Enzyme and Antioxidant Activities of Malted Bambara Groundnut as Affected by Steeping and Sprouting Times
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Materials, Reagents, and Equipment
2.2. Malted Bambara Groundnut Production Process
2.3. Physicochemical Analysis of Bambara Groundnut Malt
2.3.1. Sprout Length and Moisture Uptake of the Bambara Groundnut Green Malts
2.3.2. pH Determination of Bambara Groundnut Malts
2.3.3. Colour Determination of Bambara Groundnut Malts
2.3.4. Bambara Groundnut Malts Protein Content Determination
2.4. Determination of α- and β-Amylase Activities of BGN Malts
2.4.1. Alpha-Amylase Assay Procedure (Ceralpha Method)
2.4.2. β-Amylase Assay Procedure (Betamyl-3 Method)
2.5. Determination of Total Polyphenols and Antioxidants Activities of Bambara Groundnut Malts
2.5.1. Total Polyphenols Activity Determination by Folin–Ciocâlteu Reagent Assay (FCR) Method
2.5.2. Antioxidant Activities Determination by Ferric Reducing Antioxidant Power Assay (FRAP) Method
2.5.3. Antioxidant Activities Determination by 2,2-Diphenyl-1-Picrylhydrazyl Assay (DPPH) Method
2.6. Data Analysis
3. Results and Discussion
3.1. Water Absorption of Steeped Bambara Groundnut Seeds
3.2. Effect of Steeping and Sprouting Times on the Sprout Length of Bambara Groundnut Green Malts
3.3. Effect of Steeping and Sprouting Time on the Colour of Bambara Groundnut Malt
3.4. Effect of Steeping and Sprouting Time on the pH of Bambara Groundnut Malt
3.5. Effect of Steeping and Sprouting Time on the Protein Content of Bambara Groundnut Malt
3.6. Effect of Steeping and Sprouting Time on α- and β-Amylase Activities of Bambara Groundnut Malt
3.7. Effect of Steeping and Sprouting Time on Total Polyphenol Activities of Bambara Groundnut Malt
3.8. Effect of Steeping and Sprouting Time on the Antioxidant Activities of Bambara Groundnut Malt
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briggs, D.E. Malts and Malting; Springer Science & Business Media, Blackie Academic and Professional: London, UK, 1998. [Google Scholar]
- Lewis, M.J.; Young, T.W. Malting Technology: Malt, Specialized Malts and Non-malt Adjuncts. In Brewing; Springer: Boston, MA, USA; Aspen Publishers, Inc.: Boston, MA, USA, 2001; pp. 163–190. [Google Scholar] [CrossRef]
- Mulder, C.J. Malts and Malting. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 516–541. [Google Scholar] [CrossRef]
- MacLeod, L.; Evans, E. Barley: Malting. In Encyclopedia of Food Grains; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–433. [Google Scholar] [CrossRef]
- Baranwal, D. Malting: An indigenous technology used for improving the nutritional quality of grains—A review. Asian J. Dairy Food Res. 2017, 36, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Edney, M.J.; Izydorczyk, M.S. MALT|Malt Types and Products. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 3671–3677. [Google Scholar] [CrossRef]
- Rosentrater, K.A.; Evers, A.D. Malting, brewing, fermentation, and distilling. In Kent’s Technology of Cereals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 729–784. ISBN 978-0-08-100529-3. [Google Scholar]
- Frank, T.; Scholz, B.; Peter, S.; Engel, K.-H. Metabolite profiling of barley: Influence of the malting process. Food Chem. 2011, 124, 948–957. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. Barley. In Cereal Grains for the Food and Beverage Industries; Woodhead Publishing: Cambridge, UK, 2013; pp. 155–201e. ISBN 9780857098924. [Google Scholar]
- Møller, M.S.; Svensson, B. Enzymes in grain processing. Curr. Opin. Food Sci. 2021, 37, 153–159. [Google Scholar] [CrossRef]
- Aubert, M.K.; Coventry, S.; Shirley, N.J.; Betts, N.S.; Würschum, T.; Burton, R.A.; Tucker, M.R. Differences in hydrolytic enzyme activity accompany natural variation in mature aleurone morphology in barley (Hordeum vulgare L.). Sci. Rep. 2018, 8, 11025. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Román-Gutiérrez, A.D. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Gupta, M.; Abu-Ghannam, N.; Gallaghar, E. Barley for Brewing: Characteristic Changes during Malting, Brewing and Applications of its By-Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Lekjing, S.; Venkatachalam, K. Effects of germination time and kilning temperature on the malting characteristics, biochemical and structural properties of HomChaiya rice. RSC Adv. 2020, 10, 16254–16265. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R. Role of Enzymes in Seed Germination. Int. J. Creat. Res. Dhaka Div. Bangladesh 2018, 6, 1481–1485. [Google Scholar]
- Shu, X.-L.; Frank, T.; Shu, Q.; Engel, K. Metabolite profiling of germinating rice seeds. J. Agric. Food Chem. 2008, 56, 11612–11620. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, C.; Zhai, L.; Yu, W.; Chang, H.; Kou, X.; Zhou, F. Bioactive compounds and antioxidant activity of mung bean (Vigna radiata L.), soybean (Glycine max L.) and black bean (Phaseolus vulgaris L.) during the germination process. Czech J. Food Sci. 2016, 34, 68–78. [Google Scholar] [CrossRef]
- Lewis, M.J.; Young, T.W.; Lewis, M.J.; Young, T.W. Malting biochemistry. In Brewing; Springer: Boston, MA, USA, 2001; pp. 191–204. [Google Scholar]
- Woffenden, H.M.; Ames, J.M.; Chandra, S.; Anese, M.; Nicoli, M.C. Effect of Kilning on the Antioxidant and Pro-oxidant Activities of Pale Malts. J. Agric. Food Chem. 2002, 50, 4925–4933. [Google Scholar] [CrossRef]
- Woffenden, H.M.; Ames, J.M.; Chandra, S. Antioxidant activity, colour and flavour of crystal malt extracts. Int. Congr. Ser. 2002, 1245, 483–484. [Google Scholar] [CrossRef]
- Gorzolka, K.; Lissel, M.; Kessler, N.; Loch-Ahring, S.; Niehaus, K. Metabolite fingerprinting of barley whole seeds, endosperms, and embryos during industrial malting. J. Biotechnol. 2012, 159, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, M.M.; Zarnkow, M.; Becker, T. Effect of Drying Temperature and Time on Alpha-Amylase, Beta-Amylase, Limit Dextrinase Activities and Dimethyl Sulphide Level of Teff (Eragrostis tef) Malt. Food Bioprocess Technol. 2013, 6, 3462–3472. [Google Scholar] [CrossRef]
- López-Cortez, M.d.S.; Rosales-Martínez, P.; Arellano-Cárdenas, S.; Cornejo-Mazón, M. Antioxidants Properties and Effect of Processing Methods on Bioactive Compounds of Legumes. In Grain Legumes; InTech: London, UK, 2016. [Google Scholar]
- Van Hung, P.; Yen, N.T.H.; Phi, N.T.L.; Tien, N.P.H.; Trung, N.T.T. Nutritional composition, enzyme activities and bioactive compounds of mung bean (Vigna radiata L.) germinated under dark and light conditions. LWT 2020, 133, 110100. [Google Scholar] [CrossRef]
- Chavan, J.K.; Kadam, S.S.; Beuchat, L.R. Nutritional improvement of cereals by sprouting. Crit. Rev. Food Sci. Nutr. 1989, 28, 401–437. [Google Scholar] [CrossRef] [PubMed]
- Gharachorloo, M.; Ghiassi Tarzi, B.; Baharinia, M. The Effect of Germination on Phenolic Compounds and Antioxidant Activity of Pulses. J. Am. Oil Chem. Soc. 2013, 90, 407–411. [Google Scholar] [CrossRef]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.C.C.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Cash, K.J. Malting in the Lab and at Home: The Forgotten Step on the Path to Beer. J. Chem. Educ. 2021, 98, 1410–1414. [Google Scholar] [CrossRef]
- Mamiro, P.R.S.; Van Camp, J.; Mwikya, S.M.; Huyghebaert, A. In vitro extractability of calcium, iron, and zinc in finger millet and kidney beans during processing. J. Food Sci. 2001, 66, 1271–1275. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Rozan, P.; Lambein, F.; Frias, J.; Vidal-Valverde, C. Effects of different germination conditions on the contents of free protein and non-protein amino acids of commercial legumes. Food Chem. 2004, 86, 537–545. [Google Scholar] [CrossRef]
- Márton, M.; Mándoki, Z.; Csapó-Kiss, Z.; Csapó, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae Aliment. 2010, 3, 81–117. [Google Scholar]
- Xu, M.; Jin, Z.; Simsek, S.; Hall, C.; Rao, J.; Chen, B. Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chem. 2019, 295, 579–587. [Google Scholar] [CrossRef]
- EL-Suhaibani, M.; Ahmed, M.A.; Osman, M.A. Study of germination, soaking and cooking effects on the nutritional quality of goat pea (Securigera securidaca L.). J. King Saud Univ.-Sci. 2020, 32, 2029–2033. [Google Scholar] [CrossRef]
- Medhe, S.; Jain, S.; Anal, A.K. Effects of sprouting and cooking processes on physicochemical and functional properties of moth bean (Vigna aconitifolia) seed and flour. J. Food Sci. Technol. 2019, 56, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Sadiq, M.B.; Anal, A.K. Comparative study of physicochemical and functional properties of soaked, germinated and pressure cooked Faba bean. J. Food Sci. Technol. 2021, 59, 257–267. [Google Scholar] [CrossRef]
- Ispiryan, L.; Kuktaite, R.; Zannini, E.; Arendt, E.K. Fundamental study on changes in the FODMAP profile of cereals, pseudo-cereals, and pulses during the malting process. Food Chem. 2021, 343, 128549. [Google Scholar] [CrossRef]
- Mbithi, S.; Van Camp, J.; Rodriguez, R.; Huyghebaert, A. Effects of sprouting on nutrient and antinutrient composition of kidney beans (Phaseolus vulgaris var. Rose coco). Eur. Food Res. Technol. 2001, 212, 188–191. [Google Scholar] [CrossRef]
- Van Hung, P.; Trinh, L.N.D.; Thuy, N.T.X.; Morita, N. Changes in nutritional composition, enzyme activities and bioactive compounds of germinated buckwheat (Fagopyrum esculantum M.) under unchanged air and humidity conditions. Int. J. Food Sci. Technol. 2021, 56, 3209–3217. [Google Scholar] [CrossRef]
- Chavan, J.K.; Kadam, S.S.; Salunkhe, D.K. Changes in Tannin, Free Amino Acids, Reducing Sugars, and Starch during Seed Germination of Low and High Tannin Cultivars of Sorghum. J. Food Sci. 1981, 46, 638–639. [Google Scholar] [CrossRef]
- Winarsi, H.; Septiana, A.T.; Wulandari, S.P. Germination improves sensory, phenolic, protein content and anti-inflammatory properties of red kidney bean (Phaseolus vulgaris L.) sprouts milk. Food Res. 2020, 4, 1921–1928. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, H.; Xie, Y.; Yang, M.; Tang, J.; Wang, P.; Yang, R.; Gu, Z. Response of nutritional and functional composition, anti-nutritional factors and antioxidant activity in germinated soybean under UV-B radiation. LWT 2020, 118, 108709. [Google Scholar] [CrossRef]
- Singh, G. The Soybean: Botany, Production and Uses; CABI: Oxfordshire, UK, 2010. [Google Scholar]
- Kayembe, N.C.; van Rensburg, C.J. Germination as a processing technique for soybeans in small-scale farming. S. Afr. J. Anim. Sci. 2013, 43, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Ghani, M.; Kulkarni, K.P.; Song, J.T.; Shannon, J.G.; Lee, J.-D. Soybean Sprouts: A Review of Nutrient Composition, Health Benefits and Genetic Variation. Plant Breed. Biotechnol. 2016, 4, 398–412. [Google Scholar] [CrossRef] [Green Version]
- Hart, C. The Economic Evolution of the Soybean Industry. In The Soybean Genome; Springer Nature: Cham, Switzerland, 2017; pp. 1–9. [Google Scholar]
- Kaczmarska, K.T.; Chandra-Hioe, M.V.; Frank, D.; Arcot, J. Aroma characteristics of lupin and soybean after germination and effect of fermentation on lupin aroma. LWT-Food Sci. Technol. 2018, 87, 225–233. [Google Scholar] [CrossRef]
- Warle, B.; Riar, C.; Gaikwad, S.; Mane, V. Effect of Germination on Nutritional Quality of Soybean (Glycine max). IOSR J. Environ. Sci. Ver. II 2015, 9, 2319–2399. [Google Scholar] [CrossRef]
- Zieliński, H. Contribution of low molecular weight antioxidants to the antioxidant screen of germinated soybean seeds. Plant Foods Hum. Nutr. 2003, 58, 1–20. [Google Scholar] [CrossRef]
- Huang, X.; Cai, W.; Xu, B. Kinetic changes of nutrients and antioxidant capacities of germinated soybean (Glycine max L.) and mung bean (Vigna radiata L.) with germination time. Food Chem. 2014, 143, 268–276. [Google Scholar] [CrossRef]
- Xu, J.-G.; Hu, Q.-P. Changes in γ-aminobutyric acid content and related enzyme activities in Jindou 25 soybean (Glycine max L.) seeds during germination. LWT-Food Sci. Technol. 2014, 55, 341–346. [Google Scholar] [CrossRef]
- Mamilla, R.K.; Mishra, V.K. Effect of germination on antioxidant and ACE inhibitory activities of legumes. LWT 2017, 75, 51–58. [Google Scholar] [CrossRef]
- Chua, J.-Y.; Koh, M.K.P.; Liu, S.-Q. Gamma-aminobutyric acid. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; Volume 67, pp. 25–54. ISBN 9780128115251. [Google Scholar]
- Gan, R.-Y.; Chan, C.-L.; Yang, Q.-Q.; Li, H.-B.; Zhang, D.; Ge, Y.-Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 191–246. ISBN 9780128115251. [Google Scholar]
- Wu, F.; Xu, X. Sprouted grains-based fermented products. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–173. ISBN 9780128115251. [Google Scholar]
- Murugkar, D.A. Effect of sprouting of soybean on the chemical composition and quality of soymilk and tofu. J. Food Sci. Technol. 2014, 51, 915–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziki, D.; Gawlik-Dziki, U. Processing of germinated grains. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–90. ISBN 9780128115251. [Google Scholar]
- Jiang, S.; Cai, W.; Xu, B. Food quality improvement of soy milk made from short-time germinated soybeans. Foods 2013, 2, 198–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atiku, A.A.; Aviara, N.A.; Haque, M.A. Performance Evaluation of a Bambara Ground Nut Sheller. Agric. Eng. Int. CIGR J. 2004, VI, 1–18. [Google Scholar]
- Murevanhema, Y.Y.; Jideani, V.A. Potential of Bambara Groundnut (Vigna subterranea (L.) Verdc) Milk as a Probiotic Beverage—A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Jideani, V.A. Utilizing bambara groundnut in Value-Added products. Food Technol. 2016, 70, 48–52. [Google Scholar]
- Cleasby, P.; Massawe, F.J.; Symonds, R.S. Bambara Groundnut for Food Security in the Changing African Climate. Sustain. Agric. Rev. 2016, 19, 363–389. [Google Scholar]
- Mayes, S.; Ho, W.K.; Chai, H.H.; Gao, X.; Kundy, A.C.; Mateva, K.I.; Zahrulakmal, M.; Hahiree, M.K.I.M.; Kendabie, P.; Licea, L.C.S.; et al. Bambara groundnut: An exemplar underutilised legume for resilience under climate change. Planta 2019, 250, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Mabhaudhi, T.; Chibarabada, T.P.; Chimonyo, V.G.P.; Modi, A.T. Modelling climate change impact: A case of Bambara groundnut (Vigna subterranea). Phys. Chem. Earth 2018, 105, 25–31. [Google Scholar] [CrossRef]
- Feldman, A.; Ho, W.K.; Massawe, F.; Mayes, S. Bambara groundnut is a Climate-Resilient Crop: How Could a Drought-Tolerant and Nutritious Legume Improve Community Resilience in the Face of Climate Change? In Sustainable Solutions for Food Security; Sarkar, A., Sensarma, S.R., VanLoon, G.W., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 151–167. ISBN 9783319778785. [Google Scholar]
- Muhammad, I.; Rafii, M.Y.; Ramlee, S.I.; Nazli, M.H.; Harun, A.R.; Oladosu, Y.; Musa, I.; Arolu, F.; Chukwu, S.C.; Haliru, B.S.; et al. Exploration of Bambara Groundnut (Vigna subterranea (L.) Verdc.), an Underutilized Crop, to Aid Global Food Security: Varietal Improvement, Genetic Diversity and Processing. Agronomy 2020, 10, 766. [Google Scholar] [CrossRef]
- Heller, J.; Begemann, F.; Mushonga, J. Bambara Groundnut, Vigna subterranea (l.) Verdc. Promoting the Conservation and Use of Underutilized and Neglected Crops; IPGRI: Rome, Italy, 1997. [Google Scholar]
- Alobo, A.P. Production and organoleptic assessment of akara from Bambara groundnut (Voandzeia subterranea (L.) Thouars). Plant Foods Hum. Nutr. 1999, 53, 313–320. [Google Scholar] [CrossRef]
- Falade, K.O.; Nwajei, C.P. Physical, proximate, functional and pasting properties of four non- and γ-irradiated Bambara groundnut (Vigna subterranean) cultivars. Int. J. Food Sci. Technol. 2015, 50, 640–651. [Google Scholar] [CrossRef]
- Hardy, Z.; Jideani, A. Functional and Nutritional Characteristics of Bambara Groundnut Milk Powder as an Ingredient in Yoghurt. Master’s Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2016. [Google Scholar]
- Pahane, M.M.; Tatsadjieu, L.N.; Bernard, C.; Njintang, N.Y. Production, nutritional and biological value of bambara groundnut (Vigna subterranea) milk and yoghurt. J. Food Meas. Charact. 2017, 11, 1613–1622. [Google Scholar] [CrossRef]
- Uvere, P.O.; Uwaegbute, A.C.; Adedeji, E.M. Effects of malting on the milling performance and acceptability of Bambara groundnut (Voandzeia subterranea Thouars) seeds and products. Plant Foods Hum. Nutr. 1999, 54, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Akpapunam, M.A.; Igbedioh, S.O.; Aremo, I. Effect of malting time on chemical composition and functional properties of soybean and Bambara groundnut flours. Int. J. Food Sci. Nutr. 1996, 47, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Nyau, V.; Prakash, S.; Rodrigues, J.; Farrant, J. Profiling of phenolic compounds in sprouted common beans and Bambara groundnuts. J. Food Res. 2017, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Chinma, C.E.; Abu, J.O.; Asikwe, B.N.; Sunday, T.; Adebo, O.A. Effect of germination on the physicochemical, nutritional, functional, thermal properties and in vitro digestibility of Bambara groundnut flours. LWT 2021, 140, 110749. [Google Scholar] [CrossRef]
- Oyedeji, A.B.; Chinma, C.E.; Green, E.; Adebo, O.A. Metabolite data of germinated Bambara groundnut flour and starch extracted with two different solvents. Data Br. 2021, 38, 107288. [Google Scholar] [CrossRef]
- Acquah, C.; Ohemeng-Boahen, G.; Power, K.A.; Tosh, S.M. The Effect of Processing on Bioactive Compounds and Nutritional Qualities of Pulses in Meeting the Sustainable Development Goal 2. Front. Sustain. Food Syst. 2021, 5, 166. [Google Scholar] [CrossRef]
- Obizoba, I.C.; Egbuna, H.I. Effect of germination and fermentation on the nutritional quality of Bambara nut (Voandzeia subterranea L. Thouars) and its product (milk). Plant Foods Hum. Nutr. 1992, 42, 13–23. [Google Scholar] [CrossRef]
- Jom, K.N.; Frank, T.; Engel, K.-H. A metabolite profiling approach to follow the sprouting process of mung beans (Vigna radiata). Metabolomics 2011, 7, 102–117. [Google Scholar] [CrossRef]
- Usansa, U.; Sompong, N.; Wanapu, C.; Boonkerd, N.; Teaumroong, N. The influences of steeping duration and temperature on the α- and β-amylase activities of six thai rice malt cultivars (Oryza sativa l. Indica). J. Inst. Brew. 2009, 115, 140–147. [Google Scholar] [CrossRef]
- Alsalman, F.B.; Ramaswamy, H. Reduction in soaking time and anti-nutritional factors by high pressure processing of chickpeas. J. Food Sci. Technol. 2020, 57, 2572–2585. [Google Scholar] [CrossRef]
- Atudorei, D.; Stroe, S.-G.; Codină, G.G. Impact of Germination on the Microstructural and Physicochemical Properties of Different Legume Types. Plants 2021, 10, 592. [Google Scholar] [CrossRef]
- Sofi, S.A.; Singh, J.; Chhikara, N.; Panghal, A.; Gat, Y. Quality characterization of gluten free noodles enriched with chickpea protein isolate. Food Biosci. 2020, 36, 100626. [Google Scholar] [CrossRef]
- Panghal, A.; Kaur, R.; Janghu, S.; Sharma, P.; Sharma, P.; Chhikara, N. Nutritional, phytochemical, functional and sensorial attributes of Syzygium cumini L. pulp incorporated pasta. Food Chem. 2019, 289, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Gaithersburg, M. (Ed.) AOAC 990.03-2002, Protein (Crude) in Animal Feed. Combustion Met. In Office Methods of Analysis of AOAC International Method; Association of Official Analytical Chemists: Washington, DC, USA, 2000; p. 990. [Google Scholar]
- Awobusuyi, T.D.; Siwela, M. Nutritional properties and consumer’s acceptance of provitamin a-biofortified amahewu combined with Bambara (Vigna subterranea) flour. Nutrients 2019, 11, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montanuci, F.D.; Ribani, M.; de Matos Jorge, L.M.; Matos Jorge, R.M. Effect of steeping time and temperature on malting process. J. Food Process Eng. 2017, 40, e12519. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Effect of soaking, boiling, and steaming on total phenolic contentand antioxidant activities of cool season food legumes. Food Chem. 2008, 110, 1–13. [Google Scholar] [CrossRef]
- Nemzer, B.; Lin, Y.; Huang, D. Antioxidants in sprouts of grains. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–68. [Google Scholar]
- Rico, D.; Peñas, E.; del Carmen García, M.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted barley flour as a nutritious and functional ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef] [Green Version]
- Mubaiwa, J.; Fogliano, V.; Chidewe, C.; Linnemann, A.R. Hard-to-cook phenomenon in Bambara groundnut (Vigna subterranea (L.) Verdc.) processing: Options to improve its role in providing food security. Food Rev. Int. 2017, 33, 167–194. [Google Scholar] [CrossRef] [Green Version]
- Mandizvo, T.; Odindo, A.O. Seed coat structural and imbibitional characteristics of dark and light coloured Bambara groundnut (Vigna subterranea L.) landraces. Heliyon 2019, 5, 1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, B.G.; Hughes, J.S.; Rasmussen, H.P. Seed Microstructure: Review of Water Imbibition in Legumes. Food Struct. 1985, 4, 115–124. [Google Scholar]
- Miano, A.C.; Augusto, P.E.D. The Hydration of Grains: A Critical Review from Description of Phenomena to Process Improvements. Compr. Rev. Food Sci. Food Saf. 2018, 17, 352–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onwurafor, E.U.; Uzodinma, E.O.; Uchegbu, N.N.; Ani, J.C.; Umunnakwe, I.L.; Ziegler, G. Effect of malting periods on the nutrient composition, antinutrient content and pasting properties of mungbean flour. Agro-Science 2020, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Owusu-Mensah, E.; Oduro, I.; Sarfo, K.J. Steeping: A way of improving the malting of rice grain. J. Food Biochem. 2011, 35, 80–91. [Google Scholar] [CrossRef]
- Tajoddin, M.; Manohar, S.; Lalitha, J. Effect of Soaking and Germination on Polyphenol Content and Polyphenol Oxidase Activity of Mung Bean (Phaseolus aureus L.) Cultivars Differing in Seed Color. Int. J. Food Prop. 2014, 17, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Mun, J.-H.; Kim, I.-D.; Dhungana, S.K.; Park, Y.-S.; Kim, J.-H.; Shin, D.-H. Yield and quality characteristics of Korean red bean sprouts produced with different time of seed soaking. Food Sci. Biotechnol. 2020, 29, 197–206. [Google Scholar] [CrossRef]
- Wijngaard, H.H.H.; Ulmer, H.M.M.; Neumann, M.; Arendt, E.K.K. The Effect of Steeping Time on the Final Malt Quality of Buckwheat. J. Inst. Brew. 2005, 111, 275–281. [Google Scholar] [CrossRef]
- Chandraprabha, S.; Sharon, C.L. Optimisation of conditions for barnyard millet germination. Plant Arch. 2021, 21, 1676–1680. [Google Scholar] [CrossRef]
- Neylon, E.; Arendt, E.K.; Lynch, K.M.; Zannini, E.; Bazzoli, P.; Monin, T.; Sahin, A.W. Rootlets, a Malting By-Product with Great Potential. Fermentation 2020, 6, 117. [Google Scholar] [CrossRef]
- Mosher, M.; Trantham, K. Malting and Water. In Brewing Science: A Multidisciplinary Approach; Springer Nature: Cham, Switzerland, 2021; pp. 133–166. [Google Scholar] [CrossRef]
- Xu, M.; Rao, J.; Chen, B. Phenolic compounds in germinated cereal and pulse seeds: Classification, transformation, and metabolic process. Crit. Rev. Food Sci. Nutr. 2020, 60, 740–759. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, I.L.; Tayade, P.T. Development of appropriate process for sprouting of soybeans. J. Food Res. Technol. 2013, 1, 36–44. [Google Scholar]
- Hudson, O.P. Malting Technology. J. Inst. Brew. 1986, 92, 115–122. [Google Scholar] [CrossRef]
- Guerrero, B.G. Effects of Amino Acid Profile, Endoprotease Activities and Wort Quality on Fermentability under Different Malting and Brewing Conditions. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2009. [Google Scholar]
- Islam, M.; Hassan, M.; Sarker, S.; Rahman, A.; Fakir, M. Light and temperature effects on sprout yield and its proximate composition and vitamin C content in Lignosus and Mung beans. J. Bangladesh Agric. Univ. 2017, 15, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, R.; Yadav, R.B.; Yadav, B.S.; Yadav, R. Quality characteristics of pearl millet malt as affected by steeping temperature and germination period. Qual. Assur. Saf. Crop. Foods 2018, 10, 41–50. [Google Scholar] [CrossRef]
- Devi, C.B.; Kushwaha, A.; Kumar, A. Sprouting characteristics and associated changes in nutritional composition of cowpea (Vigna unguiculata). J. Food Sci. Technol. 2015, 52, 6821–6827. [Google Scholar] [CrossRef]
- Murugkar, D.; Jha, K. Effect of sprouting on nutritional and functional characteristics of soybean. J. Food Sci. Technol. 2009, 46, 240–243. [Google Scholar]
- Guido, L.; Moreira, M. Malting. In Engineering Aspects of Cereal and Cereal-Based Products; Guine, R.D.P.F., dos Reis Correia, P., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 51–70. [Google Scholar]
- Ikram, A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Tufail, T.; Hussain, M.; Anjum, F.M. Nutritional and end-use perspectives of sprouted grains: A comprehensive review. Food Sci. Nutr. 2021, 9, 4617–4628. [Google Scholar] [CrossRef]
- Islam, M.Z.; Shim, M.; Jeong, S.; Lee, Y. Effects of soaking and sprouting on bioactive compounds of black and red pigmented rice cultivars. Int. J. Food Sci. Technol. 2022, 57, 201–209. [Google Scholar] [CrossRef]
- Liu, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity Inhibits Rice Seed Germination by Reducing α-Amylase Activity via Decreased Bioactive Gibberellin Content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef]
- Bayram, M.; Kaya, A.; Öner, M.D. Water absorption, leaching and color changes during the soaking for production of soy-bulgur. J. Food Process Eng. 2004, 27, 119–141. [Google Scholar] [CrossRef]
- Wood, J.A.; Knights, E.J.; Campbell, G.M.; Harden, S.; Choct, M. Enzyme pre-milling treatments improved milling performance of chickpeas by targeting mechanisms of seed coat and cotyledon adhesion with various effects on dhal quality. J. Sci. Food Agric. 2022, 102, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Odunmbaku, L.A.; Sobowale, S.S.; Adenekan, M.K.; Oloyede, T.; Adebiyi, J.A.; Adebo, O.A. Influence of steeping duration, drying temperature, and duration on the chemical composition of sorghum starch. Food Sci. Nutr. 2018, 6, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.A.; Nahla, A.; Al-Askalany, S.A.; Thabet, H.A. Effect of domestic processing methods of some legumes on phytochemicals content and bioavailability of some minerals. J. Am. Sci. 2014, 10, 276–288. [Google Scholar]
- Osuji, C.M.; Ofoedu, C.E.; Omeire, G.C.; Ojukwu, M. Colour analysis of syrup from malted and unmalted rice of different varieties. Croat. J. food Sci. Technol. 2020, 12, 130–138. [Google Scholar] [CrossRef]
- Dugulin, C.A.; De Rouck, G.; Cook, D.J. Green Malt for a Green Future–Feasibility and Challenges of Brewing Using Freshly Germinated (Unkilned) Malt: A Review. J. Am. Soc. Brew. Chem. 2021, 79, 315–332. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Kortei, N.K.; Akonor, P.T. Correlation between hue-angle and colour lightness of gamma irradiated mushrooms. Ann. Food Sci. Technol. 2015, 16, 98–103. [Google Scholar]
- Turner, H.M.; Elmore, L.; Walling, J.; Lachowiec, J.; Mangel, D.; Fischer, A.; Sherman, J. Effect of steeping regime on barley malt quality and its impacts on breeding program selection. J. Am. Soc. Brew. Chem. 2019, 77, 267–281. [Google Scholar] [CrossRef]
- Ramashia, S.E.; Gwata, E.T.; Meddows-Taylor, S.; Anyasi, T.A.; Jideani, A.I.O. Some physical and functional properties of finger millet (Eleusine coracana) obtained in sub-Saharan Africa. Food Res. Int. 2018, 104, 110–118. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Gonçalves, L.M.; Guido, L.F. Overall Antioxidant Properties of Malt and How They Are Influenced by the Individual Constituents of Barley and the Malting Process. Compr. Rev. Food Sci. Food Saf. 2016, 15, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Kun, S.; Hegyesné Vecseri, B.; Kun-Farkas, G. Study of antioxidant activity during the malting and brewing process. J. Food Sci. Technol. 2019, 56, 3801–3809. [Google Scholar] [CrossRef] [Green Version]
- Bettenhausen, H.M.; Benson, A.; Fisk, S.; Herb, D.; Hernandez, J.; Lim, J.; Queisser, S.H.; Shellhammer, T.H.; Vega, V.; Yao, L.; et al. Variation in Sensory Attributes and Volatile Compounds in Beers Brewed from Genetically Distinct Malts: An Integrated Sensory and Non-Targeted Metabolomics Approach. J. Am. Soc. Brew. Chem. 2020, 78, 136–152. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Rafiee, S.; Emam-Djomeh, Z.; Keyhani, A. Kinetic Models for Colour Changes in Kiwifruit Slices During Hot Air Drying. World J. Agric. Sci. 2008, 4, 376–383. [Google Scholar]
- Bhol, S.; Sowriappan, J.D.B. Influence of malted finger millet and red kidney bean flour on quality characteristics of developed bread. LWT-Food Sci. Technol. 2014, 55, 294–300. [Google Scholar] [CrossRef]
- Jha, S.N. Colour Measurements and Modeling. In Nondestructive Evaluation of Food Quality; Jha, S.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–40. ISBN 978-3-642-15795-0. [Google Scholar]
- Chung, H.-J.; Cho, D.-W.; Park, J.-D.; Kweon, D.-K.; Lim, S.-T. In vitro starch digestibility and pasting properties of germinated brown rice after hydrothermal treatments. J. Cereal Sci. 2012, 56, 451–456. [Google Scholar] [CrossRef]
- Setia, R. Impacts of Germination on the Physicochemical Properties, Nutritional Quality and Bread Making Performance of Yellow Pea and Faba Bean Flours. Ph.D. Thesis, University of Saskatchewan, Saskatoon, Canada, 2019. [Google Scholar]
- Liu, Y.; Xu, M.; Wu, H.; Jing, L.; Gong, B.; Gou, M.; Zhao, K.; Li, W. The compositional, physicochemical and functional properties of germinated mung bean flour and its addition on quality of wheat flour noodle. J. Food Sci. Technol. 2018, 55, 5142–5152. [Google Scholar] [CrossRef]
- Asuk, A.A.; Ugwu, M.N.; Idole, B. The Effect of Different Malting Periods on the Nutritional Composition of Malted Sorghum-Soy Composite Flour. J. Food Sci. Nutr. Res. 2020, 3, 217–230. [Google Scholar] [CrossRef]
- Gqaleni, P. Nutritional Value of Bambara Groundnut (Vigna subterranea (L.) Verdc.): A Human and Animal Perspective. Ph.D. Thesis, University of KwaZulu, Natal Pietermaritzburg, South Africa, 2014. [Google Scholar]
- Harris, T.; Jideani, V.; Le Roes-Hill, M. Flavonoids and tannin composition of Bambara groundnut (Vigna subterranea) of Mpumalanga, South Africa. Heliyon 2018, 4, e00833. [Google Scholar] [CrossRef] [Green Version]
- Olamiti, G.; Takalani, T.K.; Beswa, D.; Jideani, A.I.O. Effect of malting and fermentation on colour, thermal properties, functional groups and crystallinity level of flours from pearl millet (Pennisetum glaucum) and sorghum (Sorghum bicolor). Heliyon 2020, 6, e05467. [Google Scholar] [CrossRef]
- Melgosa, M.; Huertas, R.; Yebra, A.; Pérez, M.M. Are chroma tolerances dependent on hue-angle? Color Res. Appl. 2004, 29, 420–427. [Google Scholar] [CrossRef]
- Simons, C. Color Determination in Food. Available online: https://cwsimons.com/color-determination-in-food/ (accessed on 16 March 2021).
- Parr, H.; Bolat, I.; Cook, D. Modelling flavour formation in roasted malt substrates under controlled conditions of time and temperature. Food Chem. 2021, 337, 127641. [Google Scholar] [CrossRef] [PubMed]
- Döehler. Malt extract—Natural, Intensive Brown Tones for Innovative Products. Available online: www.doehler.com (accessed on 27 May 2021).
- Döhler. Healthy, Sustainable and Natural: These are the Trends for the Food and Beverage Industry in 2021. Available online: www.doehler.com (accessed on 27 May 2021).
- Bamforth, C.W. Brewing Materials and Processes; Bamforth, C.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780127999548. [Google Scholar]
- Ranaweera, S.; Ampemohotti, T.; Aranchchige, U. Advantages and considerations for the applications of natural food pigments in the food industry. J. Res. Technol. Eng. 2020, 1, 8–15. [Google Scholar]
- Obadina, A.O.; Oyewole, O.B.; Awojobi, T.M. Effect of steeping time of milled grains on the quality of Kunnu-Zaki (A Nigerian beverage). Afr. J. Food Sci. 2008, 2, 033–036. [Google Scholar]
- South, J.B. Changes in organic acid levels during malting. J. Inst. Brew. 1996, 102, 161–166. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Bubolz, V.K.; da Silva, J.; Dittgen, C.L.; Ziegler, V.; de Oliveira Raphaelli, C.; de Oliveira, M. Changes in the chemical composition and bioactive compounds of chickpea (Cicer arietinum L.) fortified by germination. LWT 2019, 111, 363–369. [Google Scholar] [CrossRef]
- Nefale, F.E.; Mashau, M.E. Effect of Germination Period on the Physicochemical, Functional and Sensory Properties of Finger Millet Flour and Porridge. Asian J. Appl. Sci. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Adedeji, O.E.; Oyinloye, O.D.; Ocheme, O.B. Effects of germination time on the functional properties of maize flour and the degree of gelatinization of its cookies. African J. Food Sci. 2014, 8, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Handa, V.; Kumar, V.; Panghal, A.; Suri, S.; Kaur, J. Effect of soaking and germination on physicochemical and functional attributes of horsegram flour. J. Food Sci. Technol. 2017, 54, 4229–4239. [Google Scholar] [CrossRef]
- Okoth, J.K.; Ochola, S.; Gikonyo, N.K.; Makokha, A. Optimization of the Period of Steeping and Germination for Amaranth Grain. J. Agric. Food. Tech 2011, 6, 101–105. [Google Scholar]
- Widjajaseputra, A.I.; Widyastuti, T.E.W.; Trisnawati, C.Y. Potency of mung bean with different soaking times as protein source for breastfeeding women in Indonesia. Food Res. 2019, 3, 501–505. [Google Scholar] [CrossRef]
- Asouzu, A.I.; Umerah, N.N. Effects of Malting on Nutritional Characteristics of Pigeon Pea (Cajanus cajan). Asian J. Biochem. Genet. Mol. Biol. 2020, 3, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Dattatray, T.R.; Surendra Babu, A.; Jagan Mohan, R. Effect of pre-treatments on sprouting rate and nutritional quality of green gram (Vigna radiata L.) malt. J. Food Process. Preserv. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Urbano, G.; Aranda, P.; Vílchez, A.; Aranda, C.; Cabrera, L.; Porres, J.M.; López-Jurado, M.; G Urbano, P.A.A.V. Effects of germination on the composition and nutritive value of proteins in Pisum sativum L. Food Chem. 2005, 93, 671–679. [Google Scholar] [CrossRef]
- Shah, S.A.; Zeb, A.; Masood, T.; Noreen, N.; Abbas, S.J.; Samiullah, M.; Alim, A.; Muhammad, A. Effects of sprouting time on biochemical and nutritional qualities of Mungbean varieties. Afr. J. Agric. Res. 2011, 6, 5091–5098. [Google Scholar] [CrossRef]
- Bau, H.-M.; Villaume, C.; Nicolas, J.; Mejean, L. Effect of germination on chemical composition, biochemical constituents and antinutritional factors of soya bean (Glycine max) seeds. J. Sci. Food Agric. 1997, 73, 1–9. [Google Scholar] [CrossRef]
- Gulewicz, P.; Martínez-Villaluenga, C.; Frias, J.; Ciesiołka, D.; Gulewicz, K.; Vidal-Valverde, C. Effect of germination on the protein fraction composition of different lupin seeds. Food Chem. 2008, 107, 830–844. [Google Scholar] [CrossRef]
- Dipnaik, K.; Bathere, D. Effect of soaking and sprouting on protein content and transaminase activity in pulses. Int. J. Res. Med. Sci. Dipnaik K al. Int J Res Med Sci 2017, 5, 4271–4276. [Google Scholar] [CrossRef] [Green Version]
- Onwuka, U.N.; Ezemba, C.O. Chemical and functional properties of flour from sprouted and un-sprouted legumes (African yam bean, Bambara). J. Agric. Eng. Technol. 2013, 21, 43–51. [Google Scholar]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Trugo, L.C.; Muzquiz, M.; Pedrosa, M.M.; Ayet, G.; Burbano, C.; Cuadrado, C.; Cavieres, E. Influence of malting on selected components of soya bean, black bean, chickpea and barley. Food Chem. 1999, 65, 85–90. [Google Scholar] [CrossRef]
- Aguilera, Y.; Liébana, R.; Herrera, T.; Rebollo-Hernanz, M.; Sanchez-Puelles, C.; Benítez, V.; Martín-Cabrejas, M.A. Effect of illumination on the content of melatonin, phenolic compounds, and antioxidant activity during germination of lentils (Lens culinaris L.) and kidney beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2014, 62, 10736–10743. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.-A.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghavidel, R.A.; Prakash, J.; Davoodi, M.G. Asessment of enzymatic changes in some legume seeds during germination. Agro FOOD Ind Hi Tech 2011, 22, 45–47. [Google Scholar]
- Tuan, P.A.; Sun, M.; Nguyen, T.-N.; Park, S.; Ayele, B.T. Molecular mechanisms of seed germination. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–24. ISBN 9780128115251. [Google Scholar]
- Chiu, K.-Y. Changes in Microstructure, Germination, Sprout Growth, Phytochemical and Microbial Quality of Ultrasonication Treated Adzuki Bean Seeds. Agronomy 2021, 11, 1093. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X. Antagonistic regulation of ABA and GA in metabolism and signaling pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Yousif, A.M.; Evans, D.E. Changes in malt quality during production in two commercial malt houses. J. Inst. Brew. 2020, 126, 233–252. [Google Scholar] [CrossRef]
- Ali, A.S.; Elozeiri, A.A. Metabolic Processes during Seed Germination. In Advances in Seed Biology; InTech: London, UK, 2017; ISBN 978-953-51-3622-4. [Google Scholar]
- Patricia, B.; Phiarais, N.; Wijngaard, H.H.; Arendt, E.K.; Brew, J.I. The Impact of Kilning on Enzymatic Activity of Buckwheat Malt. J. Inst. Brew. 2005, 111, 290–298. [Google Scholar]
- Akillioglu, H.G.; Karakaya, S. Changes in total phenols, total flavonoids, and antioxidant activities of common beans and pinto beans after soaking, cooking, and in vitro digestion process. Food Sci. Biotechnol. 2010, 19, 633–639. [Google Scholar] [CrossRef]
- López-Amorós, M.L.; Hernández, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Duodu, K.G. Effects of processing on phenolic phytochemicals in cereals and legumes. Cereal Foods World 2014, 59, 64–70. [Google Scholar] [CrossRef]
- Mazahib, A.M.; Nuha, M.O.; Salawa, I.S.; Babiker, E.E. Some nutritional attributes of Bambara groundnut as influenced by domestic processing. Int. Food Res. J. 2013, 20, 1165–1171. [Google Scholar]
- Eshraq, B.; Mona, A.; Sayed, A.; Emam, A. Effect of Soaking, Cooking and Germination on Chemical Constituents and Bioactive Compounds as well as their Cytotoxic Activities of Black Bean Extracts. Nat. Prod. Chem Res. 2016, 4, 237. [Google Scholar] [CrossRef] [Green Version]
- Adebiyi, J.A.; Njobeh, P.B.; Kayitesi, E. Assessment of nutritional and phytochemical quality of Dawadawa (an African fermented condiment) produced from Bambara groundnut (Vigna subterranea). Microchem. J. 2019, 149, 104034. [Google Scholar] [CrossRef]
- Mba, O.I.; Kwofie, E.M.; Ngadi, M. Kinetic modelling of polyphenol degradation during common beans soaking and cooking. Heliyon 2019, 5, e01613. [Google Scholar] [CrossRef] [Green Version]
- Barimalaa, I.S.; Anoghalu, S.C. Effect of Processing on Certain Antinutrients in Bambara Groundnut (Vigna subterranea) Cotyledons. J. Sci Food Agric. 1997, 73, 186–188. [Google Scholar] [CrossRef]
- Naveena, N.; Bhaskarachary, K. Effects of soaking and germination of total and individual polyphenols content in the commonly consumed millets and legumes in India. Int. J. Food Nutr. Sci. 2013, 2, 12–19. [Google Scholar]
- Khang, D.; Dung, T.; Elzaawely, A.; Xuan, T. Phenolic Profiles and Antioxidant Activity of Germinated Legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Effect of hydrothermal processing on changes of insoluble-bound phenolics of lentils. J. Funct. Foods 2017, 38, 716–722. [Google Scholar] [CrossRef]
- Dueñas, M.; Hernández, T.; Estrella, I.; Fernández, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Liu, Y.; Su, C.; Saleh, A.S.M.; Wu, H.; Zhao, K.; Zhang, G.; Jiang, H.; Yan, W.; Li, W. Effect of germination duration on structural and physicochemical properties of mung bean starch. Int. J. Biol. Macromol. 2020, 154, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.M.; Hassan, A.A.; Mansour, E.H.; Fahmy, H.A.; El-Bedawey, A.E.F.A. Melatonin, phenolics content and antioxidant activity of germinated selected legumes and their fractions. J. Saudi Soc. Agric. Sci. 2019, 18, 294–301. [Google Scholar] [CrossRef]
- Schendel, R.R. Phenol content in sprouted grains. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–315. ISBN 9780128115251. [Google Scholar]
- Sofi, S.A.; Singh, J.; Muzaffar, K.; Mir, S.A.; Dar, B.N. Effect of germination time on physico-chemical, functional, pasting, rheology and electrophoretic characteristics of chickpea flour. J. Food Meas. Charact. 2020, 14, 2380–2392. [Google Scholar] [CrossRef]
- Borges-Martínez, E.; Gallardo-Velázquez, T.; Cardador-Martínez, A.; Moguel-Concha, D.; Osorio-Revilla, G.; Ruiz-Ruiz, J.C.; Martínez, C.J. Phenolic compounds profile and antioxidant activity of pea (Pisum sativum L.) and black bean (Phaseolus vulgaris L.) sprouts. Food Sci. Technol. 2021, 42. [Google Scholar] [CrossRef]
- Fouad, A.A.; Rehab, F.M.A. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris Medik.) sprouts. Acta Sci. Pol. Technol. Aliment. 2015, 14, 233–246. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Li, T.; Tang, K.; Liu, R.H. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J. Agric. Food Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef]
- Winarsi, H.; Bestari, Y.P.; Masluki, N.I.L.; Septiana, A.T. The effect of long germination on the levels and types of non-enzymatic antioxidants in red kidney bean sprouts. EProceeding ICMA 2021, 1, 22–31. [Google Scholar] [CrossRef]
- Nyau, V.; Prakash, S.; Rodrigues, J.; Farrant, J. Antioxidant activities of Bambara groundnuts as assessed by FRAP and DPPH assays. Am. J. Food Nutr. 2015, 3, 7–11. [Google Scholar] [CrossRef]
- Garretson, L.; Tyl, C.; Marti, A. Effect of Processing on Antioxidant Activity, Total Phenols, and Total Flavonoids of Pigmented Heirloom Beans. J. Food Qual. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Mohamed, E.A.; Yagoub, A.E.A.; Mohamed, A.R.; Elfadil, B.E. Effect of Processing Methods on Alkaloids, Phytate, Phenolics, Antioxidants Activity and Minerals of Newly Developed Lupin (Lupinus albus L.) Cultivar. J. Food Process. Preserv. 2017, 41, e12960. [Google Scholar] [CrossRef]
- Erba, D.; Angelino, D.; Marti, A.; Manini, F.; Faoro, F.; Morreale, F.; Pellegrini, N.; Casiraghi, M.C. Effect of sprouting on nutritional quality of pulses. Int. J. Food Sci. Nutr. 2019, 70, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Díaz, M.F.; Jiménez, T.; Benítez, V.; Herrera, T.; Cuadrado, C.; Martín-Pedrosa, M.; Martín-Cabrejas, M.A.; Jiménez, T.; Benítez, V.; et al. Changes in nonnutritional factors and antioxidant activity during germination of nonconventional legumes. J. Agric. Food Chem. 2013, 61, 8120–8125. [Google Scholar] [CrossRef] [PubMed]
- Pejin, J.; Grujić, O.; Canadanovic-Brunet, J.; Vujic, D.; Tumbas, V. Investigation of phenolic acids content and antioxidant activity in malt production. J. Am. Soc. Brew. Chem. 2009, 67, 81–88. [Google Scholar] [CrossRef]
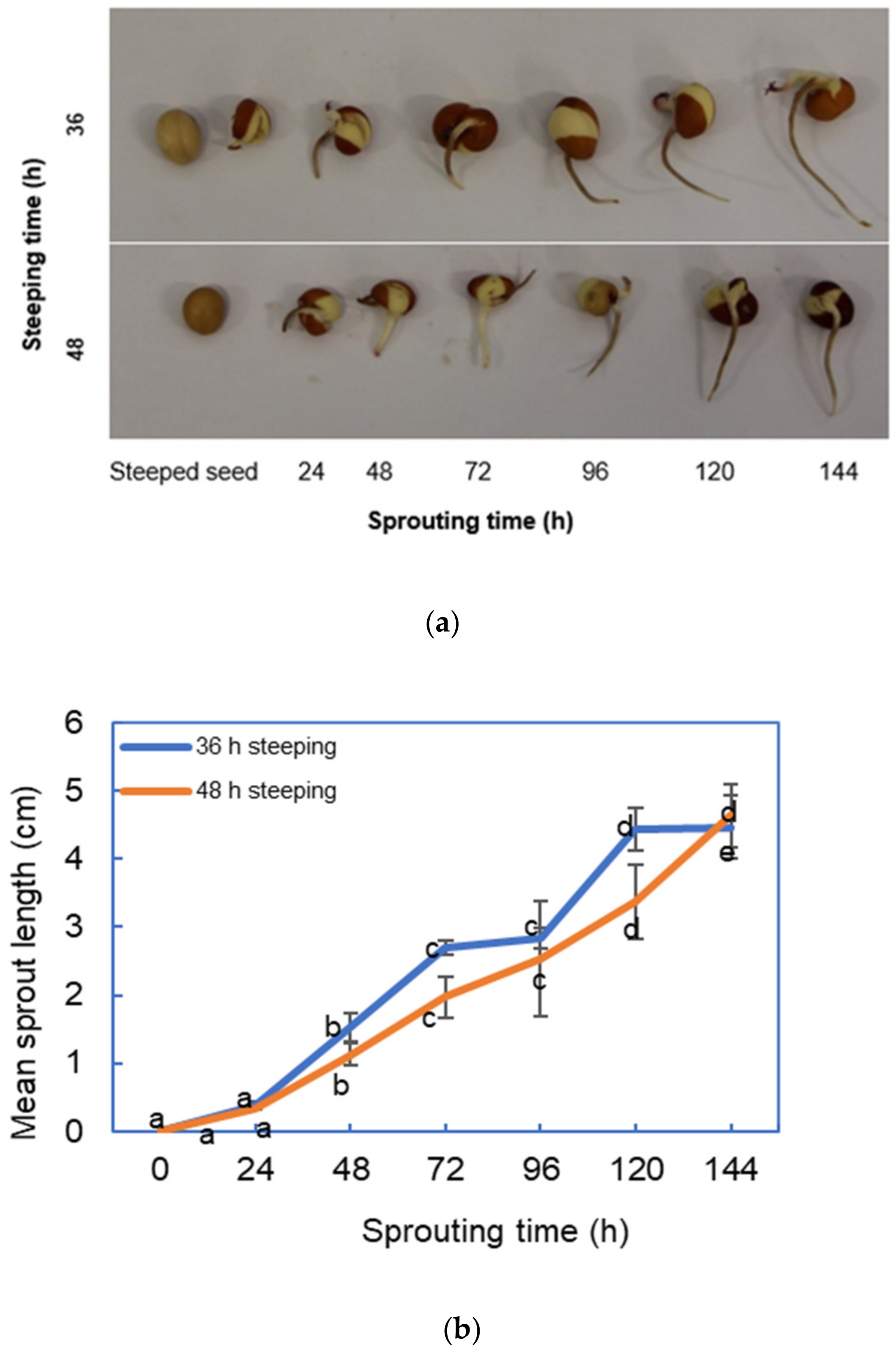

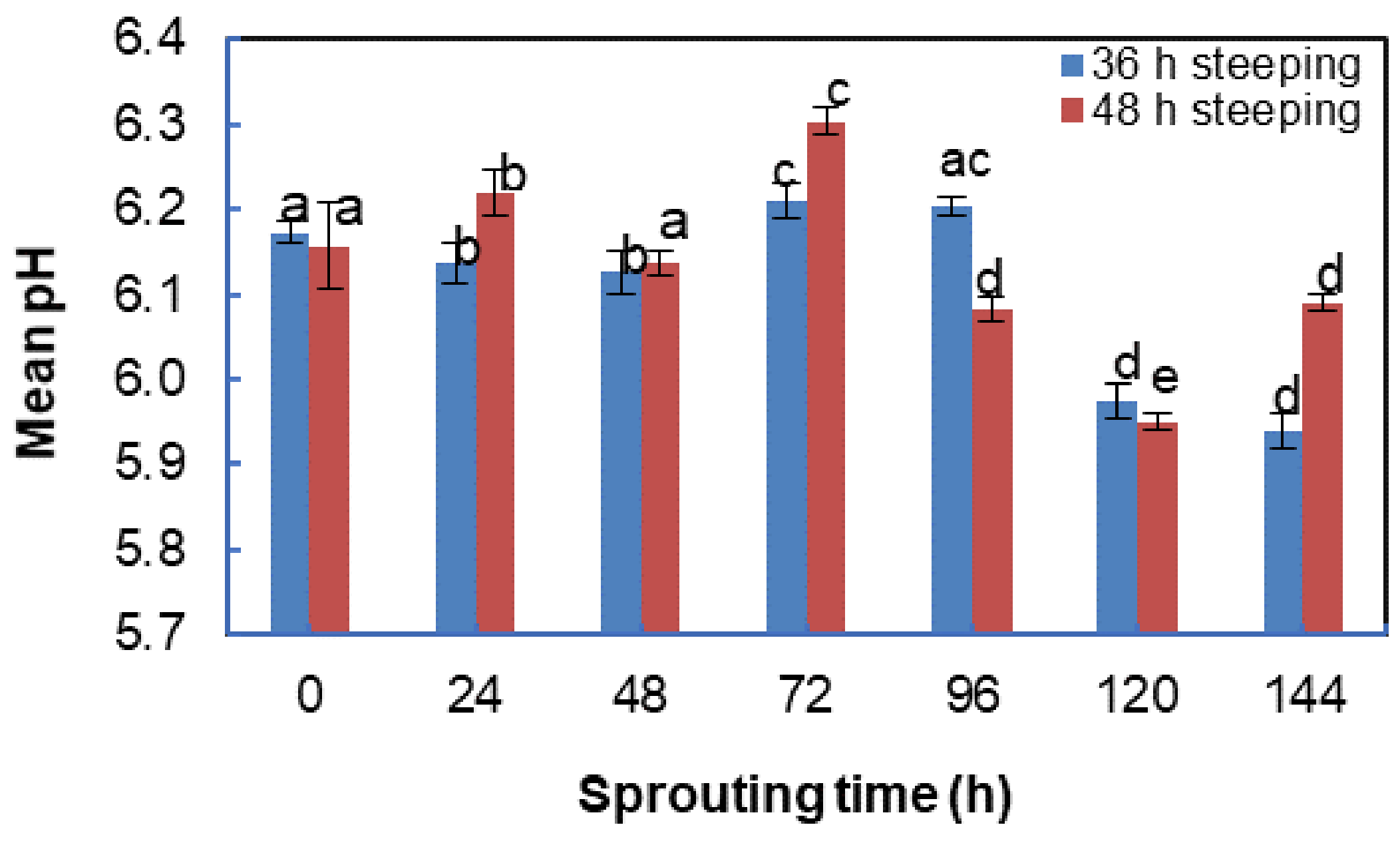
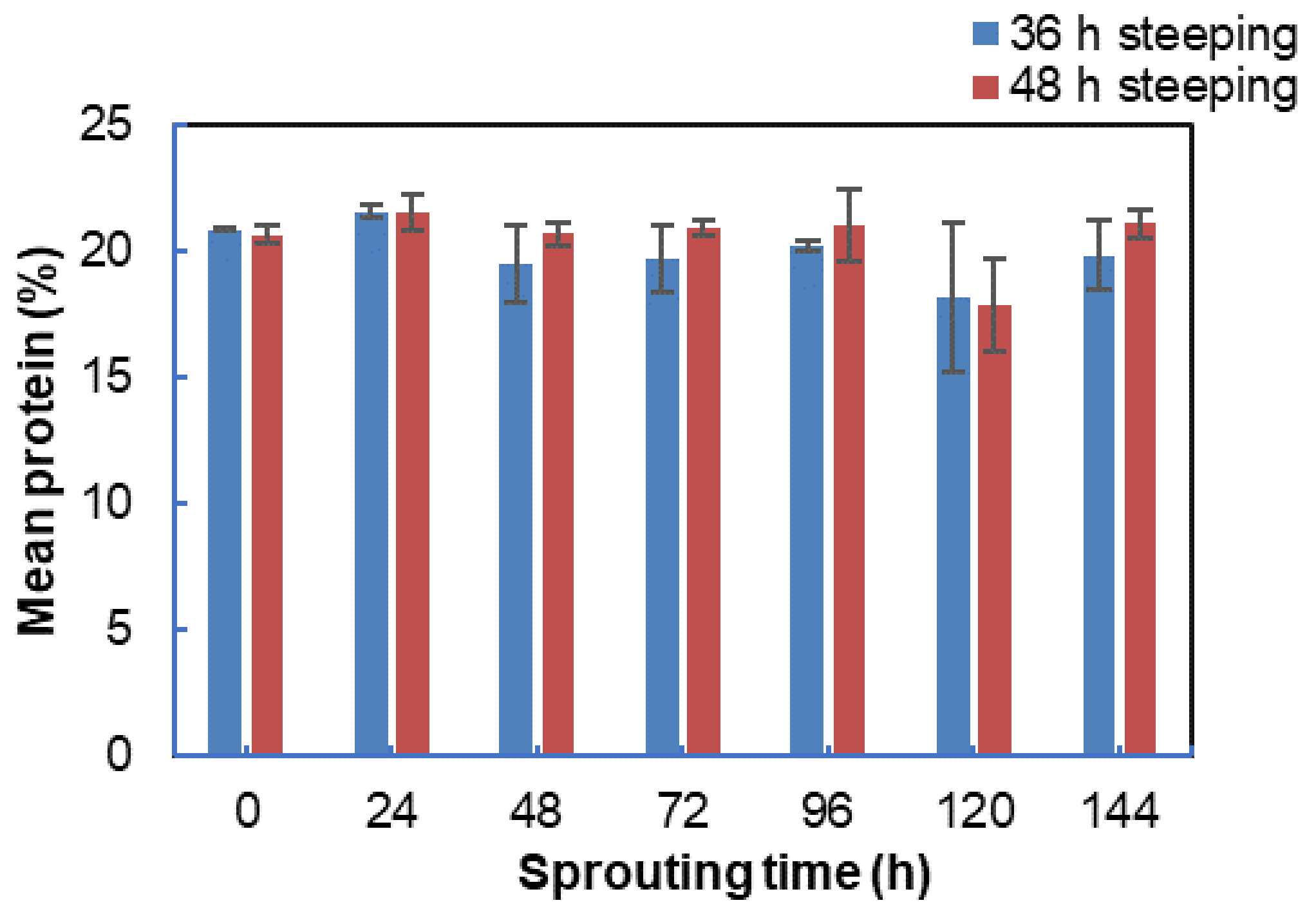
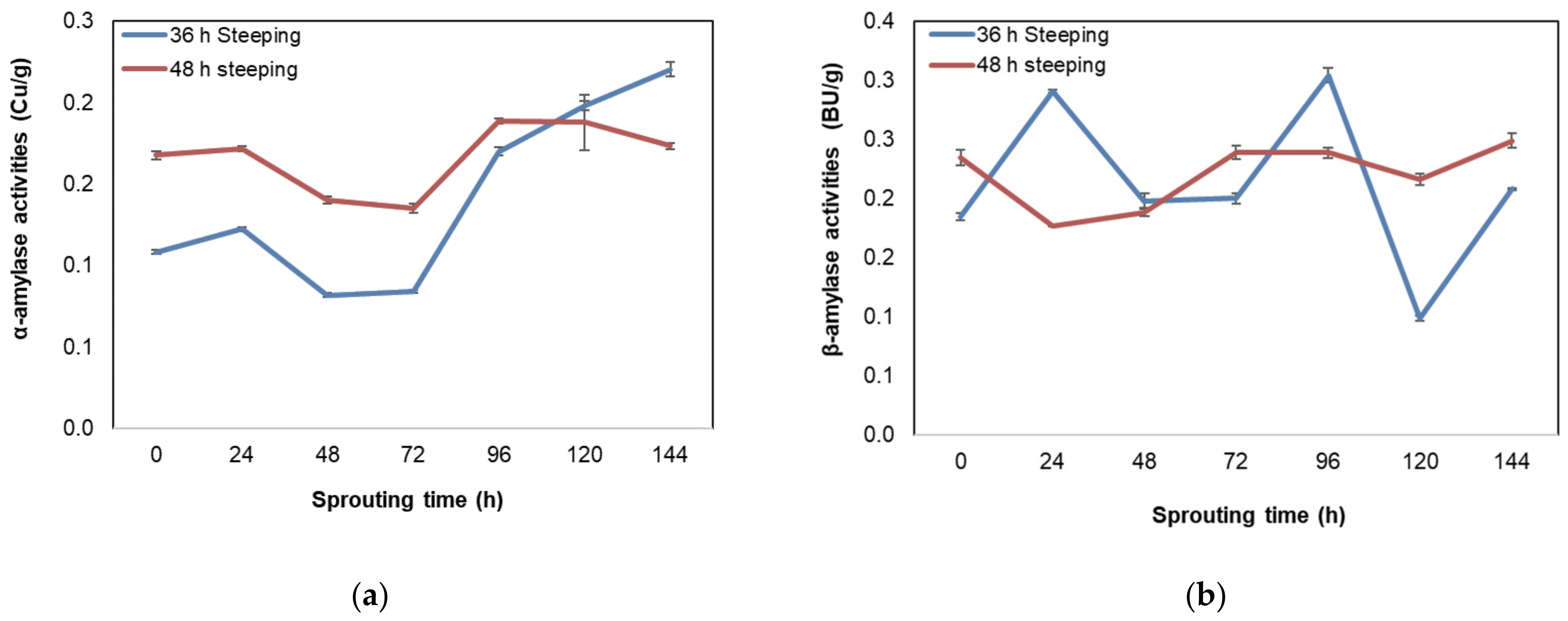


| Steeping Time (h) | ||
|---|---|---|
| Colour Parameters | 36 | 48 |
| Lightness (L*) | 76.11 ± 6.02 a | 75.60 ± 4.09 b |
| Redness (a*) | 2.93 ± 2.27 a | 3.42 ± 1.27 a |
| Yellowness (b*) | 11.45 ± 2.05 a | 12.81 ± 2.57 b |
| Chroma (C*) | 12.02 ± 2.08 a | 13.71 ± 10.40 a |
| Hue Angle (h°) | 75.67 ± 10.40 a | 71.10 ± 15.58 a |
| 36 h Steeping | |||||
|---|---|---|---|---|---|
| Sprouting Time (h) | L* | a* | b* | Chroma | Hue Angle (h°) |
| 0 | 82.67 ± 0.43 a | 0.76 ± 1.08 a | 8.08 ± 0.88 a | 8.16 ± 0.82 a | 82.11 ± 4.32 a |
| 24 | 81.59 ± 1.00 b | 2.40 ± 1.63 a | 10.05 ± 0.33 b | 10.42 ± 0.34 b | 76.64 ± 9.01 ab |
| 48 | 79.56 ± 0.08 c | 1.63 ± 1.15 a | 12.46 ± 0.86 c | 12.60 ± 0.87 cd | 82.53 ± 5.09 a |
| 72 | 79.15 ± 0.07 c | 1.45 ± 0.32 a | 12.83 ± 0.51 c | 12.91 ± 0.53 cd | 83.57 ± 1.26 a |
| 96 | 74.50 ± 0.54 d | 2.49 ± 1.40 a | 13.21 ± 1.13 c | 13.50 ± 0.80 ef | 79.06 ± 6.97 ab |
| 120 | 67.27 ± 0.31 e | 5.58 ± 0.71 b | 13.33 ± 0.70 c | 14.47 ± 0.58 f | 67.27 ± 3.23 bc |
| 144 | 68.02 ± 0.17 e | 6.22 ± 1.83 b | 10.18 ± 1.25 b | 12.06 ± 0.56 c | 58.51 ± 10.16 c |
| 48 h Steeping | |||||
| 0 | 79.97 ± 0.05 a | 3.56 ± 0.31 abc | 8.18 ± 1.40 a | 13.20 ± 2.22 abc | 66.21 ± 3.65 a |
| 24 | 80.14 ± 1.12 a | 2.43 ± 0.52 cd | 11.57 ± 1.04 b | 6.55 ± 2.64 ab | 54.15 ± 39.56 a |
| 48 | 78.17 ± 0.26 b | 1.70 ± 0.97 d | 14.83 ± 0.70 c | 3.96 ± 3.45 a | 83.56 ± 3.40 a |
| 72 | 74.43 ± 0.43 d | 4.23 ± 0.68 ab | 13.34 ± 0.44 bc | 18.67 ± 6.02 cd | 72.48 ± 2.12 a |
| 96 | 75.49 ± 0.31 c | 3.79 ± 1.55 abc | 14.56 ± 2.70 bc | 16.48 ± 11.27 bcd | 74.78 ± 7.19 a |
| 120 | 68.22 ± 0.74 f | 5.00 ± 0.65 a | 14.02 ± 1.14 bc | 25.78 ± 6.75 d | 70.23 ± 3.92 a |
| 144 | 72.77 ± 0.07 e | 3.22 ± 0.84 bcd | 13.14 ± 2.38 bc | 11.34 ± 5.41 abc | 76.31 ± 1.57 a |
| Amylase Activities | Steeping Time (h) | |
|---|---|---|
| 36 | 48 | |
| α-amylase (CU/g) | 0.14 ± 0.05 a | 0.17 ± 0.02 b |
| β-amylase (BU/g) | 0.21 ± 0.07 a | 0.22 ± 0.03 b |
| Antioxidant Assay | Sprouting Time (h) | Steeping Time (h) 1 | |
|---|---|---|---|
| 36 | 48 | ||
| FRAP umol (AAE/g) | 0 | 5.14 ± 0.38 a | 3.90 ± 0.17 a |
| 24 | 5.12 ± 0.38 a | 3.80 ± 0.16 a | |
| 48 | 4.53 ± 0.06 b | 3.80 ± 0.19 a | |
| 72 | 4.47 ± 0.24 b | 4.93 ± 0.48 bc | |
| 96 | 3.60 ± 0.02 c | 4.60 ± 0.17 b | |
| 120 | 4.30 ± 0.10 b | 5.21 ± 0.09 c | |
| 144 | 4.72 ± 0.13 ab | 5.14 ± 0.55 bc | |
| DPPH umol (TE/g) | 0 | 3.68 ± 1.11 a | 4.57 ± 0.99 a |
| 24 | 3.52 ± 0.80 a | 4.47 ± 0.80 a | |
| 48 | 3.75 ± 1.52 a | 5.04 ± 0.21 a | |
| 72 | 4.38 ± 1.66ab | 5.44 ± 1.64 a | |
| 96 | 4.94 ± 0.98 ab | 6.11 ± 1.06 a | |
| 120 | 6.25 ± 0.12 b | 7.44 ± 0.41 b | |
| 144 | 5.62 ± 1.59 ab | 6.08 ± 0.19 ab | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adetokunboh, A.H.; Obilana, A.O.; Jideani, V.A. Enzyme and Antioxidant Activities of Malted Bambara Groundnut as Affected by Steeping and Sprouting Times. Foods 2022, 11, 783. https://doi.org/10.3390/foods11060783
Adetokunboh AH, Obilana AO, Jideani VA. Enzyme and Antioxidant Activities of Malted Bambara Groundnut as Affected by Steeping and Sprouting Times. Foods. 2022; 11(6):783. https://doi.org/10.3390/foods11060783
Chicago/Turabian StyleAdetokunboh, Adeola Helen, Anthony O. Obilana, and Victoria A. Jideani. 2022. "Enzyme and Antioxidant Activities of Malted Bambara Groundnut as Affected by Steeping and Sprouting Times" Foods 11, no. 6: 783. https://doi.org/10.3390/foods11060783






