Capsaicin, the Spicy Ingredient of Chili Peppers: Effects on Gastrointestinal Tract and Composition of Gut Microbiota at Various Dosages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Histopathology and Measurement of Inflammatory Cytokine Levels in GI Tissues
2.3. Measurement of Serum Neuropeptide
2.4. Determination of Levels of SCFAs in Cecal Content
2.5. 16S rRNA Gene Sequencing
2.6. Statistical Analysis
3. Results
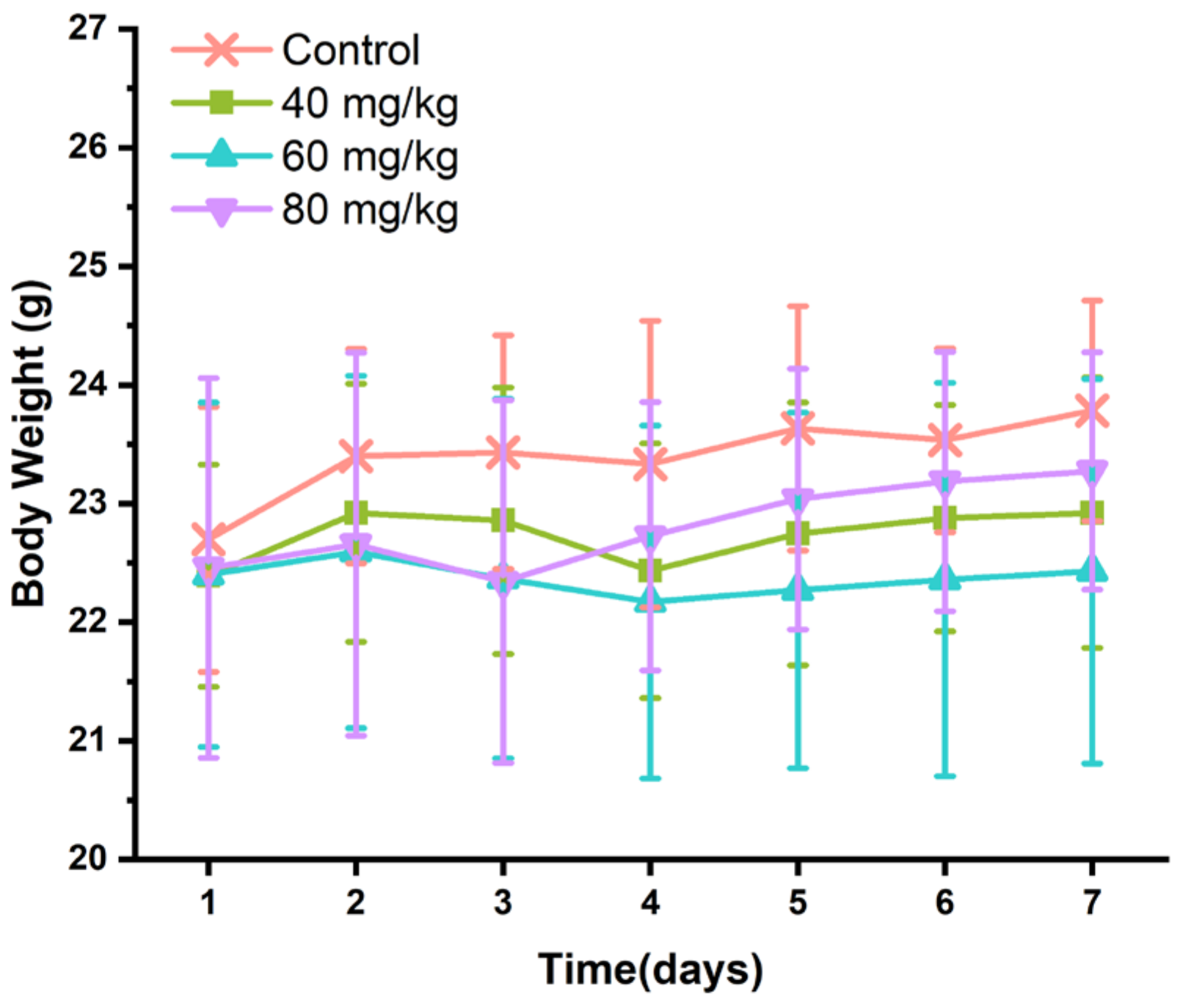
3.1. Effects of CAP on Body Weight and Food and Water Intake
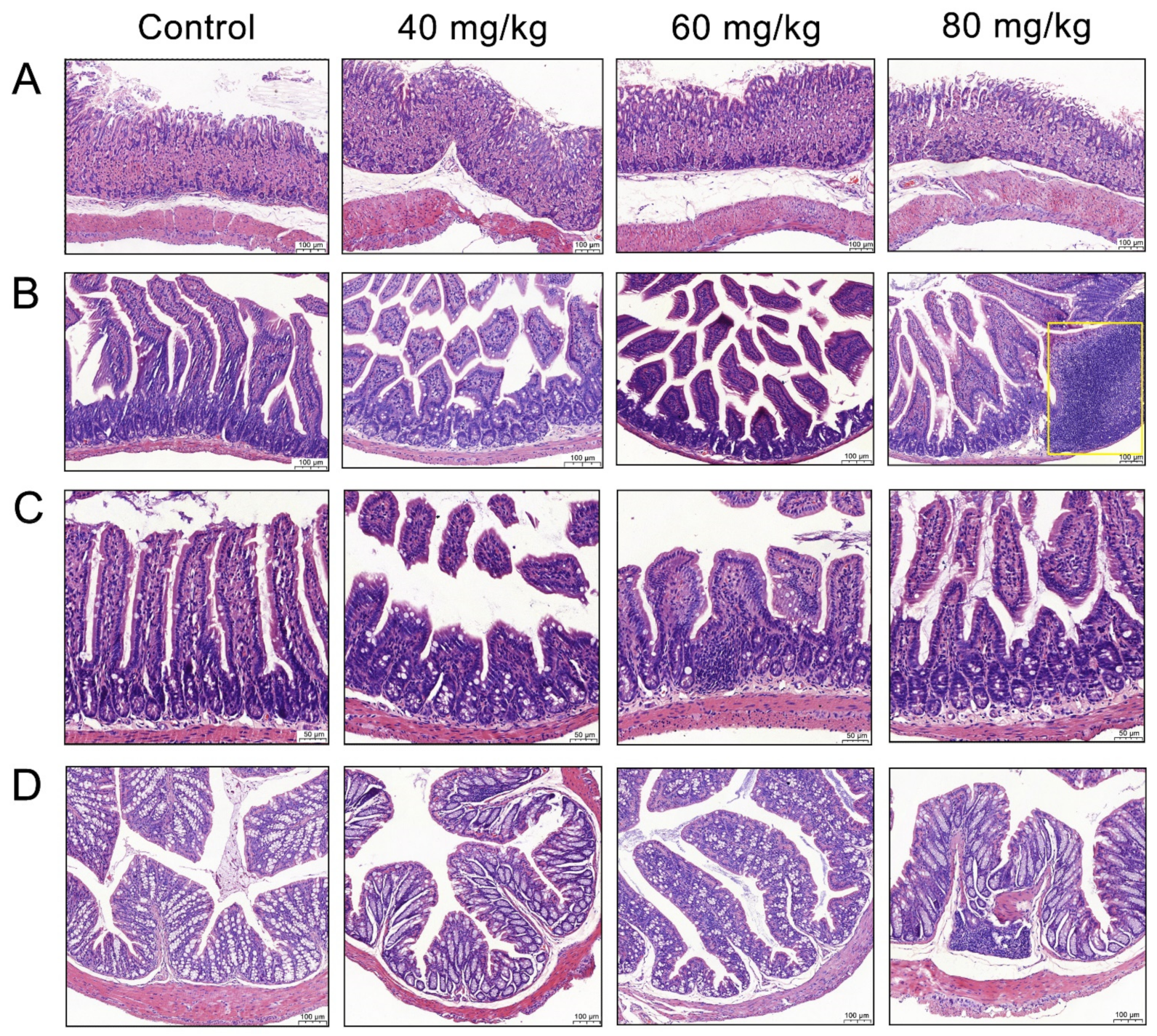
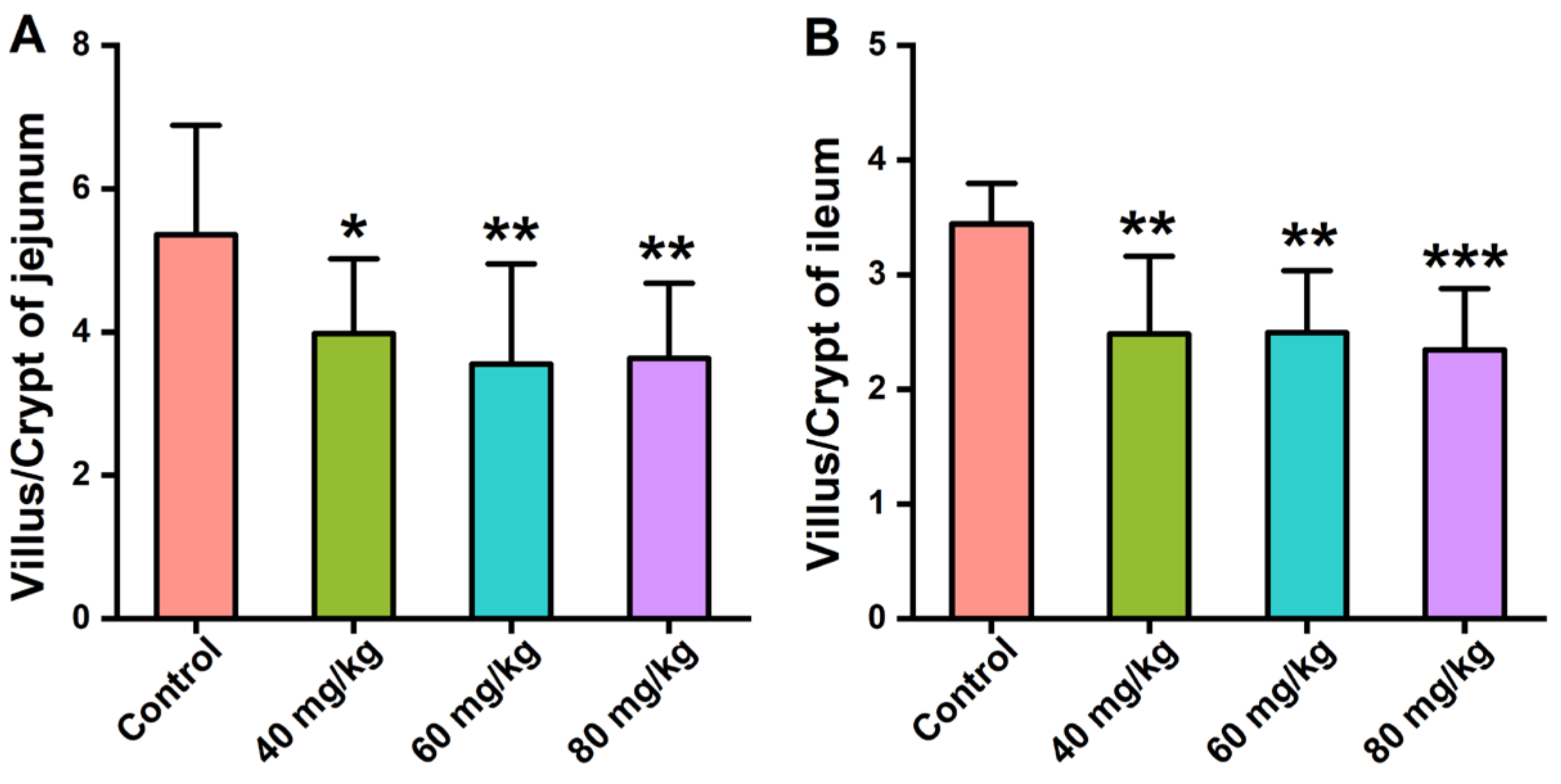
3.2. Effects of CAP Administration on the Histopathology of GI Tissues
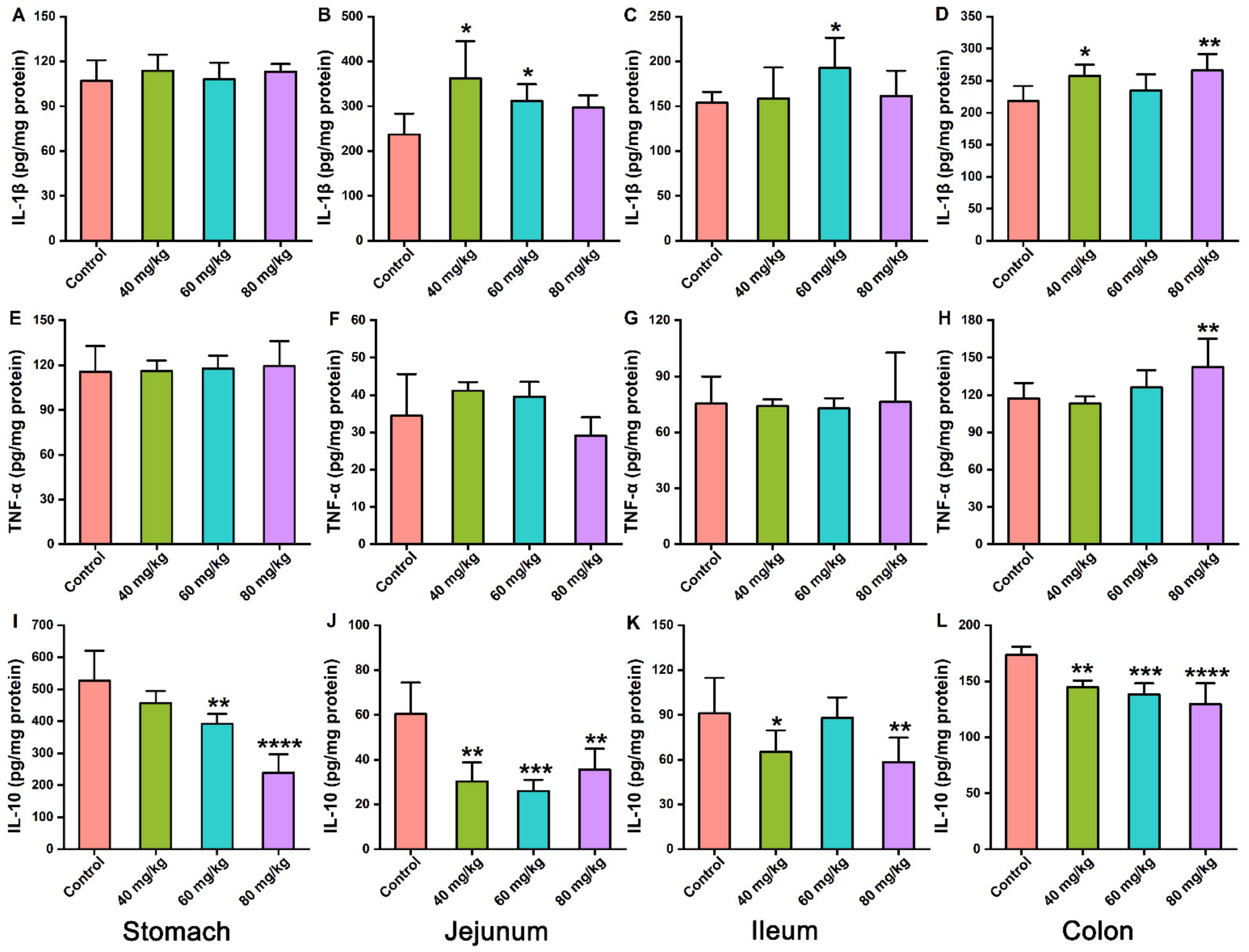
3.3. Effects of CAP on the Levels of Inflammatory Cytokines in Gastrointestinal Tissues
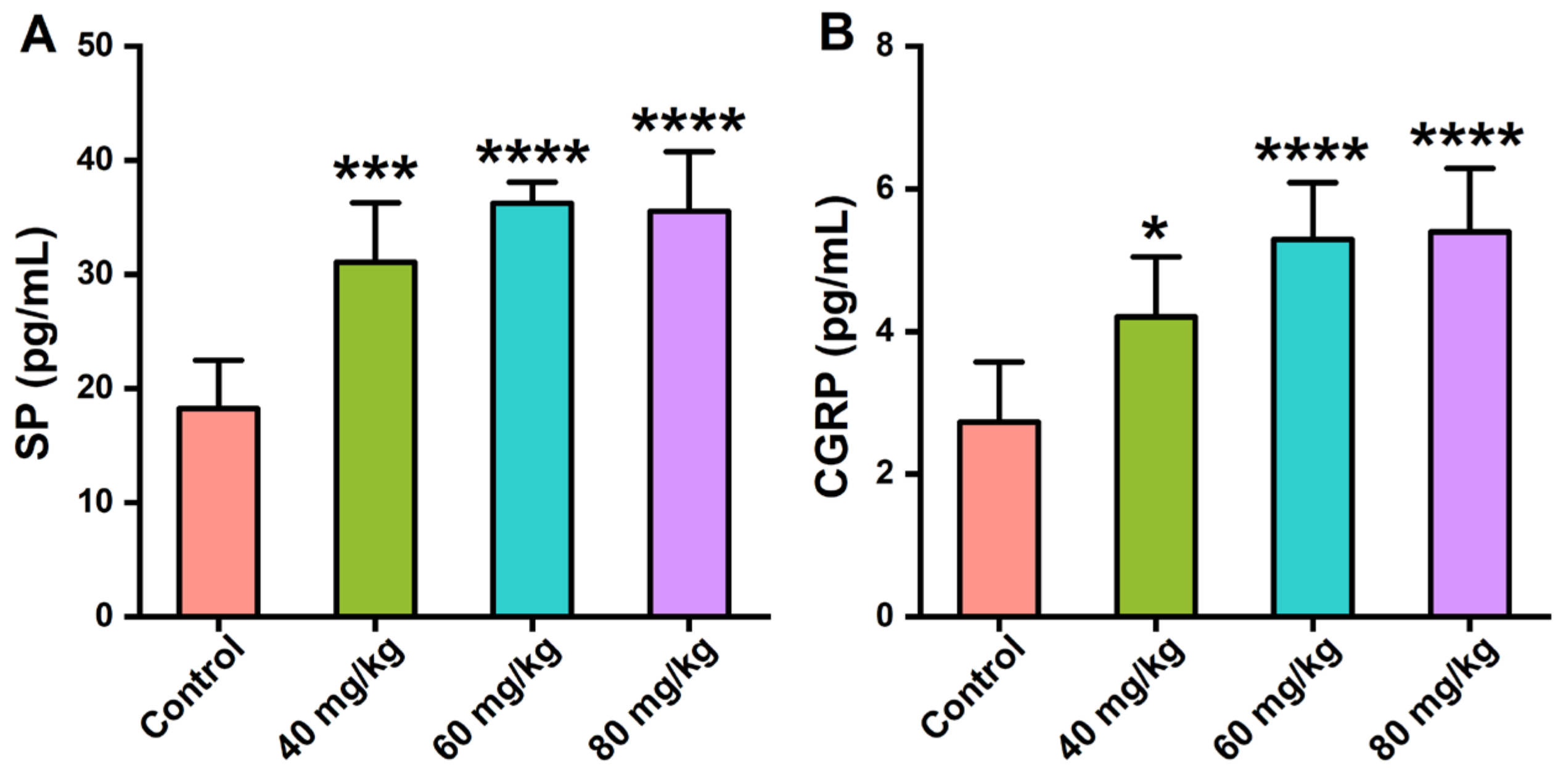
3.4. Effects of CAP on the Neuropeptide Levels in Serum
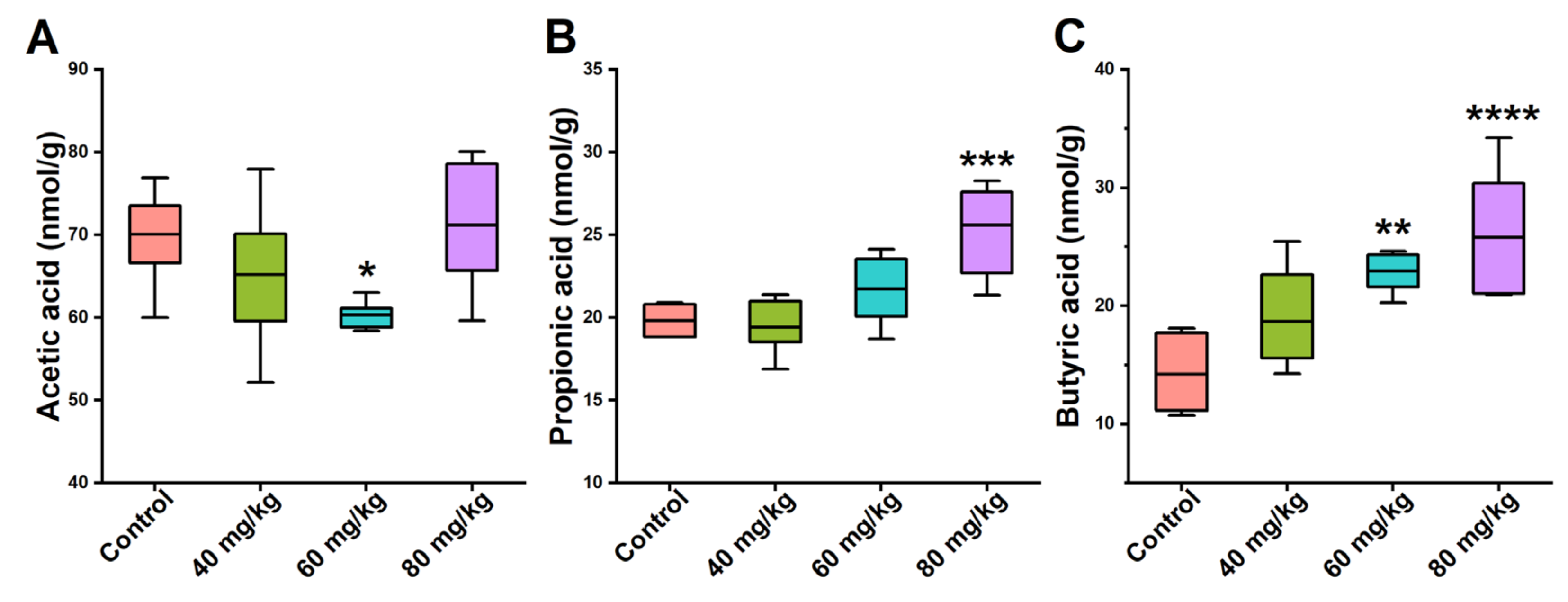
3.5. Effects of CAP on the Levels of Cecal SCFAs
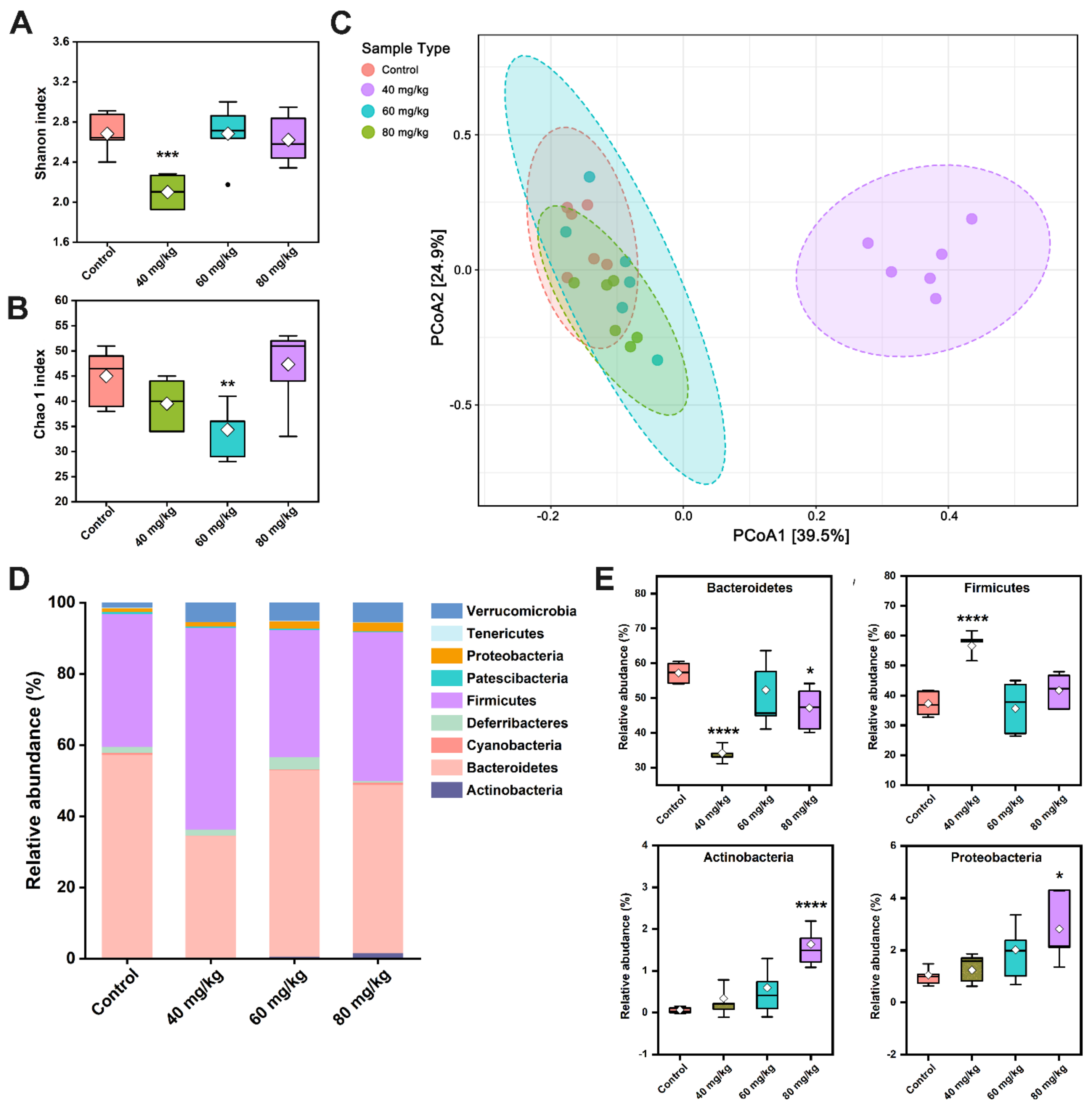
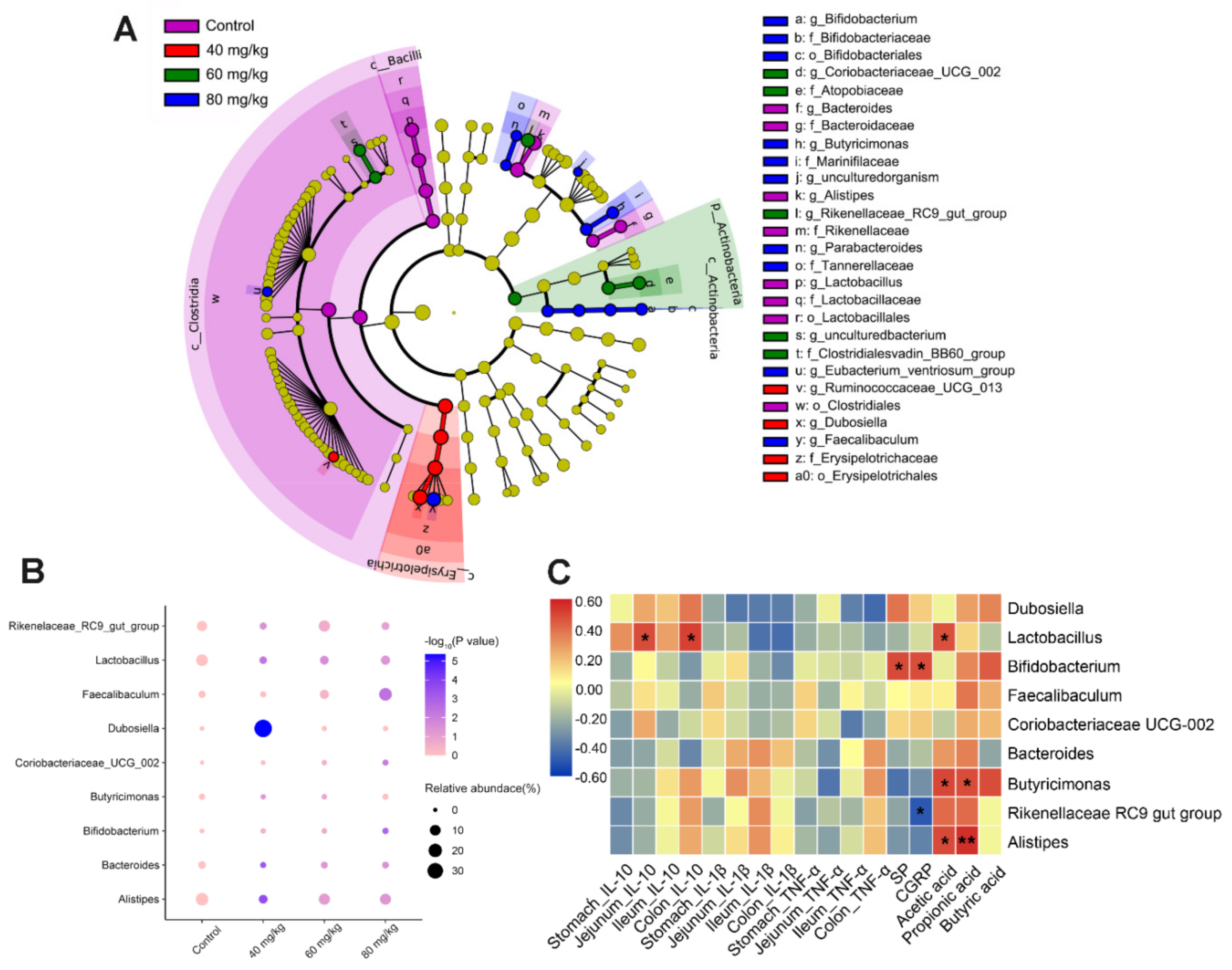
3.6. CAP Modulates the Composition of Gut Microbiota
3.7. Spearman’s Correlation Analysis between Gut Microbiota, Cytokines, Neuropeptides, and SCFAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CAP | Capsaicin |
| CGRP | Calcitonin gene-related peptide |
| GC-MS | Gas chromatograph-mass spectrometry |
| GI | Gastrointestinal |
| H&E | Hematoxylin and eosin |
| LDA | Linear discriminant analysis |
| LEfSe | Linear discriminant analysis effect size |
| OTU | Operational taxonomic unit |
| PCoA | Principal coordinates analysis |
| SCFAs | Short-chain fatty acids |
| SEM | Standard error of the mean |
| SP | Substance P |
References
- Fujiwake, H.; Suzuki, T.; Oka, S.; Iwai, K. Enzymatic formation of capsaicinoid from vanillylamine and iso-type fatty acids by cell-free extracts of Capsicum annuum var. annuum cv. Karayatsubusa. Agric. Biol. Chem. 1980, 44, 2907–2912. [Google Scholar] [CrossRef]
- Srinivasan, K. Biological Activities of Red Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.; Vogelsang, H. Characterization of sensations induced by capsaicin in the upper gastrointestinal tract. Neurogastroenterol. Motil. 2007, 19, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Mozsik, G.; Vincze, A.; Szolcsanyi, J. Four response stages of capsaicin-sensitive primary afferent neurons to capsaicin and its analog: Gastric acid secretion, gastric mucosal damage and protection. J. Gastroenterol. Hepatol. 2001, 16, 1093–1097. [Google Scholar] [CrossRef]
- Lu, M.; Cao, Y.; Ho, C.-T.; Huang, Q. Development of Organogel-Derived Capsaicin Nanoemulsion with Improved Bioaccessibility and Reduced Gastric Mucosa Irritation. J. Agric. Food Chem. 2016, 64, 4735–4741. [Google Scholar] [CrossRef]
- Lu, M.; Cao, Y.; Ho, C.-T.; Huang, Q. The enhanced anti-obesity effect and reduced gastric mucosa irritation of capsaicin-loaded nanoemulsions. Food Funct. 2017, 8, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, S.; Liu, X.; Xiao, C. Fabrication of capsaicin emulsions: Improving the stability of system and relieving the irritation to the gastrointestinal tract of rats. J. Sci. Food Agric. 2019, 100, 129–138. [Google Scholar] [CrossRef]
- Tsukura, Y.; Mori, M.; Hirotani, Y.; Ikeda, K.; Amano, F.; Kato, R.; Ijiri, Y.; Tanaka, K. Effects of capsaicin on cellular damage and monolayer permeability in human intestinal Caco-2 cells. Biol. Pharm. Bull. 2007, 30, 1982–1986. [Google Scholar] [CrossRef] [Green Version]
- Umeda, K.; Ikenouchi, J.; Katahira-Tayama, S.; Furuse, K.; Sasaki, H.; Nakayama, M.; Matsui, T.; Tsukita, S.; Furuse, M.; Tsukita, S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 2006, 126, 741–754. [Google Scholar] [CrossRef] [Green Version]
- Drewes, A.M.; Schipper, K.P.; Dimcevski, G.; Petersen, P.; Gregersen, H.; Funch-Jensen, P.; Arendt-Nielsen, L. Gut pain and hyperalgesia induced by capsaicin: A human experimental model. Pain 2003, 104, 333–341. [Google Scholar] [CrossRef]
- Van Avesaat, M.; Troost, F.J.; Westerterp-Plantenga, M.S.; Helyes, Z.; Le Roux, C.W.; Dekker, J.; Masclee, A.A.M.; Keszthelyi, D. Capsaicin-induced satiety is associated with gastrointestinal distress but not with the release of satiety hormones. Am. J. Clin. Nutr. 2016, 103, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wanrooij, S.J.M.; Wouters, M.M.; Van Oudenhove, L.; Vanbrabant, W.; Mondelaers, S.; Kollmann, P.; Kreutz, F.; Schemann, M.; Boeckxstaens, G.E. Sensitivity Testing in Irritable Bowel Syndrome With Rectal Capsaicin Stimulations: Role of TRPV1 Upregulation and Sensitization in Visceral Hypersensitivity? Am. J. Gastroenterol. 2014, 109, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Baboota, R.K.; Murtaza, N.; Jagtap, S.; Singh, D.P.; Karmase, A.; Kaur, J.; Bhutani, K.K.; Boparai, R.K.; Premkumar, L.S.; Kondepudi, K.K.; et al. Capsaicin-induced transcriptional changes in hypothalamus and alterations in gut microbial count in high fat diet fed mice. J. Nutr. Biochem. 2014, 25, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, C.; Tang, Y.; Yin, H.; Liu, X. Capsaicin has an anti-obesity effect through alterations in gut microbiota populations and short-chain fatty acid concentrations. Food Nutr. Res. 2020, 64, 14. [Google Scholar] [CrossRef] [Green Version]
- Dieleman, L.A.; Palmen, M.; Akol, H.; Bloemena, E.; Pena, A.S.; Meuwissen, S.G.M.; van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef]
- Mao, B.; Li, D.; Ai, C.; Zhao, J.; Zhang, H.; Chen, W. Lactulose Differently Modulates the Composition of Luminal and Mucosal Microbiota in C57BL/6J Mice. J. Agric. Food Chem. 2016, 64, 6240–6247. [Google Scholar] [CrossRef]
- Kwon, Y. Estimation of Dietary Capsaicinoid Exposure in Korea and Assessment of Its Health Effects. Nutrients 2021, 13, 2461. [Google Scholar] [CrossRef]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, Y.; Wan, J.; Yang, X.; Firempong, C.K.; Yu, J.; Xu, X. Enhanced oral bioavailability, reduced irritation and increased hypolipidemic activity of self-assembled capsaicin prodrug nanoparticles. J. Funct. Foods 2018, 44, 137–145. [Google Scholar] [CrossRef]
- Evrard, B.; Coudeyras, S.; Dosgilbert, A.; Charbonnel, N.; Alame, J.; Tridon, A.; Forestier, C. Dose-Dependent Immunomodulation of Human Dendritic Cells by the Probiotic Lactobacillus rhamnosus Lcr35. PLoS ONE 2011, 6, e18735. [Google Scholar] [CrossRef]
- Horiuchi, T.; Mitoma, H.; Harashima, S.-I.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnova, M.G.; Kiselev, S.L.; Gnuchev, N.V.; Birchall, J.P.; Pearson, J.P. Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion. Eur. Cytokine Netw. 2002, 13, 161–172. [Google Scholar] [PubMed]
- Xiang, Q.; Guo, W.; Tang, X.; Cui, S.; Zhang, F.; Liu, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. Capsaicin—The spicy ingredient of chili peppers: A review of the gastrointestinal effects and mechanisms. Trends Food Sci. Technol. 2021, 116, 755–765. [Google Scholar] [CrossRef]
- Hoffmann, P.; Mazurkiewicz, J.; Holtmann, G.; Gerken, G.; Eysselein, V.E.; Goebell, H. Capsaicin-sensitive nerve fibres induce epithelial cell proliferation, inflammatory cell immigration and transforming growth factor-alpha expression in the rat colonic mucosa in vivo. Scand. J. Gastroenterol. 2002, 37, 414–422. [Google Scholar] [CrossRef]
- Engel, M.A.; Becker, C.; Reeh, P.W.; Neurath, M.F. Role of Sensory Neurons in Colitis: Increasing Evidence for a Neuroimmune Link in the Gut. Inflamm. Bowel Dis. 2011, 17, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.; Pedersen, A.E.; Kristensen, N.N.; de Felipe Fernandez, C.; Claesson, M.H. Blockage of the Neurokinin 1 Receptor and Capsaicin-Induced Ablation of the Enteric Afferent Nerves Protect SCID Mice Against T-Cell-Induced Chronic Colitis. Inflamm. Bowel Dis. 2009, 15, 1174–1182. [Google Scholar] [CrossRef]
- Song, J.; Ren, H.; Gao, Y.; Lee, C.; Li, S.F.; Zhang, F.; Li, L.; Chen, H. Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice. Front. Physiol. 2017, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Song, J.; Wang, H.; Zhang, Y.; Xin, J.; Suo, H. Qingke beta-glucan synergizes with a beta-glucan-utilizing Lactobacillus strain to relieve capsaicin-induced gastrointestinal injury in mice. Int. J. Biol. Macromol. 2021, 174, 289–299. [Google Scholar] [CrossRef]
- Reeves, A.E.; Theriot, C.M.; Bergin, I.L.; Huffnagle, G.B.; Schloss, P.D.; Young, V.B. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile infection. Gut Microbes 2011, 2, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Holzer, P. Neuropeptides, Microbiota, and Behavior. In Gut Microbiome and Behavior; Cryan, J.F., Clarke, G., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2016; Volume 131, pp. 67–89. [Google Scholar]
- Sperandio, V.; Torres, A.G.; Jarvis, B.; Nataro, J.P.; Kaper, J.B. Bacteria-host communication: The language of hormones. Proc. Natl. Acad. Sci. USA 2003, 100, 8951–8956. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.; Zhang, Y.; Zhu, X.; Liu, K.; Wang, X.; Chen, M.; Wang, J.; Chen, H.; Hui, S.; Huang, L.; et al. Healthy Subjects Differentially Respond to Dietary Capsaicin Correlating with Specific Gut Enterotypes. J. Clin. Endocrinol. Metab. 2016, 101, 4681–4689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miquel, S.; Martin, R.; Rossi, O.; Bermudez-Humaran, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS ONE 2017, 12, e0188634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Huang, X.; Chen, Y.; Zhang, D.; Chen, D.; Chen, L.; Lin, J. Study on the Effect of Capsaicin on the Intestinal Flora through High-Throughput Sequencing. ACS Omega 2020, 5, 1246–1253. [Google Scholar] [CrossRef]
- Sakamoto, M.; Takagaki, A.; Matsumoto, K.; Kato, Y.; Goto, K.; Benno, Y. Butyricimonas synergistica gen. nov., sp. nov and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family ‘Porphyromonadaceae’ isolated from rat faeces. Int. J. Syst. Evol. Microbiol. 2009, 59, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cao, X.; Fang, X.; Guo, A.; Li, E. Inhibitory effects of fermented Ougan (Citrus reticulata cv. Suavissima) juice on high-fat diet-induced obesity associated with white adipose tissue browning and gut microbiota modulation in mice. Food Funct. 2021, 12, 9300–9314. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, G.; Wang, C.; Xu, X.; Li, C. Specific Microbiota Dynamically Regulate the Bidirectional Gut-Brain Axis Communications in Mice Fed Meat Protein Diets. J. Agric. Food Chem. 2019, 67, 1003–1017. [Google Scholar] [CrossRef]
| Group | Daily Gavage Treatment | Volume | Sample Size |
|---|---|---|---|
| Control | Vehicle | 200 μL | n = 6 |
| 40 mg/kg | CAP at a concentration of 40 mg/kg bodyweight | 200 μL | n = 6 |
| 60 mg/kg | CAP at a concentration of 60 mg/kg bodyweight | 200 μL | n = 6 |
| 80 mg/kg | CAP at a concentration of 80 mg/kg bodyweight | 200 μL | n = 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Q.; Tang, X.; Cui, S.; Zhang, Q.; Liu, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. Capsaicin, the Spicy Ingredient of Chili Peppers: Effects on Gastrointestinal Tract and Composition of Gut Microbiota at Various Dosages. Foods 2022, 11, 686. https://doi.org/10.3390/foods11050686
Xiang Q, Tang X, Cui S, Zhang Q, Liu X, Zhao J, Zhang H, Mao B, Chen W. Capsaicin, the Spicy Ingredient of Chili Peppers: Effects on Gastrointestinal Tract and Composition of Gut Microbiota at Various Dosages. Foods. 2022; 11(5):686. https://doi.org/10.3390/foods11050686
Chicago/Turabian StyleXiang, Qunran, Xin Tang, Shumao Cui, Qiuxiang Zhang, Xiaoming Liu, Jianxin Zhao, Hao Zhang, Bingyong Mao, and Wei Chen. 2022. "Capsaicin, the Spicy Ingredient of Chili Peppers: Effects on Gastrointestinal Tract and Composition of Gut Microbiota at Various Dosages" Foods 11, no. 5: 686. https://doi.org/10.3390/foods11050686
APA StyleXiang, Q., Tang, X., Cui, S., Zhang, Q., Liu, X., Zhao, J., Zhang, H., Mao, B., & Chen, W. (2022). Capsaicin, the Spicy Ingredient of Chili Peppers: Effects on Gastrointestinal Tract and Composition of Gut Microbiota at Various Dosages. Foods, 11(5), 686. https://doi.org/10.3390/foods11050686







