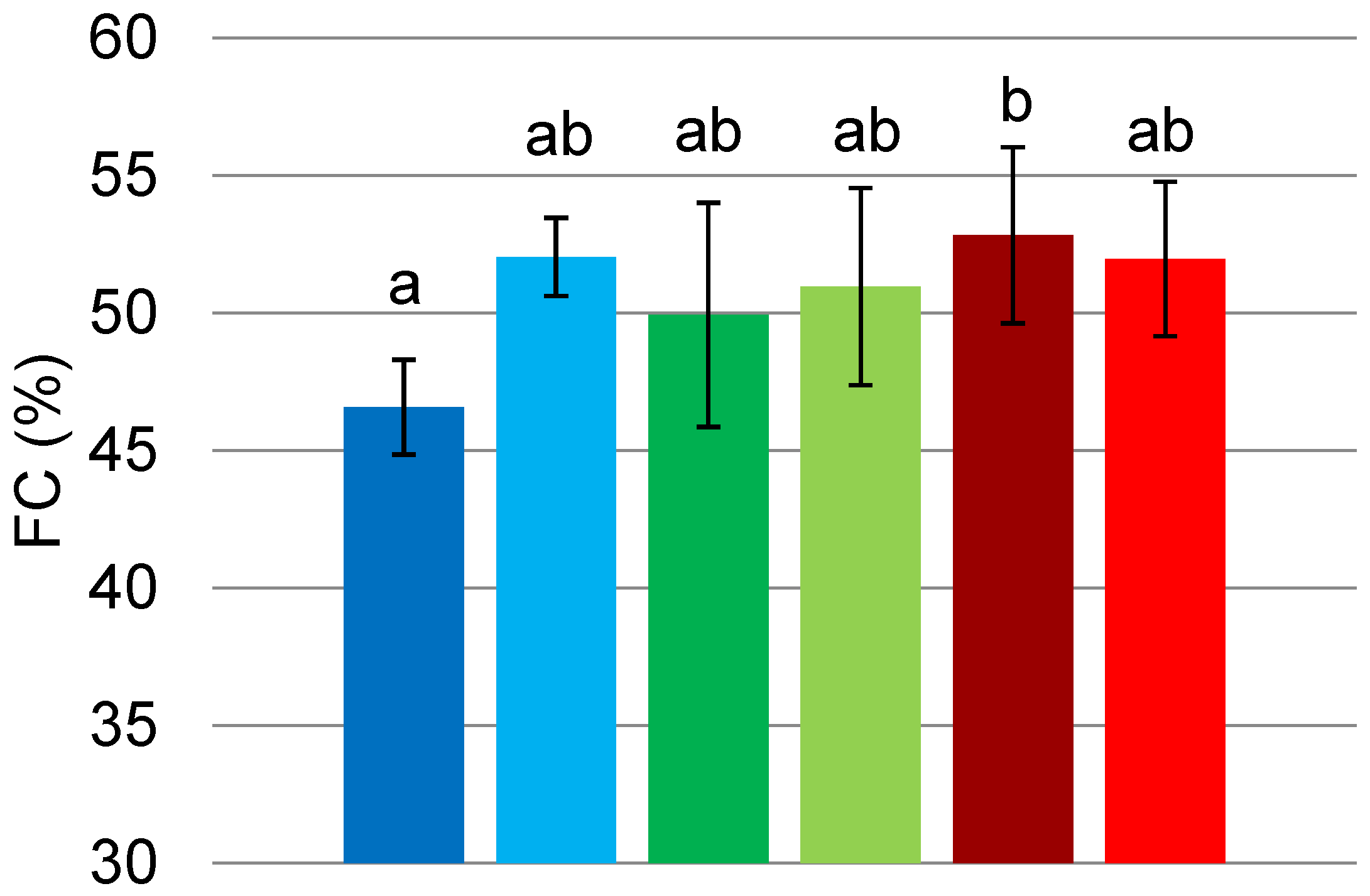
3.5.1. Foam Capacity (FC)
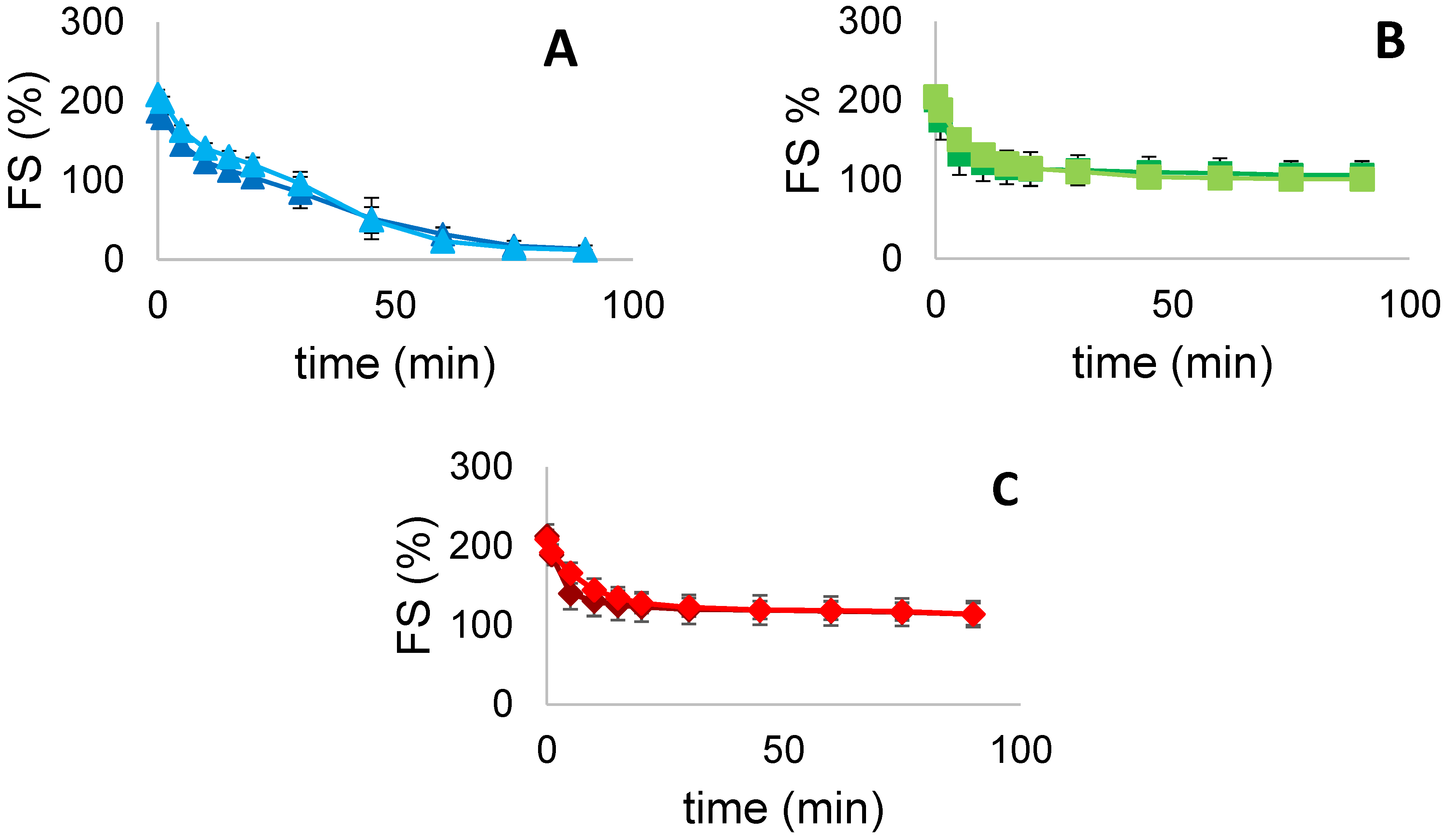
3.5.2. Foam Stability (FS)
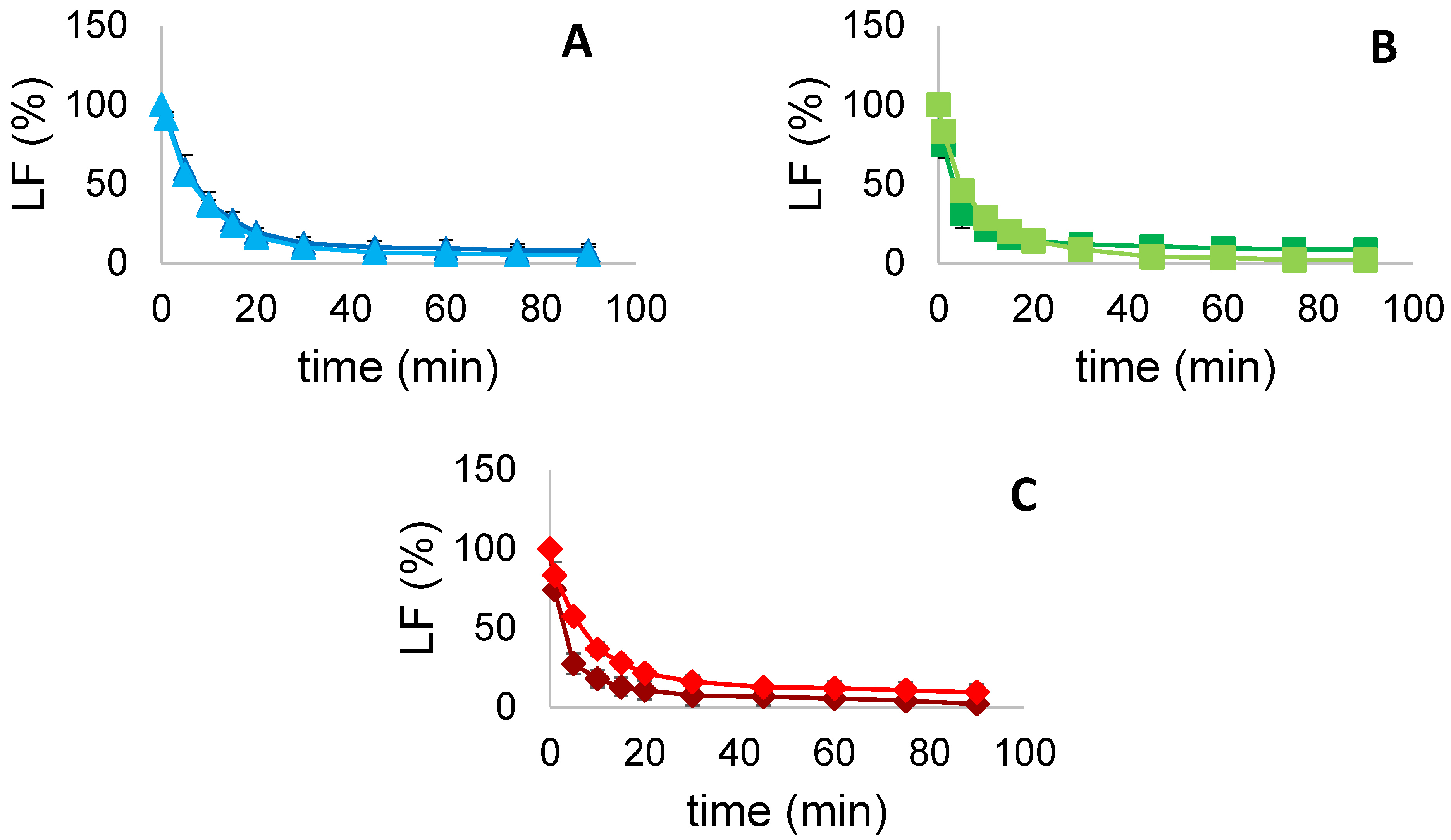
3.5.3. Liquid Fraction (LF)
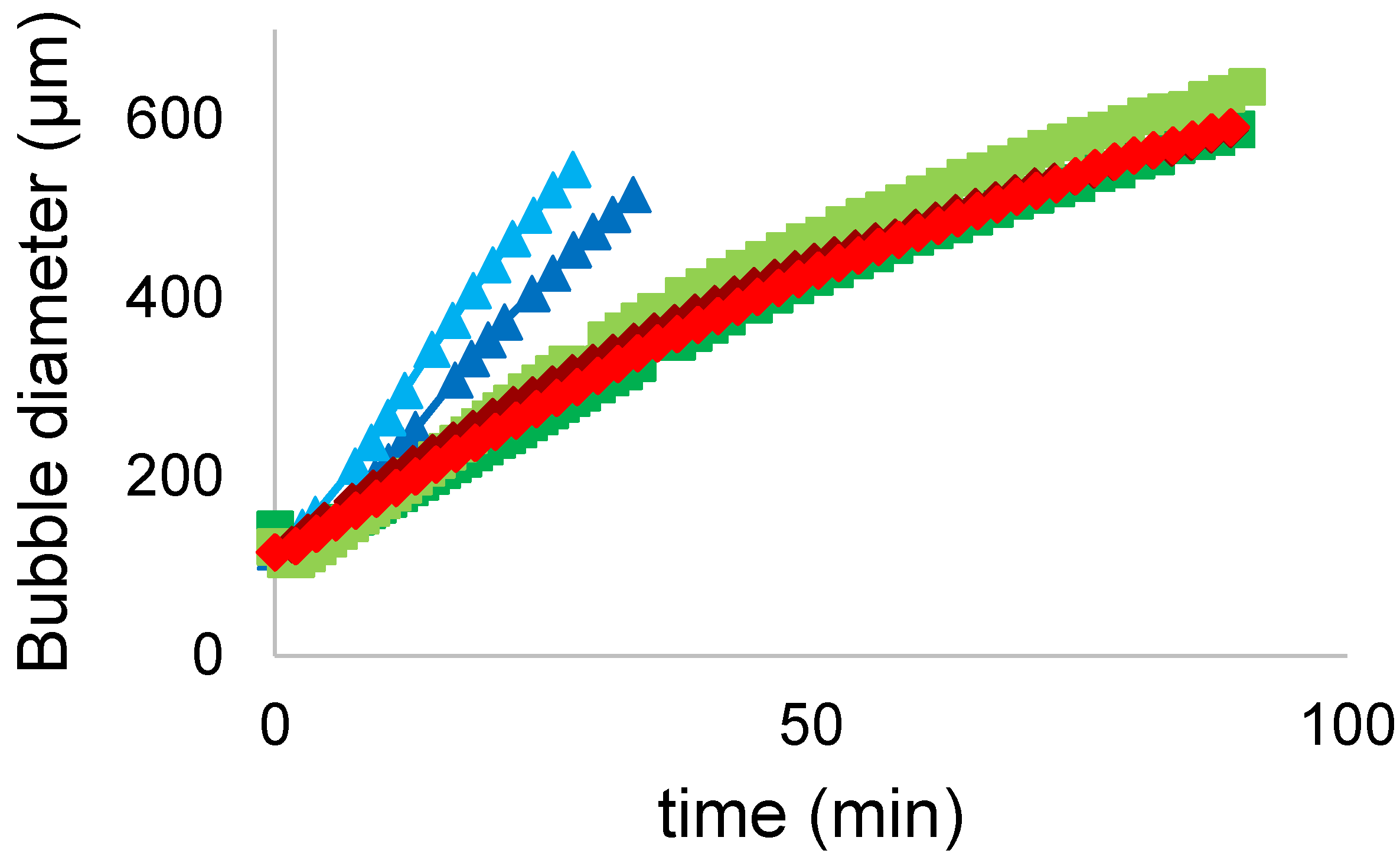
3.5.4. Turbiscan Tower
3.6. Confocal Laser Scanning Microscopy (CLSM)
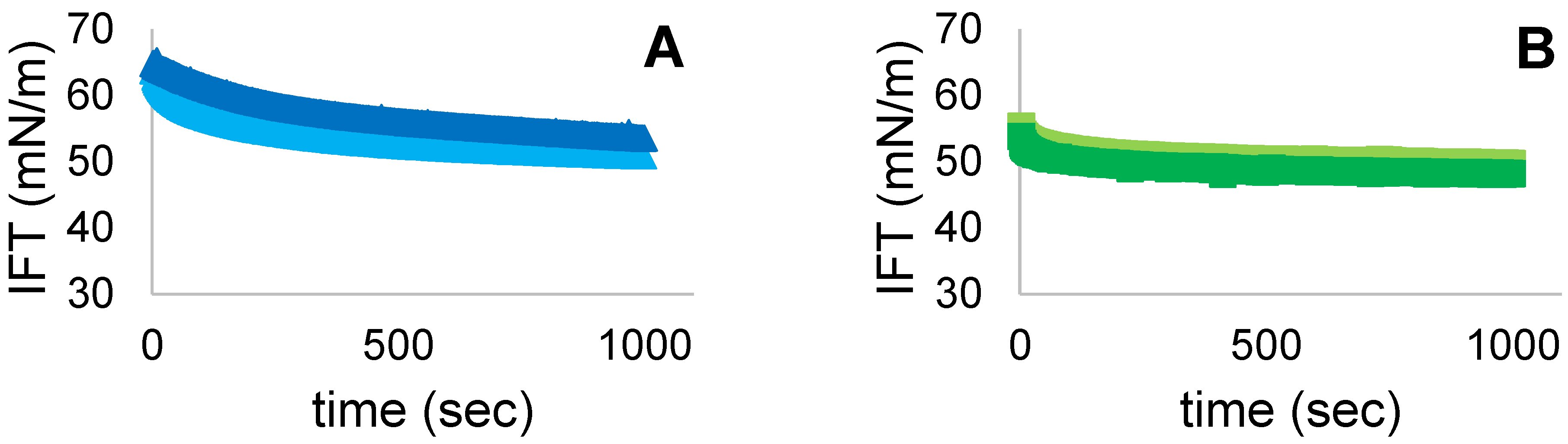
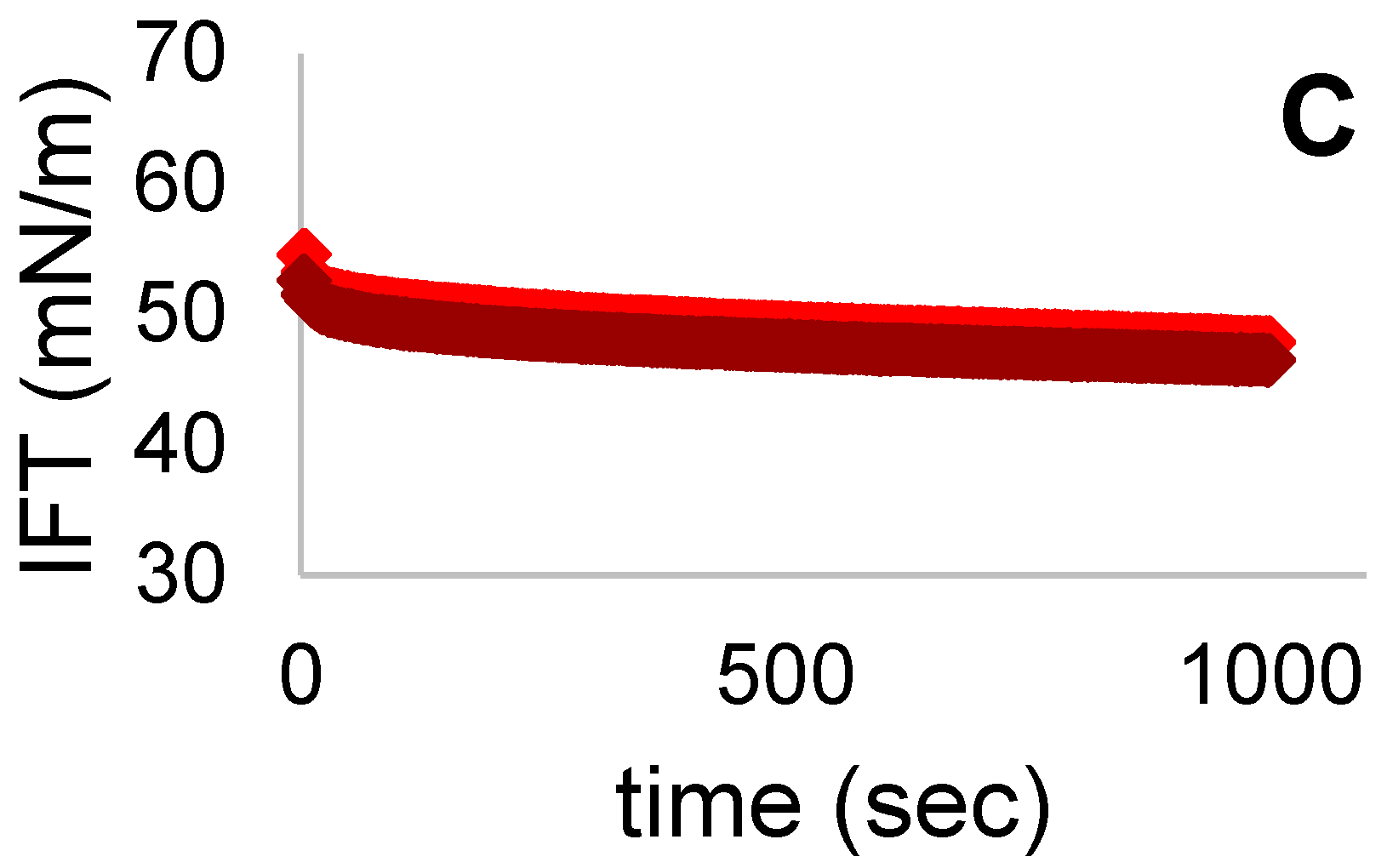
3.7. Interfacial Tension (IFT)
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zayas, J.F. Functionality of Proteins in Food. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Kralova, I.; Sjöblom, J. Surfactants used in food industry: A review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Tomadoni, B.; Capello, C.; Valencia, G.A.; Gutiérrez, T.J. Self-assembled proteins for food applications: A review. Trends Food Sci. Technol. 2020, 101, 1–16. [Google Scholar] [CrossRef]
- Benjakul, S.; Kittiphattanabawon, P.; Regenstein, J.M. Fish Gelatin. In Food Biochemistry and Food Processing, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2012. [Google Scholar] [CrossRef]
- Benjakul, S.; Kittiphattanabawon, P. Gelatin. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–127. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef]
- Casanova, F.; Mohammadifar, M.A.; Jahromi, M.; Petersen, H.O.; Eybye, K.L.; Kobbelgaard, S.; Jakobsen, G.; Jessen, F. Physico-chemical, structural and techno-functional properties of gelatin from saithe (Pollachius virens) skin. Int. J. Biol. Macromol. 2020, 156, 918–927. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; Münch, K.; Sagis, L.; Schroën, K.; Berton-Carabin, C.C. Synergistic stabilisation of emulsions by blends of dairy and soluble pea proteins: Contribution of the interfacial composition. Food Hydrocoll. 2019, 97, 105206. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; Sagis, L.; Schroën, K.; Berton-Carabin, C.C. Behavior of plant-dairy protein blends at air-water and oil-water interfaces. Colloids Surf. B Biointerfaces 2020, 192, 111015. [Google Scholar] [CrossRef]
- Vall-llosera, M.; Jessen, F.; Henriet, P.; Marie, R.; Jahromi, M.; Sloth, J.J.; Mohammadifar, M.A.; Petersen, H.O.; Jørgensen, B.M.; Casanova, F. Physical Stability and Interfacial Properties of Oil in Water Emulsion Stabilized with Pea Protein and Fish Skin Gelatin. Food Biophys. 2020, 16, 139–151. [Google Scholar] [CrossRef]
- Lionberger, R.A.; Russel, W.B. A Smoluchowski theory with simple approximations for hydrodynamic interactions in concentrated dispersions. J. Rheol. 1997, 41, 399–425. [Google Scholar] [CrossRef]
- Lock, J.A.; Gouesbet, G. Generalized Lorenz-Mie theory and applications. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 800–807. [Google Scholar] [CrossRef]
- Sakurai, K.; Konuma, T.; Yagi, M.; Goto, Y. Structural dynamics and folding of β-lactoglobulin probed by heteronuclear NMR. Biochim. Biophys. Acta Gen. Subj. 2009, 1970, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Klost, M.; Giménez-Ribes, G.; Drusch, S. Enzymatic hydrolysis of pea protein: Interactions and protein fractions involved in fermentation induced gels and their influence on rheological properties. Food Hydrocoll. 2020, 105, 105793. [Google Scholar] [CrossRef]
- Silva, M.; Zisu, B.; Chandrapala, J. Influence of low-frequency ultrasound on the physico-chemical and structural characteristics of milk systems with varying casein to whey protein ratios. Ultrason. Sonochem. 2018, 49, 268–276. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y. Recent progresses of understanding the viscosity of concentrated protein solutions. Curr. Opin. Chem. Eng. 2017, 16, 48–55. [Google Scholar] [CrossRef]
- Ahmed, J.; Ptaszek, P.; Basu, S. Food Rheology: Scientific Development and Importance to Food Industry. In Advances in Food Rheology and Its Applications; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar] [CrossRef]
- Sharma, V.; Jaishankar, A.; Wang, Y.C.; McKinley, G.H. Rheology of globular proteins: Apparent yield stress, high shear rate viscosity and interfacial viscoelasticity of bovine serum albumin solutions. Soft Matter 2011, 7, 5150–5160. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Morales, R.; Martínez, K.D.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M.R. Modification of foaming properties of soy protein isolate by high ultrasound intensity: Particle size effect. Ultrason. Sonochem. 2015, 26, 2416–2420. [Google Scholar] [CrossRef]
- Li, R.; Chen, X.E.; Chang, Y.; Zhang, L.; Zhang, Y.; Zhu, Y.; Wang, T. Increase of bubble size playing a critical role in foam-induced protein aggregation: Aggregation of BSA in foam fractionation. Chem. Eng. Sci. 2017, 174, 387–395. [Google Scholar] [CrossRef]
- Jarpa-parra, M.; Tian, Z.; Temelli, F.; Zeng, H.; Chen, L. Understanding the stability mechanisms of lentil legumin-like protein and polysaccharide foams. Food Hydrocoll. 2016, 61, 903–913. [Google Scholar] [CrossRef]
- Delahaije, R.J.B.M.; Wierenga, P.A.; Giuseppin, M.L.F.; Gruppen, H. Improved emulsion stability by succinylation of patatin is caused by partial unfolding rather than charge effects. J. Colloid Interface Sci. 2014, 430, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wierenga, P.A.; Meinders, M.B.J.; Egmond, M.R.; Voragen, A.G.J.; De Jongh, H.H.J. Quantitative description of the relation between protein net charge and protein adsorption to air-water interfaces. J. Phys. Chem. B 2005, 109, 16946–16952. [Google Scholar] [CrossRef] [PubMed]


| pH | FG | PPI | MIX |
|---|---|---|---|
| Untreated | 7.13 ± 0.1 a | 7.34 ± 0.2 b | 7.27 ± 0.1 d |
| Sonicated | 7.14 ± 0.1 a | 7.24 ± 0.1 c | 7.15 ± 0.3 e |
| Sample | Fraction 1 | Fraction 2 | Fraction 3 | Fraction 4 | |
|---|---|---|---|---|---|
| Mw (kDa) | |||||
| FG | Untreated | 33.2 | |||
| Sonicated | 29.8 | ||||
| PPI | Untreated | 879.4 | 164.4 | 61.2 | 44.1 |
| Sonicated | 820.3 | 167.6 | 67.1 | 49.4 | |
| MIX | Untreated | 1048.1 | 208.9 | 102.0 | 84.9 |
| Sonicated | 2269.5 | 428.4 | 188.7 | 136.7 | |
| Power Law | m (mPa.s) * | n ** | R2 |
|---|---|---|---|
| FG untreated | 1.3 | 1.1 | 0.99 |
| FG sonicated | 1 | 1.1 | 0.95 |
| PPI untreated | 1.5 | 1 | 0.98 |
| PPI sonicated | 1 | 1.1 | 0.98 |
| MIX untreated | 1.2 | 1.1 | 0.99 |
| MIX sonicated | 1.3 | 1 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odelli, D.; Sarigiannidou, K.; Soliani, A.; Marie, R.; Mohammadifar, M.A.; Jessen, F.; Spigno, G.; Vall-llosera, M.; de Carvalho, A.F.; Verni, M.; et al. Interaction between Fish Skin Gelatin and Pea Protein at Air-Water Interface after Ultrasound Treatment. Foods 2022, 11, 659. https://doi.org/10.3390/foods11050659
Odelli D, Sarigiannidou K, Soliani A, Marie R, Mohammadifar MA, Jessen F, Spigno G, Vall-llosera M, de Carvalho AF, Verni M, et al. Interaction between Fish Skin Gelatin and Pea Protein at Air-Water Interface after Ultrasound Treatment. Foods. 2022; 11(5):659. https://doi.org/10.3390/foods11050659
Chicago/Turabian StyleOdelli, Davide, Krystalia Sarigiannidou, Alberto Soliani, Rodolphe Marie, Mohammad Amin Mohammadifar, Flemming Jessen, Giorgia Spigno, Mar Vall-llosera, Antonio Fernandes de Carvalho, Michela Verni, and et al. 2022. "Interaction between Fish Skin Gelatin and Pea Protein at Air-Water Interface after Ultrasound Treatment" Foods 11, no. 5: 659. https://doi.org/10.3390/foods11050659
APA StyleOdelli, D., Sarigiannidou, K., Soliani, A., Marie, R., Mohammadifar, M. A., Jessen, F., Spigno, G., Vall-llosera, M., de Carvalho, A. F., Verni, M., & Casanova, F. (2022). Interaction between Fish Skin Gelatin and Pea Protein at Air-Water Interface after Ultrasound Treatment. Foods, 11(5), 659. https://doi.org/10.3390/foods11050659











