The Effect of Irrigation-Initiation Timing on the Phenolic Composition and Overall Quality of Cabernet Sauvignon Wines Grown in a Semi-Arid Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Irrigation Treatments, Calculation of Irrigation Amounts
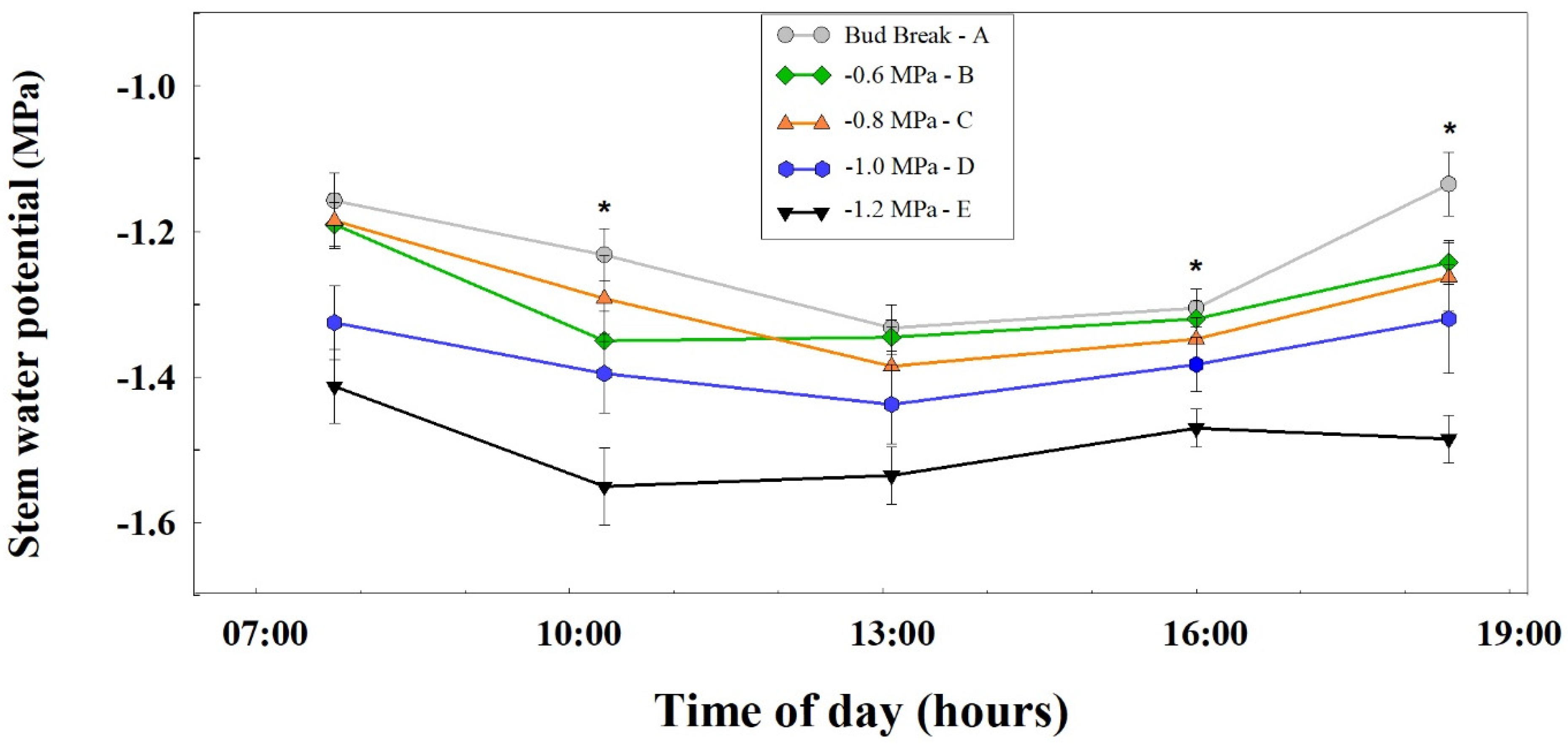
2.3. Midday Stem Water Potential and Yield Measurements
2.4. Wine Vinification
2.5. Must and Wine Analysis
2.6. Wine Sensory Evaluation
2.7. Determination of Polyphenols
2.8. Determination of Glycosylated Anthocyanins
2.9. Statistical Analyses
3. Results and Discussion
3.1. Irrigation Application
3.2. Vine Water Status
3.3. Yield Parmeters
3.4. Must Parmeters
3.5. Wine Parmeters
3.6. Wine Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drappier, J.; Thibon, C.; Rabot, A.; Geny-Denis, L. Relationship between wine composition and temperature: Impact on Bordeaux wine typicity in the context of global warming—Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.J.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term climate change: Projections, commitments and irreversibility. In Climate Change 2013 the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 1029–1136. ISBN 9781107415324. [Google Scholar]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizildeniz, T.; Mekni, I.; Santesteban, H.; Pascual, I.; Morales, F.; Irigoyen, J.J. Effects of climate change including elevated CO2 concentration, temperature and water deficit on growth, water status, and yield quality of grapevine (Vitis vinifera L.) cultivars. Agric. Water Manag. 2015, 159, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Parry, M.L.; Canziani, O.; Palutikof, J.; Van der Linden, P.; Hanson, C. (Eds.) Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007; pp. 541–580. [Google Scholar]
- Matthews, M.A.; Ishii, R.; Anderson, M.M.; O’Mahony, M.O. Dependence of wine sensory attributes on vine water status. J. Sci. Food Agric. 1990, 51, 321–335. [Google Scholar] [CrossRef]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Bravdo, B.; Hepner, Y.; Loinger, C.; Cohen, S.; Tabacman, H. Effect of irrigation and crop level on growth, yield and wine quality of Cabernet Sauvignon. Am. J. Enol. Vitic. 1985, 36, 132–139. [Google Scholar]
- Munitz, S.; Netzer, Y.; Schwartz, A. Sustained and regulated deficit irrigation of field-grown Merlot grapevines. Aust. J. Grape Wine Res. 2016, 23, 87–94. [Google Scholar] [CrossRef]
- Chaves, M.M.; Santos, T.P.; Souza, C.R.; Ortuño, M.F.; Rodrigues, M.L.; Lopes, C.M.; Maroco, J.P.; Pereira, J.S. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann. Appl. Biol. 2007, 150, 237–252. [Google Scholar] [CrossRef]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Smithyman, R.P.R.R.P.; Mills, L.J.L.J. Interactive effects of deficit irrigation and crop load on Cabernet Sauvignon in an arid climate. Am. J. Enol. Vitic. 2008, 59, 221–234. [Google Scholar] [CrossRef]
- Netzer, Y.; Munitz, S.; Shtein, I.; Schwartz, A. Structural memory in grapevines: Early season water availability affects late season drought stress severity. Eur. J. Agron. 2019, 105, 96–103. [Google Scholar] [CrossRef]
- Girona, J.; Marsal, J.; Mata, M.; Del Campo, J.; Basile, B. Phenological sensitivity of berry growth and composition of Tempranillo grapevines (Vitis vinifera L.) to water stress. Aust. J. Grape Wine Res. 2009, 15, 268–277. [Google Scholar] [CrossRef]
- Smithyman, R.; Wample, R. Water deficit and crop level influences on photosynthetic strain and blackleaf symptom development in Concord grapevines. Am. J. Enol. Vitic. 2001, 52, 364–375. [Google Scholar]
- Romero, P.; Fernández-Fernández, J.I.; Martinez-Cutillas, A. Physiological thresholds for efficient regulated deficit-irrigation management in winegrapes grown under semiarid conditions. Am. J. Enol. Vitic. 2010, 61, 300–312. [Google Scholar]
- Grimes, D.W.; Williams, L.E. Irrigation Effects on Plant Water Relations and Productivity of Thompson Seedless Grapevines. Crop Sci. 1990, 30, 255–260. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Cifre, J.; Bota, J.; Flexas, J. A ten-year study on the physiology of two Spanish grapevine cultivars under field conditions: Effects of water availability from leaf photosynthesis to grape yield and quality. Funct. Plant Biol. 2003, 30, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.). Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Munitz, S.; Schwartz, A.; Netzer, Y. Effect of timing of irrigation initiation on vegetative growth, physiology and yield parameters in Cabernet Sauvignon grapevines. Aust. J. Grape Wine Res. 2020, 26, 220–232. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Ollé, D.; Guiraud, J.L.; Souquet, J.M.; Terrier, N.; Ageorges, A.; Cheynier, V.; Verries, C. Effect of pre- and post-veraison water deficit on proanthocyanidin and anthocyanin accumulation during Shiraz berry development. Aust. J. Grape Wine Res. 2011, 17, 90–100. [Google Scholar] [CrossRef]
- Zarrouk, O.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Santos, R.R.; Pinheiro, C.; Costa, J.M.; Lopes, C.; Chaves, M.M. Impact of irrigation regime on berry development and flavonoids composition in Aragonez (Syn. Tempranillo) grapevine. Agric. Water Manag. 2012, 114, 18–29. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Response of grapevine cv. ‘Tempranillo’ to timing and amount of irrigation: Water relations, vine growth, yield and berry and wine composition. Irrig. Sci. 2010, 28, 113–125. [Google Scholar] [CrossRef]
- Romero, P.; Gil-Muñoz, R.; del Amor, F.M.; Valdés, E.; Fernández, J.I.; Martinez-Cutillas, A. Regulated Deficit Irrigation based upon optimum water status improves phenolic composition in Monastrell grapes and wines. Agric. Water Manag. 2013, 121, 85–101. [Google Scholar] [CrossRef]
- Williams, L.E.; Araujo, F.J. Correlations among predawn leaf, midday leaf, and midday stem water potential and their correlations with other measures of soil and plant water status in Vitis vinifera. J. Am. Soc. 2002, 127, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Santesteban, L.G.; Miranda, C.; Royo, J.B. Regulated deficit irrigation effects on growth, yield, grape quality and individual anthocyanin composition in Vitis vinifera L. cv. “Tempranillo”. Agric. Water Manag. 2011, 98, 1171–1179. [Google Scholar] [CrossRef]
- Fernandes-Silva, A.; Oliveira, M.; Paço, T.A.; Ferreira, I. Deficit Irrigation in Mediterranean Fruit Trees and Grapevines: Water Stress Indicators and Crop Responses. In Irrigation in Agroecosystems; Ondrašek, G., Ed.; IntechOpen: London, UK, 2019; pp. 1–35. ISBN 9789537619992. [Google Scholar]
- Barbagallo, M.G.; Vesco, G.; Di Lorenzo, R.; Lo Bianco, R.; Pisciotta, A. Soil and regulated deficit irrigation affect growth, yield and quality of ‘nero d’avola’ grapes in a semi-arid environment. Plants 2021, 10, 641. [Google Scholar] [CrossRef]
- Hardie, W.; Considine, J. Response of grapes to water-deficit stress in particular stages of development. Am. J. Enol. Vitic. 1976, 27, 55–61. [Google Scholar]
- Kennedy, J. Understanding grape berry development. Pract. Winer. Vineyard 2002, 4, 1–5. [Google Scholar]
- Munitz, S.; Netzer, Y.; Shetin, I.; Schwartz, A. Water availability dynamics have long-term effects on mature stem structure in Vitis vinifera. Am. J. Bot. 2018, 105, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munitz, S.; Schwartz, A.; Netzer, Y. Water consumption, crop coefficient and leaf area relations of a Vitis vinifera cv. “Cabernet Sauvignon” vineyard. Agric. Water Manag. 2019, 219, 86–94. [Google Scholar] [CrossRef]
- Van Es, H.M.; Gomes, C.P.; Sellmann, M.; van Es, C.L. Spatially-Balanced Complete Block designs for field experiments. Geoderma 2007, 140, 346–352. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; Food and Agriculture Organization: Rome, Italy, 1998. [Google Scholar]
- Netzer, Y.; Yao, C.; Shenker, M.; Bravdo, B.A.; Schwartz, A. Water use and the development of seasonal crop coefficients for Superior Seedless grapevines trained to an open-gable trellis system. Irrig. Sci. 2009, 27, 109–120. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. OIV Standard for International Wine Competitions and Spiritous Beverages of Vitivinicultural Origin (OIV-Concours 332A-2009). Available online: https://www.oiv.int/public/medias/1848/oiv-concours-332a-2009-fr-avec-signature.pdf (accessed on 13 February 2022).
- Rosenzweig, T.; Skalka, N.; Rozenberg, K.; Elyasiyan, U.; Pinkus, A.; Green, B.; Stanevsky, M.; Drori, E. Red wine and wine pomace reduced the development of insulin resistance and liver steatosis in HFD-fed mice. J. Funct. Foods 2017, 34, 379–389. [Google Scholar] [CrossRef]
- De Andrade, R.H.S.; Do Nascimento, L.S.; Pereira, G.E.; Hallwass, F.; Paim, A.P.S. Anthocyanic composition of Brazilian red wines and use of HPLC-UV-Vis associated to chemometrics to distinguish wines from different regions. Microchem. J. 2013, 110, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Opazo, C.; Ortega-Farias, S.; Fuentes, S. Effects of grapevine (Vitis vinifera L.) water status on water consumption, vegetative growth and grape quality: An irrigation scheduling application to achieve regulated deficit irrigation. Agric. Water Manag. 2010, 97, 956–964. [Google Scholar] [CrossRef]
- Buesa, I.; Pérez, D.; Castel, J.; Intrigliolo, D.; Castel, J. Effect of deficit irrigation on vine performance and grape composition of Vitis vinifera L. cv. Muscat of Alexandria. Aust. J. Grape Wine Res. 2017, 23, 251–259. [Google Scholar] [CrossRef]
- Shellie, K. Above Ground Drip Application Practices Alter Water Productivity of Malbec Grapevines under Sustained Deficit. J. Agric. Sci. 2017, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effects of water stress on vegetative growth and ‘merlot’ grapevine yield in a semi-arid mediterranean climate. Horticulturae 2020, 6, 95. [Google Scholar] [CrossRef]
- Buesa, I.; Intrigliolo, D.S.; Castel, J.R.; Vilanova, M. Influence of water regime on grape aromatic composition of Muscat of Alexandria in a semiarid climate. Sci. Hortic. (Amst.) 2021, 290, 110525. [Google Scholar] [CrossRef]
- Ohana-Levi, N.; Munitz, S.; Ben-Gal, A.; Schwartz, A.; Peeters, A.; Netzer, Y.; Schwarz, A.; Peeters, A.; Netzer, Y.; Schwartz, A.; et al. Multiseasonal grapevine water consumption—Drivers and forecasting. Agric. For. Meteorol. 2020, 280, 1–12. [Google Scholar] [CrossRef]
- Williams, L.E.; Ayars, J.E. Grapevine water use and the crop coefficient are linear functions of the shaded area measured beneath the canopy. Agric. For. Meteorol. 2005, 132, 201–211. [Google Scholar] [CrossRef]
- Ohana-Levi, N.; Munitz, S.; Ben-Gal, A.; Netzer, Y. Evaluation of within-season grapevine evapotranspiration patterns and drivers using generalized additive models. Agric. Water Manag. 2020, 228, 105808. [Google Scholar] [CrossRef]
- Choné, X.; Van Leeuwen, C.; Dubourdieu, D.; Gaudillere, J.P. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Patakas, A.; Noitsakis, B.; Chouzouri, A. Optimization of irrigation water use in grapevines using the relationship between transpiration and plant water status. Agric. Ecosyst. Environ. 2005, 106, 253–259. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Marín, D.; Sesma, B.; Intrigliolo, D.S.; Mirás-Avalos, J.M.; Escalona, J.M.; Montoro, A.; de Herralde, F.; Baeza, P.; et al. Discrimination ability of leaf and stem water potential at different times of the day through a meta-analysis in grapevine (Vitis vinifera L.). Agric. Water Manag. 2019, 221, 202–210. [Google Scholar] [CrossRef]
- Mirás-avalos, J.M.; Intrigliolo, D.S. Grape Composition under Abiotic Constrains: Water Stress and Salinity. Front. Plant Sci. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Castellarin, S.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [Green Version]
- Savoi, S.; Herrera, J.C.; Carlin, S.; Lotti, C.; Bucchetti, B.; Peterlunger, E.; Castellarin, S.D.; Mattivi, F. From grape berries to wines: Drought impacts on key secondary metabolites. Oeno One 2020, 54, 569–582. [Google Scholar] [CrossRef]
- Cook, M.G.; Zhang, Y.; Nelson, C.J.; Gambetta, G.; Kennedy, J.A.; Kurtural, S.K. Anthocyanin composition of merlot is ameliorated by light microclimate and irrigation in central California. Am. J. Enol. Vitic. 2015, 66, 266–278. [Google Scholar] [CrossRef]
- Hochberg, U.; Degu, A.; Cramer, G.R.; Rachmilevitch, S.; Fait, A. Cultivar specific metabolic changes in grapevines berry skins in relation to deficit irrigation and hydraulic behavior. Plant Physiol. Biochem. 2015, 88, 42–52. [Google Scholar] [CrossRef]
- Deluc, L.G.; Grimplet, J.; Wheatley, M.D.; Tillett, R.L.; Quilici, D.R.; Osborne, C.; Schooley, D.A.; Schlauch, K.A.; Cushman, J.C.; Cramer, G.R. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genom. 2007, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagnari, F.; Galieni, A.; Pisante, M. Drought stress effects on crop quality. Water Stress Crop Plants A Sustain. Approach 2016, 2, 375–392. [Google Scholar] [CrossRef]
- Ju, Y.; Yang, B.; He, S.; Tu, T.; Min, Z.; Fang, Y.; Sun, X. Anthocyanin accumulation and biosynthesis are modulated by regulated deficit irrigation in Cabernet Sauvignon (Vitis vinifera L.) grapes and wines. Plant Physiol. Biochem. 2019, 135, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Ren, Y.; Zhao, Y.; Merkeryan, H.; Su-Zhou, C.; Li, Y.; Mei, Y.; Liu, X. An adequate regulated deficit irrigation strategy improves wine astringency perception by altering proanthocyanidin composition in Cabernet Sauvignon grapes. Sci. Hortic. (Amst.) 2021, 285, 110182. [Google Scholar] [CrossRef]
- Cáceres-Mella, A.; Ribalta-Pizarro, C.; Villalobos-González, L.; Cuneo, I.F.; Pastenes, C. Controlled water deficit modifies the phenolic composition and sensory properties in Cabernet Sauvignon wines. Sci. Hortic. (Amst.) 2018, 237, 105–111. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.B.H. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef]
- Bertelli, A.A.A.; Das, D.K. Grapes, wines, resveratrol, and heart health. J. Cardiovasc. Pharmacol. 2009, 54, 468–476. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Tradit. Med. 2021, 21, 415–431. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Wine phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Hursel, R.; Westerterp-Plantenga, M.S. Catechin- and caffeine-rich teas for control of body weight in humans. Am. J. Clin. Nutr. 2013, 98, 1682–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phyther. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Aguirre, L.; Arias, N.; Macarulla, M.T.; Gracia, A.; Portillo, M.P. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 2011, 4, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Quercetin: Antiviral significance and possible COVID-19 integrative considerations. Nat. Prod. Commun. 2020, 15, 1934578X20976293. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [Green Version]

| Treatment | Dates of Irrigation Initiation | Seasonal Irrigation (mm) |
|---|---|---|
| Budbreak | 4 April (94) | 113.1 |
| −0.6 MPa | 2 May (122) | 92.5 |
| −0.8 MPa | 7 June (158) | 44.6 |
| −1.0 MPa | 27 June (178) | 26.5 |
| −1.2 MPa | 13 July (194) | 20.1 |
| Treatment | Yield (kg/Vine) | Bunch (Number/Vine) | Bunch Mass (gr) | 100 Berry Mass (gr) | Berry (Number/Bunch) |
|---|---|---|---|---|---|
| Budbreak | 7.77 A | 59.0 | 133.5 A | 138.5 A | 83.5 |
| −0.6 MPa | 7.00 AB | 56.5 | 125.2 B | 136.6 A | 86.0 |
| −0.8 MPa | 6.12 AB | 55.7 | 109.7 AB | 126.6 AB | 77.1 |
| −1.0 MPa | 5.75 B | 50.5 | 113 AB | 120.7 B | 77.2 |
| −1.2 MPa | 5.37 B | 52.7 | 102.2 B | 117.1 B | 77.1 |
| Treatment | TSS (°Brix) | pH | TA (g/L) |
|---|---|---|---|
| Budbreak | 24.7 A | 3.48 A | 4.9 A |
| −0.6 MPa | 24.2 A | 3.48 A | 4.3 A |
| −0.8 MPa | 25.2 A | 3.51 A | 4.7 A |
| −1.0 MPa | 24.9 A | 3.46 A | 5.0 A |
| −1.2 MPa | 24.6 A | 3.51 A | 4.5 A |
| Treatment | Yellow, AU | Red, AU | Blue, AU | CI, AU | CH | Total Phenolics, (as mg Gallic Acid/L) |
|---|---|---|---|---|---|---|
| Budbreak | 3.47 B | 4.38 C | 1.25 B | 9.12 B | 0.793 A | 872 B |
| −0.6 MPa | 3.61 AB | 4.70 CB | 1.32 AB | 9.63 AB | 0.768 AB | 925 AB |
| −0.8 MPa | 3.68 AB | 4.96 CBA | 1.39 AB | 10.03 AB | 0.740 B | 950 AB |
| −1.0 MPa | 4.00 AB | 5.45 BA | 1.53 AB | 10.98 AB | 0.735 B | 975 AB |
| −1.2 MPa | 4.18 A | 5.71 A | 1.61 A | 11.48 A | 0.735 B | 1026 A |
| Treatment | Malvidin-3-O-glucoside | Delphinidin-3-O-glucoside | Cyanidin-3-O-glucoside | Petunidin-3-O-glucoside | Peonidin-3-O-glucoside |
|---|---|---|---|---|---|
| Budbreak | 66.1 B | 0.36 B | 0.69 | 4.91 B | 2.12 C |
| −0.6 MPa | 78.7 AB | 0.34 B | 0.77 | 4.91 B | 2.35 CB |
| −0.8 MPa | 90.2 AB | 0.50 AB | 0.65 | 7.60 AB | 2.87 AB |
| −1.0 MPa | 84.5 AB | 0.59 AB | 0.71 | 7.68 A | 2.81 AB |
| −1.2 MPa | 94.2 A | 0.83 A | 0.79 | 9.08 A | 3.21 A |
| Treatment | Gallic Acid | Catechin | Epicatechin | Vanillin | p-Coumaric Acid | Vanillic Acid | Quercetin | Caffeic Acid |
|---|---|---|---|---|---|---|---|---|
| Budbreak | 20.02 | 116.5 B | 48.1 B | 2.24 B | 4.33 | 2.27 B | 1.15 B | 8.23 |
| −0.6 MPa | 17.69 | 142.3 AB | 53.3 AB | 2.68 AB | 4.24 | 2.36 AB | 1.31 B | 7.44 |
| −0.8 MPa | 21.53 | 154.5 AB | 48.7 B | 3.76 A | 4.26 | 2.83 AB | 1.69 AB | 7.46 |
| −1.0 MPa | 24.54 | 144.3 AB | 49.6 B | 3.24 AB | 4.37 | 2.94 A | 1.62 AB | 6.91 |
| −1.2 MPa | 26.96 | 162.8 A | 56.2 A | 3.55 A | 4.47 | 2.99 A | 2.04 A | 6.60 |
| Treatment | Color Quality | Color Intensity | Smell Intensity | Smell Genu | Smell Quality | Taste Intensity | Taste Genu | Taste Quality | After Taste | General | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Budbreak | 3.9 BC | 7.8 B | 6.8 | 4.9 | 13.8 | 6.8 | 4.9 AB | 18.4 | 6.9 | 9.9 | 84.4 C |
| −0.6 MPa | 3.6 C | 8.2 B | 7.0 | 4.8 | 13.8 | 6.8 | 4.6 B | 19.0 | 6.8 | 9.9 | 84.7 BC |
| −0.8 MPa | 4.2 B | 8.5 B | 6.9 | 5.1 | 14.1 | 7.0 | 5.0 AB | 18.9 | 7.0 | 9.9 | 86.8 AB |
| −1.0 MPa | 4.3 B | 8.5 B | 6.9 | 4.6 | 14.0 | 6.9 | 4.8 AB | 19.3 | 6.7 | 9.8 | 86.0 AB |
| −1.2 MPa | 4.8 A | 9.6 A | 7.2 | 5.0 | 14.0 | 7.2 | 5.1 A | 19.1 | 7.0 | 10.0 | 89.2 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drori, E.; Munitz, S.; Pinkus, A.; Stanevsky, M.; Netzer, Y. The Effect of Irrigation-Initiation Timing on the Phenolic Composition and Overall Quality of Cabernet Sauvignon Wines Grown in a Semi-Arid Climate. Foods 2022, 11, 770. https://doi.org/10.3390/foods11050770
Drori E, Munitz S, Pinkus A, Stanevsky M, Netzer Y. The Effect of Irrigation-Initiation Timing on the Phenolic Composition and Overall Quality of Cabernet Sauvignon Wines Grown in a Semi-Arid Climate. Foods. 2022; 11(5):770. https://doi.org/10.3390/foods11050770
Chicago/Turabian StyleDrori, Elyashiv, Sarel Munitz, Ania Pinkus, Maria Stanevsky, and Yishai Netzer. 2022. "The Effect of Irrigation-Initiation Timing on the Phenolic Composition and Overall Quality of Cabernet Sauvignon Wines Grown in a Semi-Arid Climate" Foods 11, no. 5: 770. https://doi.org/10.3390/foods11050770
APA StyleDrori, E., Munitz, S., Pinkus, A., Stanevsky, M., & Netzer, Y. (2022). The Effect of Irrigation-Initiation Timing on the Phenolic Composition and Overall Quality of Cabernet Sauvignon Wines Grown in a Semi-Arid Climate. Foods, 11(5), 770. https://doi.org/10.3390/foods11050770






