Regular Consumption of Green Coffee Phenol, Oat β-Glucan and Green Coffee Phenol/Oat β-Glucan Supplements Does Not Change Body Composition in Subjects with Overweight and Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
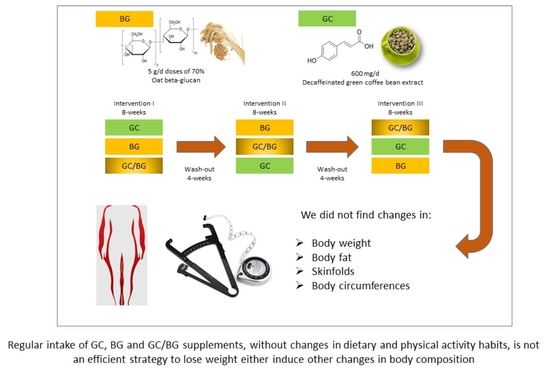
2.2. Food Supplements
2.3. Study Design
2.4. Dietary Assessment
2.5. Anthropometric, Physical Activity and Resting Energy Expenditure Analysis
2.6. Statistical Analysis
3. Results
3.1. Dietary Intake
3.2. Weight, BMI, Total Body Fat and Intracellular and Extracellular Water
3.3. Body Skinfold Measurements
3.4. Body Circumference Measurements
3.5. Physical Activity and Resting Energy Expenditure
4. Discussion
4.1. Dietary and Physical Activity Habits Analysis
4.2. Effects of Food Supplements BG and GC on Body Composition
4.3. Effects of the Food Supplement BG/GC on Body Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Fact Sheets: Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 December 2021).
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. World Obesity Federation. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Stuby, J.; Gravestock, I.; Wolfram, E.; Pichierri, G.; Steurer, J.; Burgstaller, J.M. Appetite-suppressing and satiety-increasing bioactive phytochemicals: A systematic review. Nutrients 2019, 11, 2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuliana, N.D.; Jahangir, M.; Korthout, H.; Choi, Y.H.; Kim, H.K.; Verpoorte, R. Comprehensive review on herbal medicine for energy intake suppression. Obes. Rev. 2011, 12, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Panopoulou, N.; Turunen, K.; Spiliotis, V.; Kyriacou, A. Prebiotic potential of barley derived b-glucan at low intake levels: A double-blinded, placebo controlled clinical study. Food Res. Int. 2010, 43, 1086–1092. [Google Scholar] [CrossRef]
- Arena, M.P.; Caggianiello, G.; Fiocco, D.; Russo, P.; Torelli, M.; Spano, G.; Capozzi, V. Barley β-glucans-containing food enhances probiotic performances of beneficial bacteria. Int. J. Mol. Sci. 2014, 15, 3025–3039. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffi, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 124121. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.X.; Chen, G.R.; Xu, H.; Ge, R.S.; Lin, J. Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med. Hypoth. 2010, 74, 123–126. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef]
- Wang, Q.; Ellis, P.R. Oat β-glucan: Physico-chemical characteristics in relation to its blood-glucose and cholesterol-lowering properties. Br. J. Nutr. 2014, 112, S4–S13. [Google Scholar] [CrossRef] [Green Version]
- García-Cordero, J.; Sarria-Ruiz, B.; González-Rámila, S.; Bravo-Clemente, L.; Mateos-Briz, R. Effectiveness of hydroxycinamates and beta-glucans as dietary tools against obesity and its associated dysfunctions, and their application as nutraceuticals. Nutr. Hosp. 2020, 37, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.F.; Vieira Lobato, R.; Vasques Araújo, T.; Zangerônimo, M.G.; Vicente Sousa, R.; José Pereira, L. Effect of beta-glucans in the control of blood glucose levels of diabetic patients: A systematic review. Nutr. Hosp. 2014, 31, 170–177. [Google Scholar]
- Bozbulut, R.; Sanlier, N. Promising effects of β-glucans on glyceamic control in diabetes. Trends Food Sci. Technol. 2018, 83, 159–166. [Google Scholar] [CrossRef]
- Tessari, P.; Lante, A. A multifunctional bread rich in beta glucans and low in dtarch improves metabolic control in type 2 diabetes: A controlled trial. Nutrients 2017, 9, 297. [Google Scholar] [CrossRef]
- Bresson, J.L.; Flynn, A.; Heinonen, M.; Hulshof, K.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; Moseley, B.; et al. Scientific Opinion on the substantiation of health claims related to beta glucans and maintenance of normal blood cholesterol concentrations (ID 754, 755, 757, 801, 1465, 2934) and maintenance or achievement of a normal body weight (ID 820, 823) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1254. [Google Scholar]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on the substantiation of a health claim related to oat beta glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1885. [Google Scholar]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207. [Google Scholar]
- Rahmani, J.; Miri, A.; Černevičiūtė, R.; Thompson, J.; de Souza, N.N.; Sultana, R.; Kord Varkaneh, H.; Mousavi, S.M.; Hekmatdoost, A. Effects of cereal beta-glucan consumption on body weight, body mass index, waist circumference and total energy intake: A meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 43, 131–139. [Google Scholar] [CrossRef]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front. Microbiol. 2016, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Aoe, S.; Ichinose, Y.; Kohyama, N.; Komae, K.; Takahashi, A.; Abe, D.; Yoshioka, T.; Yanagisawa, T. Effects of high β-glucan barley on visceral fat obesity in Japanese individuals: A randomized, double-blind study. Nutrition 2017, 42, 1–6. [Google Scholar] [CrossRef]
- Reyna-Villasmil, N.; Bermúdez-Pirela, V.; Mengual-Moreno, E.; Arias, N.; Cano-Ponce, C.; Leal-Gonzalez, E.; Souki, A.; Inglett, G.E.; Israili, Z.H.; Hernández-Hernández, R.; et al. Oat-derived beta-glucan significantly improves HDLC and diminishes LDLC and non-HDL cholesterol in overweight individuals with mild hypercholesterolemia. Am. J. Ther. 2007, 14, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Santas, J.; Lázaro, E.; Cuñé, J. Effect of a polysaccharide-rich hydrolysate from Saccharomyces cerevisiae (LipiGo®) in body weight loss: Randomised, double-blind, placebo-controlled clinical trial in overweight and obese adults. J. Sci. Food Agric. 2017, 97, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Lima, N.; de Oliveira, E.; da Silva, A.P.S.; de Albuquerque Maia, L.; de Moura, E.G.; Lisboa, P.C. Effects of Ilex paraguariensis (yerba mate) treatment on leptin resistance and inflammatory parameters in obese rats primed by early weaning. Life Sci. 2014, 115, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Osaki, N.; Hase, T.; Shimotoyodome, A. Ingestion of coffee polyphenols increases postprandial release of the active glucagon-like peptide-1 (GLP-1(7-36)) amide in C57BL/6J mice. J. Nutr. Sci. 2015, 4, e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambero, A.; Ribeiro, M.L. The positive effects of yerba maté (Ilex paraguariensis) in obesity. Nutrients 2015, 7, 730–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Taher, E.A.; Sheikh, B.Y.; Anjum, S.; Saeed, A.; AlAjmi, M.F.; Moustafa, M.S.; Al-Mousawi, S.M.; Farag, M.A.; Hegazy, M.-E.F.; et al. Hydroxycinnamic acids: Natural sources, biosynthesis, possible biological activities, and roles in Islamic medicine. Stud. Nat. Prod. Chem. 2018, 55, 269–292. [Google Scholar]
- Seo, C.-R.; Yi, B.R.; Oh, S.; Kwon, S.-M.; Kim, S.; Song, N.-J.; Cho, J.Y.; Park, K.-M.; Ahn, J.-Y.; Hong, J.-W.; et al. Aqueous extracts of hulled barley containing coumaric acid and ferulic acid inhibit adipogenesis in vitro and obesity in vivo. J. Funct. Foods. 2015, 12, 208–218. [Google Scholar] [CrossRef]
- Cardile, V.; Graziano, A.C.; Venditti, A. Clinical evaluation of Moro (Citrus sinensis (L.) Osbeck) orange juice supplementation for the weight management. Nat. Prod. Res. 2015, 29, 2256–2260. [Google Scholar] [CrossRef] [Green Version]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Zuñiga, L.Y.; Aceves-de la Mora, M.C.A.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of chlorogenic acid administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance. J. Med. Food. 2018, 21, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, S.; Sarriá, B.; Mateos, R.; Bravo-Clemente, L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: Results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur. J. Nutr. 2019, 58, 865–878. [Google Scholar] [CrossRef] [Green Version]
- Sarriá, B.; Sierra-Cinos, J.L.; García-Diz, L.; Martínez-López, S.; Mateos, R.; Bravo-Clemente, L. Green/roasted coffee may reduce cardiovascular risk in hypercholesterolemic subjects by decreasing body weight, abdominal adiposity and blood pressure. Foods 2020, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Puente, M.; Mateos, R.; Seguido, M.A.; García-Cordero, J.; González, S.; Tarradas, R.M.; Bravo-Clemente, L.; Sarriá, B. Appetite and satiety effects of the acute and regular consumption of green coffee phenols and green coffee phenol/oat β-glucan nutraceuticals in subjects with overweight and obesity. Foods 2021, 10, 2511. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; García-Cordero, J.; Bravo-Clemente, L.; Sarriá, B. Evaluation of novel nutraceuticals based on the combination of oat beta-glucans and a green coffee phenolic extract to combat obesity and its comorbidities. A randomized, dose-response, parallel trial. Food Funct. 2022, 13, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Durnin, J.V.; Womersley, J.V.G.A. Body fat assessed from total body density and its estimation from 645 skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef] [Green Version]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos (Food Composition Tables), 13th ed.; Ediciones Pirámide S.A.: Madrid, Spain, 2009; ISBN 978-843-682-273-1. [Google Scholar]
- Varela-Moreiras, G. La dieta mediterránea en la España actual. Nutr. Hosp. 2014, 30, 21–28. [Google Scholar]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef] [Green Version]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Sarriá, B.; Martínez-López, S.; Sierra-Cinos, J.L.; García-Diz, L.; Mateos, R.; Bravo-Clemente, L. Regularly consuming a green/roasted coffee blend reduces the risk of metabolic síndrome. Eur. J. Nutr. 2018, 57, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.C.; Huang, C.N.; Yeh, D.M.; Wang, S.J.; Peng, C.H.; Wang, C.J. Oat prevents obesity and abdominal fat distribution, and improves liver function in humans. Plant Foods Hum. Nutr. 2013, 68, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Beck, E.J.; Tapsell, L.C.; Batterham, M.J.; Tosh, S.M.; Huang, X.F. Oat beta-glucan supplementation does not enhance the effectiveness of an energy-restricted diet in overweight women. Br. J. Nutr. 2010, 103, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Bessell, E.; Maunder, A.; Lauche, R.; Adams, J.; Sainsbury, A.; Fuller, N.R. Efficacy of dietary supplements containing isolated organic compounds for weight loss: A systematic review and meta-analysis of randomised placebo-controlled trials. Int. J. Obes. 2021, 45, 1631–1643. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Soga, S.; Ota, N.; Shimotoyodome, A. Stimulation of postprandial fat utilization in healthy humans by daily consumption of chlorogenic acids. Biosci. Biotechnol. Biochem. 2013, 77, 1633–1636. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Risi, R.; Masi, D.; Caputi, A.; Balena, A.; Rossini, G.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Manfrini, S.; et al. Current evidence to propose different food supplements for weight loss: A comprehensive review. Nutrients 2020, 12, 2873. [Google Scholar] [CrossRef]
- Wang, J.; Thornton, J.C.; Kolesnik, S.; Pierson, R.N., Jr. Anthropometry in body composition. An overview. Ann. N. Y. Acad. Sci. 2000, 904, 317–326. [Google Scholar] [CrossRef]
- Garrido-Chamorro, R.; Sirvent-Belando, J.E.; González-Lorenzo, M.; Blasco-Lafarga, C.; Roche, E. Skinfold sum: Reference values for top athletes. Int. J. Morphol. 2012, 30, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, M.; Chen, J.; Chung, S.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Jaskari, J.; Kontula, P.; Siitonen, A.; Jousimies-Somer, H.; Mattila-Sandholm, T.; Poutanen, K. Oat beta-glucan and xylan hydrolysates as selective substrates for Bifidobacterium and Lactobacillus strains. Appl. Microbiol. Biotechnol. 1998, 49, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Drzikova, B.; Dongowski, G.; Gebhardt, E. Dietary fibre-rich oat-based products affect serum lipids, microbiota, formation of short-chain fatty acids and steroids in rats. Br. J. Nutr. 2005, 94, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Snart, J.; Bibiloni, R.; Grayson, T.; Lay, C.; Zhang, H.; Allison, G.E.; Laverdiere, J.K.; Temelli, F.; Vasanthan, T.; Bell, R.; et al. Supplementation of the diet with high-viscosity beta-glucan results in enrichment for lactobacilli in the rat cecum. Appl. Environ. Microbiol. 2006, 72, 1925–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, M.; Montemurno, E.; Vannini, L.; Cosola, C.; Cavallo, N.; Gozzi, G.; Maranzano, V.; Di Cagno, R.; Gobbetti, M.; Gesualdo, L. Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl. Environ. Microbiol. 2015, 81, 7945–7956. [Google Scholar] [CrossRef] [Green Version]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hydroxycinnamic acids on gut microbiota and health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 710–737. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Cao, S.; Wang, L.; Wang, D.; Yang, H.; Feng, Y.; Wang, S.; Li, L. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget 2016, 7, 31790–31799. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lam, K.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef]
| GCP | BG | GCP/BG | p | |
|---|---|---|---|---|
| Energy (Kcal) | ||||
| Baseline | 2019 ± 100 | 2058 ± 109 | 2023 ± 112 | 0.971 |
| End | 2115 ± 118 | 1937 ± 80 | 2128 ± 115 | 0.537 |
| Rate of change | 0.08 ± 0.06 | −0.02 ± 0.04 | 0.08 ± 0.06 | 0.265 |
| Protein (g) | ||||
| Baseline | 82.8 ± 4.1 | 84.8 ± 4.7 | 79.7 ± 3.6 | 0.759 |
| End | 82.6 ± 4.7 | 85.2 ± 4.1 | 86.9 ± 4.5 | 0.796 |
| Rate of change | 0.05 ± 0.07 | 0.04 ± 0.05 | 0.12 ± 0.06 | 0.570 |
| CHO (g) | ||||
| Baseline | 199 ± 11 | 211 ± 13 | 205 ± 14 | 0.850 |
| End | 204 ± 11 | 196 ± 12 | 200 ± 13 | 0.865 |
| Rate of change | 0.09 ± 0.07 | −0.01 ± 0.07 | 0.06 ± 0.09 | 0.604 |
| Dietary Fibre (g) | ||||
| Baseline | 23.9 ± 1.9 | 23.6 ± 1.8 | 22.5 ± 1.6 | 0.813 |
| End | 22.4 ± 1.9 | 20.6 ± 1.4 | 21.9 ± 1.4 | 0.602 |
| Rate of change | 0.20 ± 0.21 | −0.03 ± 0.08 | 0.06 ± 0.07 | 0.331 |
| Fat (g) | ||||
| Baseline | 84.7 ± 5.8 | 81.9 ± 4.9 | 86.5 ± 6.3 | 0.997 |
| End | 93.6 ± 6.8 | 79.9 ± 4.3 | 96.9 ± 7.2 | 0.283 |
| Rate of change | 0.16 ± 0.08 | 0.07 ± 0.08 | 0.18 ± 0.08 | 0.363 |
| SFA (g) | ||||
| Baseline | 28.01 ± 2.2 | 26.9 ± 1.7 | 28.5 ± 2.0 | 0.798 |
| End | 30.9 ± 2.8 | 25.9 ± 1.7 | 31.5 ± 2.7 | 0.381 |
| Rate of change | 0.18 ± 0.11 | 0.08 ± 0.09 | 0.19 ± 0.11 | 0.648 |
| MUFA (g) | ||||
| Baseline | 34.5 ± 2.4 | 34.9 ± 2.3 | 34.7 ± 2.2 | 0.942 |
| End | 41.9 ± 3.2 | 34.5 ± 2.1 | 42.8 ± 3.2 | 0.185 |
| Rate of change | 0.31 ± 0.11 | 0.11 ± 0.09 | 0.29 ± 0.09 | 0.106 |
| PUFA (g) | ||||
| Baseline | 12.8 ± 1.1 | 12.8 ± 1.2 | 12.3 ± 1.2 | 0.923 |
| End | 11.7 ± 0.9 | 12.2 ± 0.9 | 12.4 ± 0.8 | 0.828 |
| Rate of change | 0.02 ± 0.09 | 0.09 ± 0.08 | 0.18 ± 0.11 | 0.304 |
| PUFA/SFA | ||||
| Baseline | 0.5 ± 0.04 | 0.4 ± 0.04 | 0.45 ± 0.04 | 0.800 |
| End | 0.42 ± 0.03 | 0.5 ± 0.03 | 0.46 ± 0.04 | 0.248 |
| Rate of change | 0.04 ± 0.09 | 0.15 ± 0.07 | 0.22 ± 0.17 | 0.845 |
| [PUFA + MUFA]/SFA | ||||
| Baseline | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 0.995 |
| End | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.1 | 0.998 |
| Rate of change | 0.14 ± 0.08 | 0.09 ± 0.05 | 0.15 ± 0.08 | 0.845 |
| Cholesterol (mg) | ||||
| Baseline | 299 ± 26 | 330 ± 29 | 329 ± 24 | 0.728 |
| End | 287 ± 29 | 325 ± 28 | 366 ± 32 | 0.205 |
| Rate of change | 0.09 ± 0.12 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.748 |
| Calcium (mg) | ||||
| Baseline | 758 ± 46 | 775 ± 50 | 688 ± 45 | 0.241 |
| End | 752 ± 70 | 727 ± 54 | 814 ± 69 | 0.376 |
| Rate of change | −0.01 ± 0.06 | −0.05 ± 0.06 | 0.25 ± 0.13 | 0.231 |
| Iron (mg) | ||||
| Baseline | 14.2 ± 0.8 | 14.9 ± 0.9 | 13.3 ± 0.7 | 0.336 |
| End | 14.4 ± 0.8 | 12.9 ± 0.6 | 15.3 ± 1.0 | 0.049 |
| Rate of change | 0.13 ± 0.10 | −0.05 ± 0.07 | 0.20 ± 0.08 | 0.095 |
| GCP | BG | GCP/BG | p | |
|---|---|---|---|---|
| Body Weight (Kg) | ||||
| Baseline | 86.4 ± 1.8 | 86.8 ± 2.3 | 87.2 ± 4.3 | 0.976 |
| End | 86.3 ± 1.8 | 86.5 ± 2.4 | 87.7 ± 4.3 | 0.936 |
| Rate of change | −0.002 ± 0.002 | −0.003 ± 0.003 | 0.006 ± 0.004 | 0.177 |
| BMI (Kg/m2) | ||||
| Baseline | 30.05 ± 0.48 | 30.09 ± 0.64 | 29.9 ± 1.1 | 0.980 |
| End | 30.1 ± 0.5 | 29.9 ± 0.6 | 31.1 ± 1.1 | 0.997 |
| Rate of change | −0.002 ± 0.002 | −0.003 ± 0.003 | 0.006 ± 0.004 | 0.177 |
| Fat (%) | ||||
| Baseline | 35.2 ± 0.9 | 35.4 ± 1.2 | 35.1 ± 1.6 | 0.988 |
| End | 35.2 ± 0.9 | 35.3 ± 1.2 | 35.2 ± 1.6 | 0.999 |
| Rate of change | 0.001 ± 0.004 | −0.002 ± 0.003 | 0.005 ± 0.005 | 0.248 |
| Fat BIA (%) | ||||
| Baseline | 29.01 ± 1.18 | 29.4 ± 1.5 | 28.9 ± 2.7 | 0.996 |
| End | 28.7 ± 1.1 | 29.6 ± 1.5 | 29.9 ± 2.8 | 0,833 |
| Rate of change | 0.003 ± 0.023 | 0.08 ± 0.08 | 0.05 ± 0.03 | 0.677 |
| Intracellular water (%) | ||||
| Baseline | 26.2 ± 0.8 | 26.8 ± 0.9 | 26.7 ± 1.2 | 0.907 |
| End | 26.6 ± 0.8 | 26.1 ± 0.8 | 26.4 ± 1.3 | 0.904 |
| Rate of change | 0.02 ± 0.02 | −0.02 ± 0.02 | −0.01 ± 0.01 | 0.224 |
| Extracellular water (%) | ||||
| Baseline | 15.7 ± 0.4 | 16.2 ± 0.5 | 16.1 ± 0.7 | 0.918 |
| End | 15.8 ± 0.4 | 15.6 ± 0.5 | 15.9 ± 0.8 | 0.925 |
| Rate of change | 0.007 ± 0.011 | −0.02 ± 0.01 | −0.01 ± 0.01 | 0.312 |
| GCP | BG | GCP/BG | p | |
|---|---|---|---|---|
| Tricipital (mm) | ||||
| Baseline | 22.9 ± 1.1 | 23.4 ± 1.3 | 23.3 ± 1.5 | 0.946 |
| End | 22.8 ± 1.04 | 23.2 ± 1.3 | 23.3 ± 1.6 | 0.943 |
| Rate of change | −0.002 ± 0.001 | −0.001 ± 0.001 | −0.003 ± 0.001 | 0.311 |
| Bicipital (mm) | ||||
| Baseline | 13.7 ± 0.8 | 13.1 ± 1.02 | 12.4 ± 1.3 | 0.73 |
| End | 13.8 ± 0.9 | 13.3 ± 1.01 | 12.3 ± 1.3 | 0.672 |
| Rate of change | 0.07 ± 0.02 | 0.03 ± 0.02 | 0.01 ± 0.04 | 0.833 |
| Subscapularis (mm) | ||||
| Baseline | 24.8 ± 1.1 | 25.4 ± 1.4 | 24.7 ± 2.3 | 0.929 |
| End | 24.7 ± 1.1 | 24.9 ± 1.4 | 25.04 ± 2.33 | 0.984 |
| Rate of change | −0.06 ± 0.01 | −0.02 ± 0.01 | 0.02 ± 0.02 | 0.183 |
| Suprailiac (mm) | ||||
| Baseline | 31.1 ± 0.9 | 31.6 ± 1.2 | 31.1 ± 2.5 | 0.943 |
| End | 31.5 ± 0.8 | 31.6 ± 1.2 | 31.5 ± 2.4 | 0.998 |
| Rate of change | 0.02 ± 0.01 | 0.004 ± 0.011 | 0.02 ± 0.02 | 0.74 |
| Leg (mm) | ||||
| Baseline | 20.9 ± 1.2 | 20.4 ± 1.4 | 19.9 ± 1.7 | 0.902 |
| End | 20.7 ± 1.1 | 21.3 ± 1.5 | 19.6 ± 1.6 | 0.716 |
| Rate of change | 0.002 ± 0.017 | 0.05 ± 0.04 | −0.01 ± 0.02 | 0.671 |
| Thigh (mm) | ||||
| Baseline | 29.1 ± 1.3 | 29.1 ± 1.5 | 29.9 ± 2.3 | 0.957 |
| End | 29.1 ± 1.3 | 27.3 ± 1.7 | 30.1 ± 2.3 | 0.63 |
| Rate of change | 0.001 ± 0.009 | −0.04 ± 0.04 | 0.01 ± 0.03 | 0.769 |
| SUMM 4 | ||||
| Baseline | 92.8 ± 3.2 | 93.28 ± 4.03 | 91.5 ± 6.6 | 0.968 |
| End | 92.7 ± 3.2 | 93.3 ± 4.1 | 92.2 ± 6.6 | 0.987 |
| Rate of change | 0.001 ± 0.007 | 0.001 ± 0.007 | 0.009 ± 0.012 | 0.149 |
| SUMM 6 | ||||
| Baseline | 142.6 ± 5.1 | 143.3 ± 6.1 | 141.3 ± 8.9 | 0.99 |
| End | 142.7 ± 5.02 | 141.6 ± 5.9 | 141.8 ± 9.01 | 0.992 |
| Rate of change | 0.002 ± 0.006 | −0.007 ± 0.009 | 0.005 ± 0.008 | 0.579 |
| GCP | BG | GCP/BG | p | |
|---|---|---|---|---|
| Waist (cm) | ||||
| Baseline | 99.9 ± 1.6 | 100.6 ± 2.2 | 103.6 ± 4.4 | 0.615 |
| End | 100.1 ± 1.6 | 100.6 ± 2.1 | 104.04 ± 4.45 | 0.584 |
| Rate of change | 0.003 ± 0.003 | 0.001 ± 0.003 | 0.004 ± 0.004 | 0.943 |
| Hip (cm) | ||||
| Baseline | 106.5 ± 1.03 | 106.4 ± 1.2 | 104.9 ± 1.6 | 0.715 |
| End | 106.4 ± 1.02 | 106.4 ± 1.2 | 105.3 ± 1.6 | 0.837 |
| Rate of change | −0.001 ± 0.001 | −0.003 ± 0.002 | 0.003 ± 0.001 | 0.105 |
| Waist-to-hip ratio | ||||
| Baseline | 0.94 ± 0.02 | 0.95 ± 0.02 | 0.99 ± 0.03 | 0.418 |
| End | 0.94 ± 0.02 | 0.95 ± 0.02 | 0.99 ± 0.04 | 0.443 |
| Rate of change | 0.004 ± 0.002 | 0.001 ± 0.004 | 0.004 ± 0.003 | 0.612 |
| Thigh (cm) | ||||
| Baseline | 57.3 ± 0.6 | 57.8 ± 0.8 | 56.6 ± 0.9 | 0.699 |
| End | 57.3 ± 0.6 | 57.2 ± 0.8 | 56.3 ± 0.8 | 0.697 |
| Rate of change | −0.005 ± 0.004 | −0.009 ± 0.004 | −0.005 ± 0.007 | 0.498 |
| Calf (cm) | ||||
| Baseline | 40.08 ± 0.54 | 40.05 ± 0.53 | 39.2 ± 0.7 | 0.635 |
| End | 40.1 ± 0.5 | 39.9 ± 0.6 | 39.5 ± 0.7 | 0.798 |
| Rate of change | 0.0007 ± 0.002 | −0.004 ± 0.005 | 0.006 ± 0.003 | 0.397 |
| Brachial (cm) | ||||
| Baseline | 33.4 ± 0.5 | 33.5 ± 0.5 | 33.1 ± 0.9 | 0.921 |
| End | 33.3 ± 0.4 | 33.4 ± 0.5 | 33.3 ± 0.9 | 0.993 |
| Rate of change | −0.0004 ± 0.0027 | −0.001 ± 0.004 | 0.007 ± 0.004 | 0.213 |
| GCP | BG | GCP/BG | p | |
|---|---|---|---|---|
| Energy Expenditure (Kcal/day) | ||||
| Baseline | 392.9 ± 52.2 | 406.87 ± 51.01 | 556.01 ± 57.55 | 0.198 |
| End | 453.9 ± 76.8 | 346 ± 42.7 | 428.7 ± 65.1 | 0.513 |
| Kcal/hour | ||||
| Baseline | 17.7 ± 2.4 | 17.7 ± 2.2 | 24.0 ± 2.5 | 0.313 |
| End | 19.4 ± 3.4 | 14.5 ± 1.8 | 18.1 ± 2.7 | 0.481 |
| MET | ||||
| Baseline | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.2 | 0.506 |
| End | 1.18 ± 0.05 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.671 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Cordero, J.; Sierra-Cinos, J.L.; Seguido, M.A.; González-Rámila, S.; Mateos, R.; Bravo-Clemente, L.; Sarriá, B. Regular Consumption of Green Coffee Phenol, Oat β-Glucan and Green Coffee Phenol/Oat β-Glucan Supplements Does Not Change Body Composition in Subjects with Overweight and Obesity. Foods 2022, 11, 679. https://doi.org/10.3390/foods11050679
García-Cordero J, Sierra-Cinos JL, Seguido MA, González-Rámila S, Mateos R, Bravo-Clemente L, Sarriá B. Regular Consumption of Green Coffee Phenol, Oat β-Glucan and Green Coffee Phenol/Oat β-Glucan Supplements Does Not Change Body Composition in Subjects with Overweight and Obesity. Foods. 2022; 11(5):679. https://doi.org/10.3390/foods11050679
Chicago/Turabian StyleGarcía-Cordero, Joaquín, José Luis Sierra-Cinos, Miguel A. Seguido, Susana González-Rámila, Raquel Mateos, Laura Bravo-Clemente, and Beatriz Sarriá. 2022. "Regular Consumption of Green Coffee Phenol, Oat β-Glucan and Green Coffee Phenol/Oat β-Glucan Supplements Does Not Change Body Composition in Subjects with Overweight and Obesity" Foods 11, no. 5: 679. https://doi.org/10.3390/foods11050679
APA StyleGarcía-Cordero, J., Sierra-Cinos, J. L., Seguido, M. A., González-Rámila, S., Mateos, R., Bravo-Clemente, L., & Sarriá, B. (2022). Regular Consumption of Green Coffee Phenol, Oat β-Glucan and Green Coffee Phenol/Oat β-Glucan Supplements Does Not Change Body Composition in Subjects with Overweight and Obesity. Foods, 11(5), 679. https://doi.org/10.3390/foods11050679