Effect of Protein Thermal Denaturation on the Texture Profile Evolution of Beijing Roast Duck
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Texture Profile Evolution
2.2.1. Shear Force
2.2.2. Texture Profile Analysis
2.3. Microstructure Analysis
2.4. Protein Thermal Stability
2.5. Intermolecular Forces of Actomyosin
2.6. Statistical Analysis
3. Results
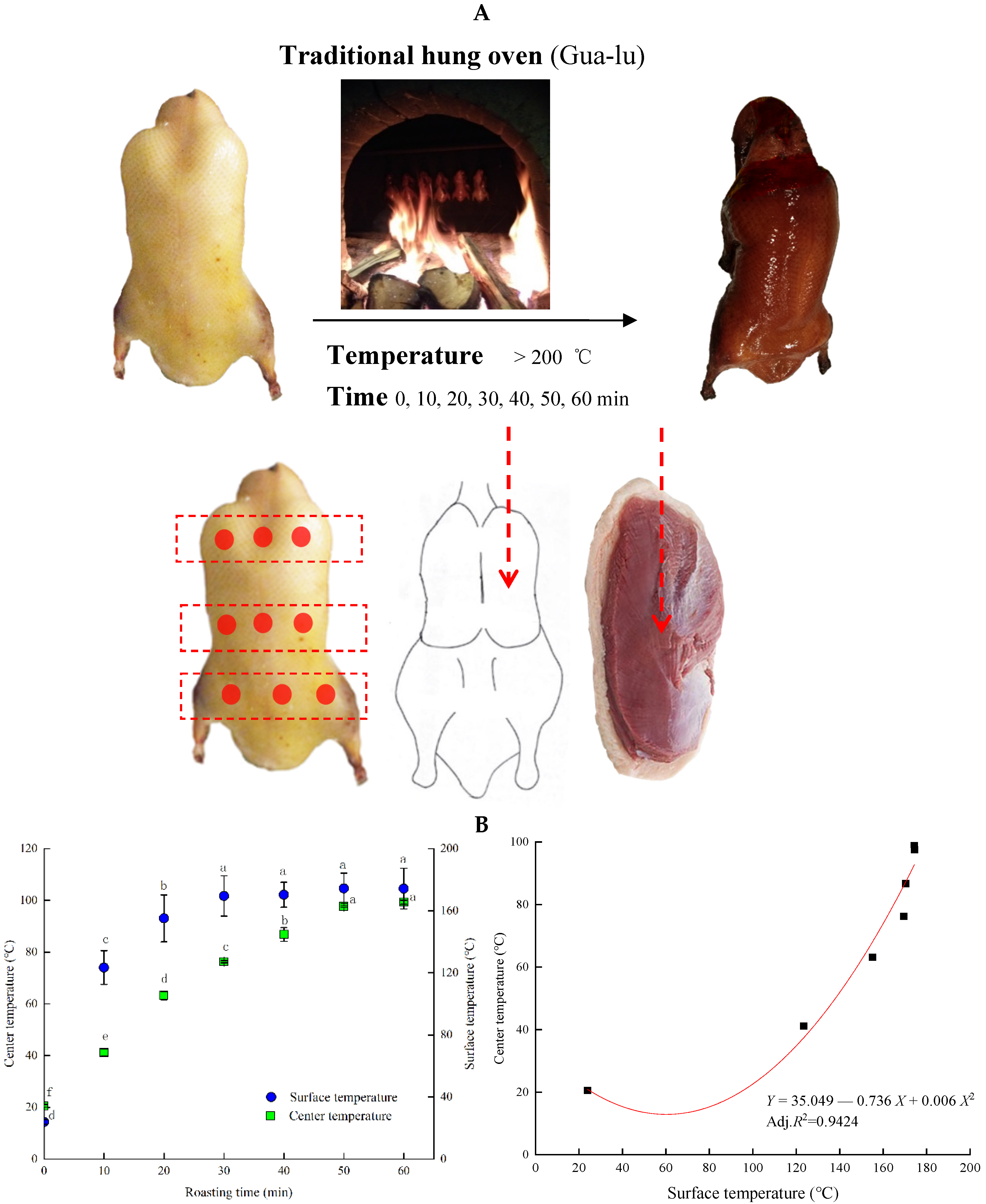
3.1. The Center and Surface Temperature of Duck Breast Meat
3.2. Changes in Shear Force and Texture Profile Analysis
3.3. Microstructure Changes
3.4. Changes in Intermolecular Forces of Myofibrillar Proteins
3.5. Analysis of Protein Thermal Stability
4. Discussion
4.1. Effects of Temperature on Protein Denaturation and Interprotein Forces
4.2. Microstructural Changes Caused by Protein Denaturation and Interprotein Forces
4.3. Mechanism of Texture Formation during Roasting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, G.; Weigel, S.; Tang, B.; Schulz, C.; Shen, J. The occurrence of polycyclic aromatic hydrocarbons in peking duck: Relevance to food safety assessment. Food Chem. 2011, 129, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Bjarnadóttir, S.G.; Hollung, K.; Høy, M.; Bendixen, E.; Codrea, M.C.; Veiseth-Kent, E. Changes in protein abundance between tender and tough meat from bovine longissimus thoracis muscle assessed by isobaric tag for relative and absolute quantitation (itraq) and 2-dimensional gel electrophoresis analysis. J. Anim. Sci. 2012, 90, 2035–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, B.; Rinnan, Å.; Ruiz-Carrascal, J. Tracking hydrophobicity state, aggregation behavior and structural modifications of pork proteins under the influence of assorted heat treatments. Food Res. Int. 2017, 101, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Huang, M.; Xu, X.; Zhou, G. Influence of heat on protein degradation, ultrastructure and eating quality indicators of pork. J. Sci. Food Agric. 2011, 91, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, D.; Pasquini, M. Influence of cooking conditions on cooking loss and tenderness of raw and marinated chicken breast meat. LWT-Food Sci. Technol. 2005, 38, 895–901. [Google Scholar] [CrossRef]
- Laville, E.; Sayd, T.; Morzel, M.; Blinet, S.; Chambon, C.; Lepetit, J.; Renand, G.; Hocquettee, J.F. Proteome changes during meat aging in tough and tender beef suggest the importance of apoptosis and protein solubility for beef aging and tenderization. J. Agric. Food Chem. 2009, 57, 10755–10764. [Google Scholar] [CrossRef]
- Paredi, G.; Sentandreu, M.A.; Mozzarelli, A.; Fadda, S.; de Almeida, A.M. Muscle and meat: New horizons and applications for proteomics on a farm to fork perspective. J. Proteom. 2013, 88, 58–82. [Google Scholar] [CrossRef]
- Yarmand, M.S.; Nikmaram, P.; Djomeh, Z.E.; Homayouni, A. Microstructural and mechanical properties of camel longissimus dorsi muscle during roasting, braising and microwave heating. Meat Sci. 2013, 95, 419–424. [Google Scholar] [CrossRef]
- Promeyrat, A.; Daudin, J.D.; Gatellier, P. Kinetics of protein physicochemical changes induced by heating in meat using mimetic models: (1) relative effects of heat and oxidants. Food Chem. 2013, 138, 581–589. [Google Scholar] [CrossRef]
- Promeyrat, A.; Gatellier, P.; Lebret, B.; Kajak-Siemaszko, K.; Aubry, L.; Sante-Lhoutellier, V. Evaluation of protein aggregation in cooked meat. Food Chem. 2010, 121, 412–417. [Google Scholar] [CrossRef]
- Yu, T.Y.; Morton, J.D.; Clerens, S.; Dyer, J.M. Proteomic investigation of protein profile changes and amino acid residue-level modification in cooked lamb longissimus thoracis et lumborum: The effect of roasting. Meat Sci. 2016, 119, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Morton, J.D.; Clerens, S.; Dyer, J.M. Proteomic investigation of protein profile changes and amino acid residue level modification in cooked lamb meat: The effect of boiling. J. Agric. Food Chem. 2015, 63, 9112–9123. [Google Scholar] [CrossRef] [PubMed]
- Huang, J. The dining experience of Beijing roast duck: A comparative study of the Chinese and English online consumer reviews. Int. J. Hosp. Manag. 2017, 66, 117–129. [Google Scholar] [CrossRef]
- Wyrwisz, J.; Poltorak, A.; Polawska, E.; Pierzchala, M.; Wierzbicka, A. The impact of heat treatment methods on the physical properties and cooking yield of selected muscles from limousine breed cattle. Anim. Sci. Pap. Rep. 2012, 30, 339–351. [Google Scholar]
- Ojha, K.S.; Granato, D.; Rajuria, G.; Barba, F.J.; Kerry, J.P.; Tiwari, B.K. Application of chemometrics to assess the influence of ultrasound frequency, lactobacillus sakei culture and drying on beef jerky manufacture: Impact on amino acid profile, organic acids, texture and colour. Food Chem. 2017, 239, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xing, T.; Chen, X.; Han, M.; Xu, X.; Zhou, G. Yield, thermal denaturation, and microstructure of proteins isolated from pale, soft, exudative chicken breast meat by using isoelectric solubilization/precipitation. Process Biochem. 2017, 58, 167–173. [Google Scholar] [CrossRef]
- Glorieux, S.; Steen, L.; Paelinck, H.; Foubert, I.; Fraeye, I. Isothermal gelation behavior of myofibrillar proteins from white and red chicken meat at different temperatures. Poult. Sci. 2017, 96, 3785–3795. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Borderías, A.J.; Montero, P. Chemical interactions of nonmuscle proteins in the network of sardine (Sardina pilchardus) muscle gels. LWT-Food Sci. Technol. 1997, 30, 602–608. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.; Xie, B.; Xiong, S. Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocoll. 2011, 25, 898–906. [Google Scholar] [CrossRef]
- Ni, N.; Wang, Z.; He, F.; Wang, L.; Pan, H.; Li, X.; Wang, Q.; Zhang, D. Gel properties and molecular forces of lamb myofibrillar protein during heat induction at different pH values. Process Biochem. 2014, 49, 631–636. [Google Scholar] [CrossRef]
- Wright, D.J.; Leach, I.B.; Wilding, P. Differential scanning calorimetric studies of muscle and its constituent proteins. J. Sci. Food Agric. 1977, 28, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.J.; Wilding, P. Differential scanning calorimetric study of muscle and its proteins: Myosin and its subfragments. J. Sci. Food Agric. 1984, 35, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Feng, J.; Cao, A.; Zhang, Y.; Lv, Y.; Li, J. Denaturation kinetics and aggregation mechanism of the sarcoplasmic and myofibril proteins from grass carp during microwave processing. Food Bioprocess Technol. 2017, 11, 417–426. [Google Scholar] [CrossRef]
- Jongberg, S.; Terkelsen, L.D.S.; Miklos, R.; Lund, M.N. Green tea extract impairs meat emulsion properties by disturbing protein disulfide cross-linking. Meat Sci. 2015, 100, 2–9. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, W.; Yang, F.; Nie, X. Protein molecular interactions involved in the gel network formation of fermented silver carp mince inoculated with Pediococcus pentosaceus. Food Chem. 2010, 120, 717–723. [Google Scholar] [CrossRef]
- Chan, J.K.; Gill, T.A.; Paulson, A. Cross-linking of myosin heavy chains from cod, herring and silver hake during thermal setting. J. Food Sci. 1992, 57, 906–912. [Google Scholar] [CrossRef]
- Lefevre, F.; Fauconneau, B.; Thompson, J.W.; Gill, T.A. Thermal denaturation and aggregation properties of atlantic salmon myofibrils and myosin from white and red muscles. J. Agric. Food Chem. 2007, 55, 4761–4770. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.; Xiong, S.; Xie, B.; Qin, L. Role of secondary structures in the gelation of porcine myosin at different pH values. Meat Sci. 2008, 80, 632–639. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, W.; Jiang, Q. Aggregation and structural changes of silver carp actomyosin as affected by mild acidification with d-gluconic acid δ-lactone. Food Chem. 2012, 134, 1005–1010. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Visessanguan, W.; Kijroongrojana, K. Thermal properties and heat-induced aggregation of natural actomyosin extracted from goatfish (Mulloidichthys martinicus) muscle as influenced by iced storage. Food Hydrocoll. 2009, 23, 1779–1784. [Google Scholar] [CrossRef]
- Pathare, P.B.; Roskilly, A.P. Quality and Energy Evaluation in Meat Cooking. Food Eng. Rev. 2016, 8, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Pan, T.; Liu, H.; Ingrid Boga, L.; Hussian, Z.; Suleman, R.; Zhang, D.; Wang, Z. The Effect of Age on the Myosin Thermal Stability and Gel Quality of Beijing Duck Breast. Food Sci. Anim. Resour. 2020, 40, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, D.; Xu, W.; Gao, F.; Zhou, G. Effect of final cooked temperature on tenderness, protein solubility and microstructure of duck breast muscle. LWT-Food Sci. Technol. 2013, 51, 266–274. [Google Scholar] [CrossRef]
- Christensen, L.; Bertram, H.C.; Aaslyng, M.D.; Christensen, M. Protein denaturation and water-protein interactions as affected by low temperature long time treatment of porcine longissimus dorsi. Meat Sci. 2011, 88, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Palka, K.; Daun, H. Changes in texture, cooking losses, and myofibrillar structure of bovine m. semitendinosus during heating. Meat Sci. 1999, 51, 237–243. [Google Scholar] [CrossRef]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Effect of heat treatment on changes in texture, structure and properties of thai indigenous chicken muscle. Food Chem. 2005, 93, 337–348. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Zu, S.; Wu, X.; Shi, A.; Zhang, J.; Wang, Q.; He, N. Effects of High Hydrostatic Pressure on the Conformational Structure and Gel Properties of Myofibrillar Protein and Meat Quality: A Review. Foods 2021, 10, 1872. [Google Scholar] [CrossRef]
- Fabre, R.; Dalzotto, G.; Perlo, F.; Bonato, P.; Tisocco, O. Cooking method effect on warner-bratzler shear force of different beef muscles. Meat Sci. 2018, 138, 10–14. [Google Scholar] [CrossRef]
- Scussat, S.; Vaulot, C.; Ott, F.; Cayot, P.; Loupiac, C. The impact of cooking on meat microstructure studied by low field nmr and neutron tomography. Food Struct. 2017, 14, 36–45. [Google Scholar] [CrossRef]
- Zaboli, G.; Huang, X.; Feng, X.; Dong, U.A. How can heat stress affect chicken meat quality?—A review. Poult. Sci. 2019, 98, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Vaskoska, R.; Ha, M.; Naqvi, Z.B.; White, J.D.; Warner, R.D. Muscle, Ageing and Temperature Influence the Changes in Texture, Cooking Loss and Shrinkage of Cooked Beef. Foods 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Llave, Y.; Shibata-Ishiwatari, N.; Watanabe, M.; Fukuoka, M.; Hamada-Sato, N.; Sakai, N. Analysis of the effects of thermal protein denaturation on the quality attributes of sous-vide cooked tuna. J. Food Process. Preserv. 2017, 42, 13347–13358. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Xiong, R.; Chang, Y.; Owens, C.M.; Meullenet, J.F. Effects of cooking methods on textural properties and water-holding capacity of broiler breast meat deboned at various postmortem times. J. Texture Stud. 2014, 45, 377–386. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Dong, H.; Xu, W.; Gao, F.; Zhou, G.; Zhang, M. Effects of different cooking regimes on the microstructure and tenderness of duck breast muscle. J. Sci. Food Agric. 2013, 93, 1979–1984. [Google Scholar] [CrossRef]




| Roasting Time/min | Hardness/g | Adhesiveness/N·s | Springiness/mm | Cohesiveness | Gumminess | Chewiness/N | Resilience |
|---|---|---|---|---|---|---|---|
| 0 | 6221.15 ± 1581.25 b | −15.06 ± 3.47 ab | 0.38 ± 0.06 c | 0.37 ± 0.07 b | 2372.63 ± 806.90 b | 966.06 ± 305.66 b | 0.24 ± 0.05 ab |
| 10 | 7719.40 ± 1606.31 ab | −14.10 ± 3.79 abc | 0.38 ± 0.08 bc | 0.37 ± 0.06 b | 3150.43 ± 1032.03 ab | 1303.33 ± 348.11 ab | 0.24 ± 0.06 ab |
| 20 | 9394.92 ± 1628.89 a | −16.40 ± 4.29 ab | 0.45 ± 0.07 a | 0.44 ± 0.05 ab | 4249.31 ± 837.18 a | 1957.54 ± 412.57 a | 0.21 ± 0.04 b |
| 30 | 6819.49 ± 1092.67 b | −8.62 ± 2.11 c | 0.51 ± 0.05 a | 0.47 ± 0.06 a | 3197.49 ± 373.18 ab | 1626.57 ± 217.55 a | 0.20 ± 0.03 b |
| 40 | 6725.24 ± 1081.84 b | −9.20 ± 3.03 bc | 0.52 ± 0.06 a | 0.48 ± 0.07 a | 3221.04 ± 513.96 ab | 1677.39 ± 322.01 ab | 0.19 ± 0.04 bc |
| 50 | 7887.75 ± 1240.04 ab | −10.04 ± 3.55 abc | 0.51 ± 0.08 a | 0.44 ± 0.04 ab | 3468.84 ± 612.06 ab | 1844.42 ± 320.75 ab | 0.16 ± 0.02 c |
| 60 | 7085.69 ± 968.19 ab | −6.43 ± 3.91 c | 0.47 ± 0.08 ab | 0.45 ± 0.06 ab | 3253.19 ± 538.99 ab | 1563.02 ± 332.22 ab | 0.15 ± 0.03 c |
| Roasting Time/min | Peak Ⅰ | Peak Ⅱ | Peak Ⅲ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature/°C | Area/mJ | ΔH/J/g | Temperature/°C | Area/mJ | ΔH/J/g | Temperature/°C | Area/mJ | ΔH/J/g | |
| 0 | 55.10 ± 0.48 a | 1.05 ± 0.62 a | 0.07 ± 0.04 a | 62.02 ± 1.10 b | 3.06 ± 0.69 b | 0.20 ± 0.05 b | 78.12 ± 0.14 ab | 4.63 ± 0.95 a | 0.31 ± 0.06 a |
| 10 | 36.99 ± 27.74 b | 0.74 ± 0.07 a | 0.05 ± 0.04 a | 61.25 ± 1.12 b | 5.78 ± 4.54 a | 0.40 ± 0.33 a | 78.29 ± 0.19 a | 4.45 ± 1.44 a | 0.30 ± 0.10 a |
| 20 | UD | UD | UD | 63.71 ± 1.13 a | 1.23± 0.39 bc | 0.08 ± 0.03 bc | 78.24 ± 0.36 ab | 3.90 ± 0.71 a | 0.25 ± 0.05 a |
| 30 | UD | UD | UD | UD | UD | UD | 78.02 ± 0.12 b | 1.85 ± 1.98 b | 0.12 ± 0.13 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Z.; Zhang, D.; Pan, T.; Liu, H.; Shen, Q.; Hui, T. Effect of Protein Thermal Denaturation on the Texture Profile Evolution of Beijing Roast Duck. Foods 2022, 11, 664. https://doi.org/10.3390/foods11050664
Liu Y, Wang Z, Zhang D, Pan T, Liu H, Shen Q, Hui T. Effect of Protein Thermal Denaturation on the Texture Profile Evolution of Beijing Roast Duck. Foods. 2022; 11(5):664. https://doi.org/10.3390/foods11050664
Chicago/Turabian StyleLiu, Yanxia, Zhenyu Wang, Dequan Zhang, Teng Pan, Huan Liu, Qingwu Shen, and Teng Hui. 2022. "Effect of Protein Thermal Denaturation on the Texture Profile Evolution of Beijing Roast Duck" Foods 11, no. 5: 664. https://doi.org/10.3390/foods11050664
APA StyleLiu, Y., Wang, Z., Zhang, D., Pan, T., Liu, H., Shen, Q., & Hui, T. (2022). Effect of Protein Thermal Denaturation on the Texture Profile Evolution of Beijing Roast Duck. Foods, 11(5), 664. https://doi.org/10.3390/foods11050664






