Cooking and In Vitro Digestion Modulate the Anti-Diabetic Properties of Red-Skinned Onion and Dark Purple Eggplant Phenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cooking Treatments and In Vitro Gastrointestinal Digestion
2.3. Preparation of the Phenolic-Rich Fractions
2.4. Characterization of the Phenolics Profile of Phenolic-Rich Fractions by Liquid Chromatography Electrospray Ionization Ion Trap Mass Spectrometer (LC-ESI-IT-MS)
2.5. Biological Activity Analysis
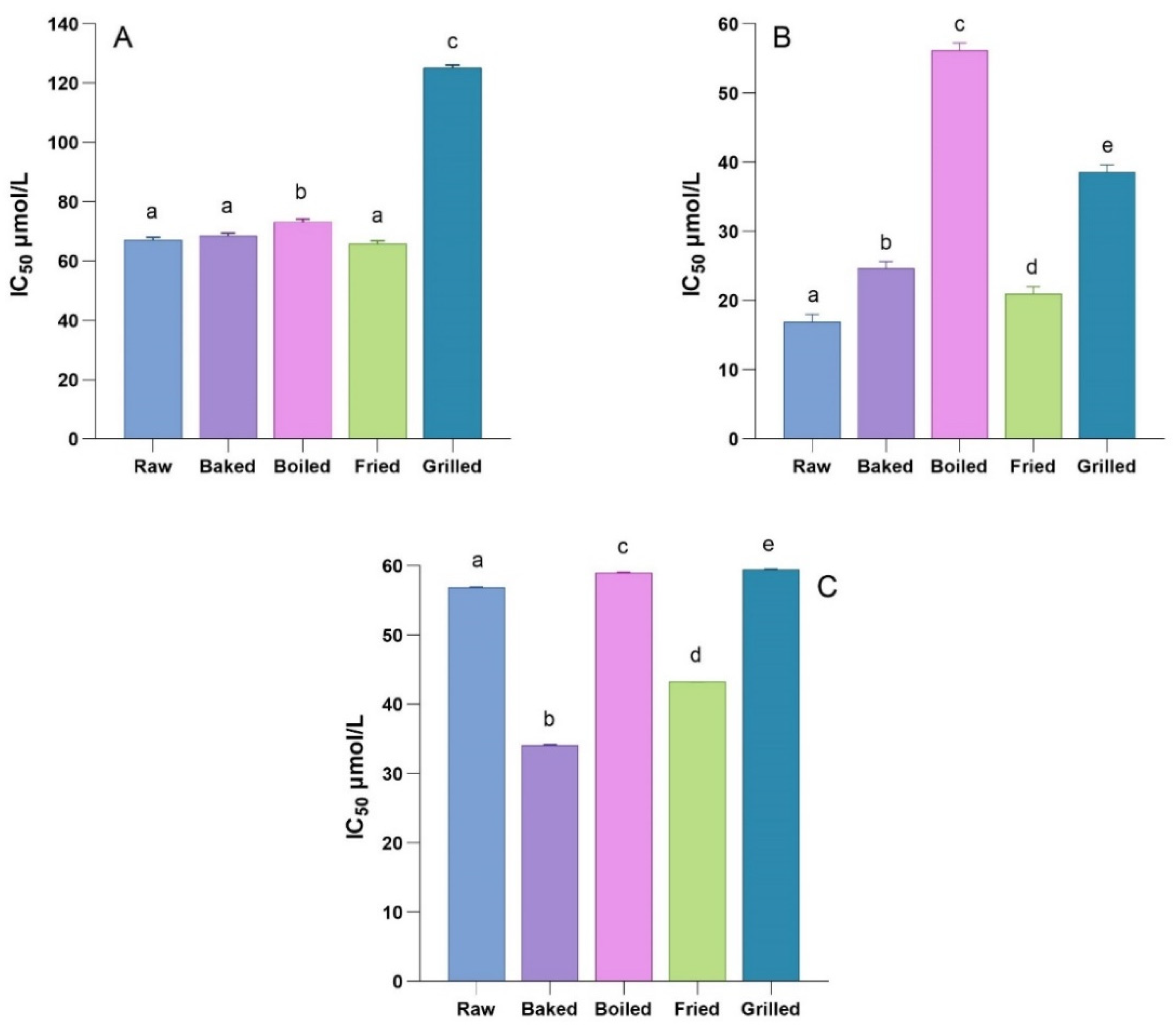
2.5.1. Measurements of α-Amylase Inhibitory Activity
2.5.2. Measurements of α-Glucosidase Inhibitory Activity
2.5.3. Measurements of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Activity
2.6. Statistics
3. Results and Discussion
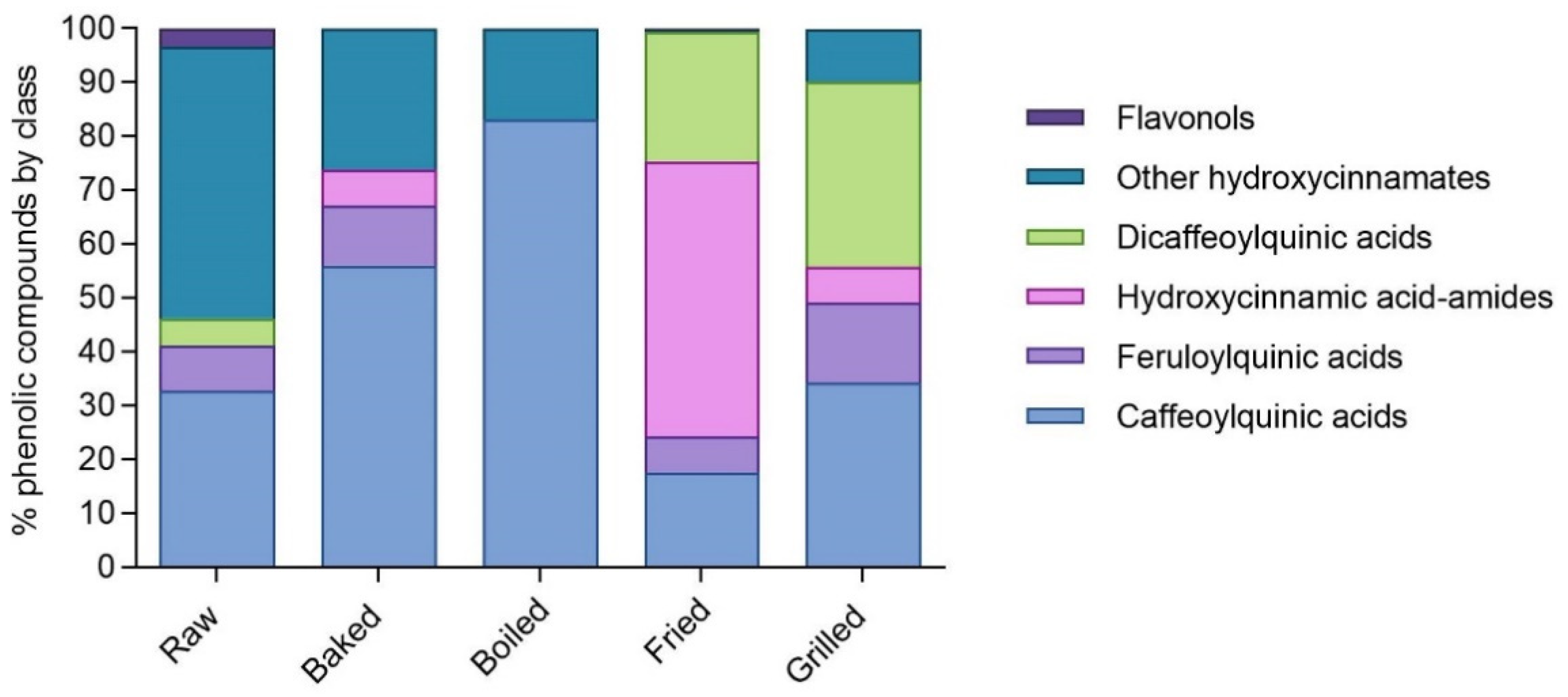
3.1. Phenolic Compounds Profile of Phenolic-Rich Fractions Extracted from Raw and Cooked In Vitro Digested Dark Purple Eggplant (DPE)
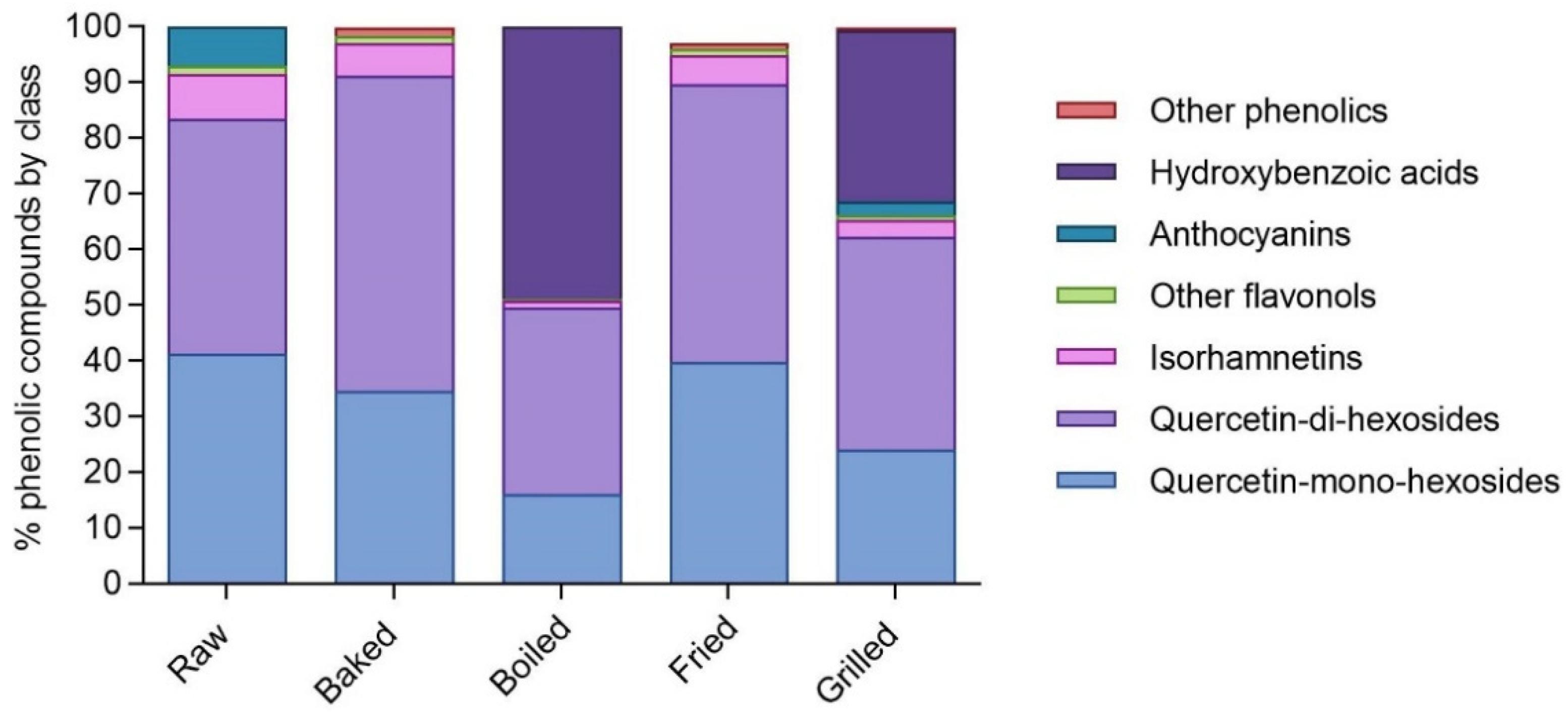
3.2. Phenolic Compounds Profile of Phenolic-Rich Fractions Extracted from Raw and Cooked In Vitro Digested Red-Skinned Onion (RSO)
3.3. Inhibitory Effect of In Vitro Digested Phenolic Rich-Fractions on α-Amylase, α-Glucosidase, and Dipeptidyl Peptidase-IV (DPP-IV) Activities
3.4. Statistical Analysis
3.4.1. Principal Component Analysis
3.4.2. Correlation and Linear Regression Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF. Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- IDF. Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Carstensen, B.; Rønn, P.F.; Jørgensen, M.E. Prevalence, incidence and mortality of type 1 and type 2 diabetes in Denmark 1996–2016. BMJ Open Diabetes Res. Care 2020, 8, e001071. [Google Scholar] [CrossRef]
- Schmidt, A.M. Diabetes mellitus and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 558–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastore, I.; Bolla, A.M.; Montefusco, L.; Lunati, M.E.; Rossi, A.; Assi, E.; Zuccotti, G.V.; Fiorina, P. The impact of diabetes mellitus on cardiovascular risk onset in children and adolescents. Int. J. Mol. Sci. 2020, 21, 4928. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.G.; Guedes, G.D.S.; Vasconcelos, S.M.D.L.; Santos, J.C.D.F. Kidney disease in diabetes mellitus: Cross-linking between hyperglycemia, redox imbalance and inflammation. Arq. Bras. Cardiol. 2019, 112, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Haratz, S.; Tanne, D. Diabetes, hyperglycemia and the management of cerebrovascular disease. Curr. Opin. Neurol. 2011, 24, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Hogger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2014, 22, 23–38. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Agunloye, O.M.; Ademosun, A.O.; Ogunsakin, B.G. Inhibitory effect of garlic, purple onion, and white onion on key enzymes linked with type 2 diabetes and hypertension. J. Diet. Suppl. 2019, 16, 105–118. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xiao, J.; Xu, B. An insight into anti-diabetic properties of dietary phytochemicals. Phytochem. Rev. 2017, 16, 535–553. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran 2017, 31, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary polyphenols, mediterranean diet, prediabetes, and type 2 diabetes: A Narrative Review of the Evidence. Oxid. Med. Cell. Longev. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Avila, J.A.; Rodrigo García, J.; González Aguilar, G.A.; de la Rosa, L.A. The antidiabetic mechanisms of polyphenols related to increased glucagon-like peptide-1 (GLP1) and insulin signaling. Molecules 2017, 22, 903. [Google Scholar] [CrossRef] [Green Version]
- Alkhalidy, H.; Wang, Y.; Liu, D. Dietary flavonoids in the prevention of T2D: An overview. Nutrients 2018, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Elya, B.; Handayani, R.; Sauriasari, R.; Azizahwati; Hasyyati, U.S.; Permana, I.T.; Permatasari, Y.I. Antidiabetic activity and phytochemical screening of extracts from indonesian plants by inhibition of alpha amylase, alpha glucosidase and dipeptidyl peptidase IV. Pakistan J. Biol. Sci. 2015, 18, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Res. Int. 2021, 145, 110383. [Google Scholar] [CrossRef]
- Abbas, G.; Al Harrasi, A.; Hussain, H.; Hamaed, A.; Supuran, C.T. The management of diabetes mellitus-imperative role of natural products against dipeptidyl peptidase-4, α-glucosidase and sodium-dependent glucose co-transporter 2 (SGLT2). Bioorg. Chem. 2019, 86, 305–315. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Antidiabetic food-derived peptides for functional feeding: Production, functionality and in vivo evidences. Foods 2020, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Cai, S.; Muhoza, B.; Qi, B.; Li, Y. Advance in dietary polyphenols as dipeptidyl peptidase-IV inhibitors to alleviate type 2 diabetes mellitus: Aspects from structure-activity relationship and characterization methods. Crit. Rev. Food Sci. Nutr. 2021, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cattivelli, A.; Conte, A.; Martini, S.; Tagliazucchi, D. Influence of cooking methods on onion phenolic compounds bioaccessibility. Foods 2021, 10, 1023. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Cattivelli, A.; Tagliazucchi, D. Domestic cooking methods affect the stability and bioaccessibility of dark purple eggplant (Solanum melongena) phenolic compounds. Food Chem. 2021, 341, 128298. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, N.; Miglio, C.; Del Rio, D.; Salvatore, S.; Serafini, M.; Brighenti, F. Effect of domestic cooking methods on the total antioxidant capacity of vegetables. Int. J. Food Sci. Nutr. 2009, 60, 12–22. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Martini, S.; Cavalchi, M.; Conte, A.; Tagliazucchi, D. The paradoxical effect of extra-virgin olive oil on oxidative phenomena during in vitro co-digestion with meat. Food Res. Int. 2018, 109, 82–90. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, A.; Tagliazucchi, D. Cocoa brew inhibits in vitro α-glucosidase activity: The role of polyphenols and high molecular weight compounds. Food Res. Int. 2014, 63, 439–445. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Martini, S.; Shamsia, S.; Helal, A.; Conte, A. Biological activities and peptidomic profile of in vitro-digested cow, camel, goat and sheep milk. Int. Dairy J. 2018, 81, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Liu, Y.; Lai, S.; Cao, H.; Guan, Y.; San Cheang, W.; Liu, B.; Zhao, K.; Miao, S.; Riviere, C.; et al. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends Food Sci. Technol. 2019, 85, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Juániz, I.; Ludwig, I.A.; Bresciani, L.; Dall’Asta, M.; Mena, P.; Del Rio, D.; Cid, C.; de Peña, M.P. Catabolism of raw and cooked green pepper (Capsicum annuum) (poly)phenolic compounds after simulated gastrointestinal digestion and faecal fermentation. J. Funct. Foods 2016, 27, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Juániz, I.; Ludwig, I.A.; Bresciani, L.; Dall’Asta, M.; Mena, P.; Del Rio, D.; Cid, C.; de Peña, M.P. Bioaccessibility of (poly)phenolic compounds of raw and cooked cardoon (Cynara cardunculus L.) after simulated gastrointestinal digestion and fermentation by human colonic microbiota. J. Funct. Foods 2017, 32, 195–207. [Google Scholar] [CrossRef]
- De Santiago, E.; Gill, C.I.R.; Carafa, I.; Tuohy, K.M.; De Peña, M.P.; Cid, C. Digestion and colonic fermentation of raw and cooked Opuntia ficus-indica cladodes impacts bioaccessibility and bioactivity. J. Agric. Food Chem. 2019, 67, 2490–2499. [Google Scholar] [CrossRef]
- De Santiago, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.P. Digestibility of (poly)phenols and antioxidant activity in raw and cooked cactus cladodes (Opuntia ficus-indica). J. Agric. Food Chem. 2018, 66, 5832–5844. [Google Scholar] [CrossRef]
- Chen, J.; Mangelinckx, S.; Ma, L.; Wang, Z.; Li, W.; De Kimpe, N. Caffeoylquinic acid derivatives isolated from the aerial parts of Gynura divaricata and their yeast α-glucosidase and PTP1B inhibitory activity. Fitoterapia 2014, 99, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.; Geng, S.; Liu, B. Three common caffeoylquinic acids as potential hypoglycemic nutraceuticals: Evaluation of α-glucosidase inhibitory activity and glucose consumption in HepG2 cells. J. Food Biochem. 2020, 44, e13361. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, Y.N.; Gao, B.; Xu, P.Y.; Inagaki, C.; Kawabata, J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem. 2008, 106, 1195–1201. [Google Scholar] [CrossRef]
- Roumani, M.; Duval, R.E.; Ropars, A.; Risler, A.; Robin, C.; Larbat, R. Phenolamides: Plant specialized metabolites with a wide range of promising pharmacological and health-promoting interests. Biomed. Pharmacother. 2020, 131, 110762. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Rizvi, S.I. Amylase inhibitory and metal chelating effects of different layers of onion (Allium cepa L.) at two different stages of maturation in vitro. Ann. Phytomedicine 2017, 6, 45–50. [Google Scholar] [CrossRef]
- Stoica, F.; Aprodu, I.; Enachi, E.; Stănciuc, N.; Condurache, N.N.; Duță, D.E.; Bahrim, G.E.; Râpeanu, G. Bioactive’s characterization, biological activities, and in silico studies of red onion (Allium cepa L.) skin extracts. Plants 2021, 10, 2330. [Google Scholar] [CrossRef]
- Metrani, R.; Singh, J.; Acharya, P.; Jayaprakasha, G.K.; Patil, B.S. Comparative metabolomics profiling of polyphenols, nutrients and antioxidant activities of two red onion (Allium cepa L.) cultivars. Plants 2020, 9, 1077. [Google Scholar] [CrossRef]
- Barber, E.; Houghton, M.J.; Williamson, G. Flavonoids as human intestinal α-glucosidase inhibitors. Foods 2021, 10, 1939. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, H.Y.; Choi, I.; Kim, J.B.; Jin, C.H.; Han, A.R. DPP-IV inhibitory potentials of flavonol glycosides isolated from the seeds of lens culinaris: In vitro and molecular docking analyses. Molecules 2018, 23, 1998. [Google Scholar] [CrossRef] [Green Version]
- Ogi, K.; Sumitani, H. Elucidation of an α-glucosidase inhibitor from the peel of Allium cepa by principal component analysis. Biosci. Biotechnol. Biochem. 2019, 83, 751–754. [Google Scholar] [CrossRef]
- Hua, F.; Zhou, P.; Wu, H.Y.; Chu, G.X.; Xie, Z.W.; Bao, G.H. Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu’an GuaPian tea: Molecular docking and interaction mechanism. Food Funct. 2018, 9, 4173–4183. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernades, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J. Enzyme Inhib. Med. Chem. 2007, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Huang, L.; Sun, C.; Zhao, D. Exploring the structure-activity relationship and interaction mechanism of flavonoids and α-glucosidase based on experimental analysis and molecular docking studies. Food Funct. 2020, 11, 3332–3350. [Google Scholar] [CrossRef] [PubMed]
- Abdel Motaal, A.; Salem, H.H.; Almaghaslah, D.; Alsayari, A.; Bin Muhsinah, A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Shati, A.A.; El-Askary, H. Flavonol glycosides: In vitro inhibition of DPPIV, aldose reductase and combating oxidative stress are potential mechanisms for mediating the antidiabetic activity of Cleome droserifolia. Molecules 2020, 25, 5864. [Google Scholar] [CrossRef] [PubMed]
- Eidenberger, T.; Selg, M.; Krennhuber, K. Inhibition of dipeptidyl peptidase activity by flavonol glycosides of guava (Psidium guajava L.): A key to the beneficial effects of guava in type II diabetes mellitus. Fitoterapia 2013, 89, 74–79. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.T.; Yin, Y.C.; Xing, S.; Li, W.N.; Fu, X.Q. Hypoglycemic effect and mechanism of isoquercitrin as an inhibitor of dipeptidyl peptidase-4 in type 2 diabetic mice. RSC Adv. 2018, 8, 14967–14974. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A review on structure–activity relationship of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef]


| In Vitro Digested Dark Purple Eggplant Phenolic-Rich Fractions | |||||
|---|---|---|---|---|---|
| Compound | Raw | Baked | Boiled | Fried | Grilled |
| Hydroxycinnamic Acids | |||||
| Caffeoylshikimic acid | n.d. | n.d. | n.d. | n.d. | 1.01 ± 0.08 |
| 3-O-Caffeoylquinic acid | 0.31 ± 0.01 c | 8.44 ± 0.03 a | 6.03 ± 0.03 b | 5.26 ± 0.03 b | 8.12 ± 0.97 a |
| 4-O-Caffeoylquinic acid | n.d. | 3.10 ± 0.11 b | 2.47 ± 0.03 c | 3.01 ± 0.03 b | 3.45 ± 0.04 a |
| 5-O-Caffeoylquinic acid trans | 2.86 ± 0.08 d | 30.29 ± 0.50 b | 19.25 ± 0.89 c | 32.26 ± 0.56 a | 33.17 ± 0.99 a |
| 5-O-Caffeoylquinic acid cis | 0.34 ± 0.00 e | 10.13 ± 0.13 c | 3.25 ± 0.12 d | 12.01 ± 0.04 b | 14.03 ± 0.58 a |
| 3-O-Feruloylquinic acid cis | n.d. | 0.53 ± 0.00 a | n.d. | n.d. | 0.12 ± 0.00 b |
| 3-O-Feruloylquinic acid trans | n.d. | 1.49 ± 0.03 b | n.d. | n.d. | 4.09 ± 0.10 a |
| 5-O-Feruloylquinic acid trans | n.d. | 2.08 ± 0.05 b | n.d. | 1.22 ± 0.01 b | 19.96 ± 1.13 a |
| 4-O-Feruloylquinic acid | 0.26 ± 0.00 b | 0.68 ± 0.02 a | n.d. | n.d. | n.d. |
| 5-O-Feruloylquinic acid cis | 0.63 ± 0.00 d | 5.66 ± 0.09 b | n.d. | 18.36 ± 0.70 a | 2.12 ± 0.03 c |
| 1,5-Di-O-caffeoylquinic acid | n.d. | n.d. | n.d. | n.d. | 2.23 ± 0.08 |
| 3,4-Di-O-caffeoylquinic acid | 0.56 ± 0.01 c | n.d. | n.d. | 17.68 ± 0.08 a | 13.16 ± 0.20 b |
| 4,5-Di-O-caffeoylquinic acid | n.d. | n.d. | n.d. | 29.27 ± 0.86 a | 8.58 ± 0.04 b |
| 3,5-Di-O-caffeoylquinic acid | n.d. | n.d. | n.d. | 24.52 ± 0.36 b | 36.13 ± 0.83 a |
| Caffeoylquinic acid dehydrodimer isomer | 1.61 ± 0.04 b | 2.79 ± 0.05 a | 0.79 ± 0.02 d | 0.92 ± 0.02 c | 1.65 ± 0.01 b |
| Caffeoylquinic acid dehydrodimer isomer | 1.36 ± 0.04 b | 1.34 ± 0.04 b | 1.36 ± 0.10 b | n.d. | 4.12 ± 0.09 a |
| Caffeoylquinic acid dehydrodimer isomer | 0.99 ± 0.02 d | 3.20 ± 0.09 b | 1.60 ± 0.08 c | n.d. | 5.32 ± 0.06 a |
| Caffeoylquinic acid dehydrodimer isomer | 1.47 ± 0.04 c | 5.48 ± 0.14 a | 0.70 ± 0.05 d | n.d. | 1.89 ± 0.06 b |
| Caffeoylquinic acid dehydrodimer isomer | n.d. | 11.49 ± 0.15 a | 1.86 ± 0.11 b | n.d. | 2.03 ± 0.07 b |
| Eggplant | |||||
| Compound | Raw | Baked | Boiled | Fried | Grilled |
| Caffeoylquinic acid dehydrodimer isomer | n.d. | n.d. | n.d. | n.d. | 0.87 ± 0.01 |
| N1,N5-Di-caffeoyl-spermidine | n.d. | 6.15 ± 0.17 | n.d. | n.d. | n.d. |
| N1,N10-Di-caffeoyl-spermidine | n.d. | n.d. | n.d. | 7.66 ± 0.03 | n.d. |
| N1,N5-Di-dihydrocaffeoyl-spermidine | n.d. | n.d. | n.d. | 107.50 ± 4.02 a | 11.58 ± 0.20 b |
| N1,N10-Dihydrocaffeoyl-caffeoyl-spermidine | n.d. | n.d. | n.d. | 37.40 ± 0.54 | n.d. |
| Total hydroxycinnamic acids | 10.39 ± 0.24 e | 92.87 ± 1.60 c | 37.32 ± 1.44 d | 297.07 ± 7.28 a | 173.63 ± 5.57 b |
| Flavonols | |||||
| Quercetin-di-O-hexoside | 0.23 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| Kaempferol-3-O-rutinoside | 0.11 ± 0.00 b | n.d. | n.d. | 0.87 ± 0.02 a | n.d. |
| Total flavonols | 0.35 ± 0.01 b | n.d. | n.d. | 0.87 ± 0.02 a | n.d. |
| Total | 10.74 ± 0.25 e | 92.87 ± 1.60 c | 37.32 ± 1.44 d | 297.95 ± 7.29 a | 173.63 ± 5.57 b |
| In Vitro Digested Red-Skinned Onion Phenolic-Rich Fractions | ||||||
|---|---|---|---|---|---|---|
| Compound | Raw | Baked | Boiled | Fried | Grilled | |
| Hydroxybenzoic acids | ||||||
| 1 | Protocatechuic acid-O-hexoside | n.d. | n.d. | 42.80 ± 0.24 b | n.d. | 51.35 ± 0.22 a |
| Total hydroxybenzoic acids | n.d. | n.d. | 42.80 ± 0.24 b | n.d. | 51.35 ± 0.22 a | |
| Flavan-3-ols | ||||||
| 2 | (Epi)catechin-3-O-hexoside isomer | n.d. | 1.55 ± 0.01 a | n.d. | 0.75 ± 0.01 c | 1.02 ± 0.00 b |
| Total flavan-3-ols | n.d. | 1.55 ± 0.01 a | n.d. | 0.75 ± 0.01 c | 1.02 ± 0.00 b | |
| Dihydro-flavonols | ||||||
| 3 | Taxifolin-O-hexoside isomer | 0.09 ± 0.00 b | n.d. | n.d. | 0.15 ± 0.00 a | n.d. |
| Total dihydro-flavonols | 0.09 ± 0.00 b | n.d. | n.d. | 0.15 ± 0.00 a | n.d. | |
| Flavonols | ||||||
| 4 | Quercetin-3-O-hexoside isomer | 11.51 ± 0.08 d | 18.70 ± 0.07 b | n.d. | 15.17 ± 0.07 c | 20.69 ± 0.06 a |
| 5 | Quercetin-4′-O-hexoside | 22.76 ± 0.11 a | 12.90 ± 0.10 d | 10.27 ± 0.01 e | 18.02 ± 0.07 b | 15.94 ± 0.01 c |
| 6 | Quercetin-3-O-glucoside | 0.91 ± 0.01 d | 1.72 ± 0.02 b | 3.81 ± 0.08 a | 1.42 ± 0.01 c | 3.71 ± 0.05 a |
| 7 | Quercetin-3-O-hexoside-7-O-hexoside | n.d. | 1.83 ± 0.00 | n.d. | n.d. | n.d. |
| 8 | Quercetin-7-O-hexoside-4′-O-hexoside | 1.78 ± 0.00 c | 2.12 ± 0.01 a | n.d. | n.d. | 1.83 ± 0.00 b |
| 9 | Quercetin-3-O-hexoside-4′-O-hexoside | 34.36 ± 0.10 d | 50.49 ± 0.10 b | 29.45 ± 0.05 e | 43.54 ± 0.16 c | 62.08 ± 0.81 a |
| 10 | Quercetin-tri-O-hexoside isomer | 0.92 ± 0.01 c | 1.29 ± 0.00 a | 0.21 ± 0.00 d | 0.94 ± 0.01 b | 1.28 ± 0.00 a |
| 11 | Kaempferol-7-O-hexoside isomer | 0.13 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| 12 | Kaempferol-3-O-hexoside isomer | 0.23 ± 0.0 | n.d. | n.d. | n.d. | n.d. |
| 13 | Isorhamnetin-3-O-hexoside isomer | n.d. | 2.33 ± 0.01 a | n.d. | 1.73 ± 0.00 b | 1.60 ± 0.00 c |
| 14 | Isorhamnetin-4′-O-hexoside | 4.45 ± 0.04 b | n.d. | n.d. | 5.37 ± 0.06 a | n.d. |
| 15 | Isorhamnetin-3-O-hexoside-4′-O-hexoside | 2.37 ± 0.00 c | 3.28 ± 0.01 b | 1.04 ± 0.01 d | n.d | 3.35 ± 0.00 a |
| Total flavonols | 79.42 ± 0.36 d | 94.66 ± 0.33 b | 44.78 ± 0.15 e | 86.19 ± 0.37 c | 110.49 ± 1.08 a | |
| Anthocyanins | ||||||
| 16 | Cyanidin-3-O-hexoside | n.d. | n.d. | n.d. | n.d. | 0.73 ± 0.00 |
| Red-skinned onion | ||||||
| Compound | Raw | Baked | Boiled | Fried | Grilled | |
| 17 | Cyanidin-O-malonyl-hexoside isomer | 2.02 ± 0.02 | n.d. | n.d. | n.d. | n.d. |
| 18 | Cyanidin-O-hexoside-O-hexoside isomer | 1.24 ± 0.00 b | n.d. | n.d. | n.d. | 1.90 ± 0.01 a |
| 19 | Cyanidin-O-hexoside-O-hexoside isomer | 0.79 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| 20 | Cyanidin-O-hexoside-O-malonyl-hexoside isomer | 1.92 ± 0.00 a | n.d. | n.d. | n.d. | 1.62 ± 0.01 b |
| Total anthocyanins | 5.98 ± 0.03 a | n.d. | n.d. | n.d. | 4.25 ± 0.02 b | |
| Total | 85.49 ± 0.39 d | 96.21 ± 0.34 b | 87.58 ± 0.39 c | 87.09 ± 0.38 c,d | 167.11 ± 1.18 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattivelli, A.; Conte, A.; Martini, S.; Tagliazucchi, D. Cooking and In Vitro Digestion Modulate the Anti-Diabetic Properties of Red-Skinned Onion and Dark Purple Eggplant Phenolic Compounds. Foods 2022, 11, 689. https://doi.org/10.3390/foods11050689
Cattivelli A, Conte A, Martini S, Tagliazucchi D. Cooking and In Vitro Digestion Modulate the Anti-Diabetic Properties of Red-Skinned Onion and Dark Purple Eggplant Phenolic Compounds. Foods. 2022; 11(5):689. https://doi.org/10.3390/foods11050689
Chicago/Turabian StyleCattivelli, Alice, Angela Conte, Serena Martini, and Davide Tagliazucchi. 2022. "Cooking and In Vitro Digestion Modulate the Anti-Diabetic Properties of Red-Skinned Onion and Dark Purple Eggplant Phenolic Compounds" Foods 11, no. 5: 689. https://doi.org/10.3390/foods11050689








