Adsorption of Quercetin on Brown Rice and Almond Protein Matrices: Effect of Quercetin Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Protein/Quercetin Microparticles
2.3. Extraction of Quercetin from Protein/Quercetin Microparticles
2.4. Reverse-Phase High Performance Liquid Chromatography (RP-HPLC)
2.5. Antioxidant Activity
2.6. Differential Scanning Calorimetry (DSC)
2.7. Fourier-Transform Infrared Spectroscopy-Attenuated Total Reflectance (FTIR-ATR)
2.8. Statistical Analysis
3. Results
3.1. Quercetin Amount and Antioxidant Activity of Protein/Quercetin Microparticles
3.2. Temperature of Denaturation of Protein Matrices and Protein/Quercetin Microparticles
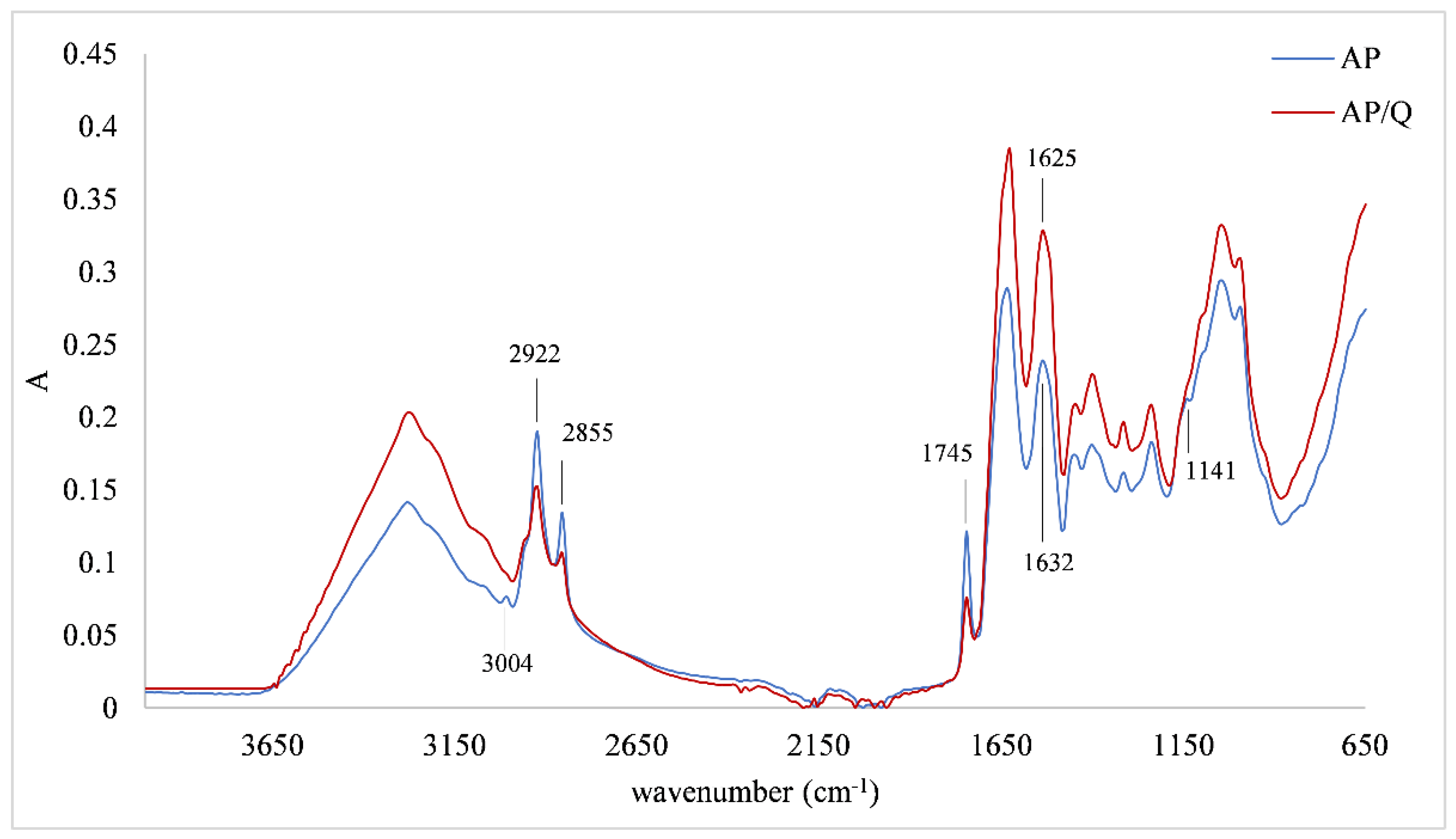
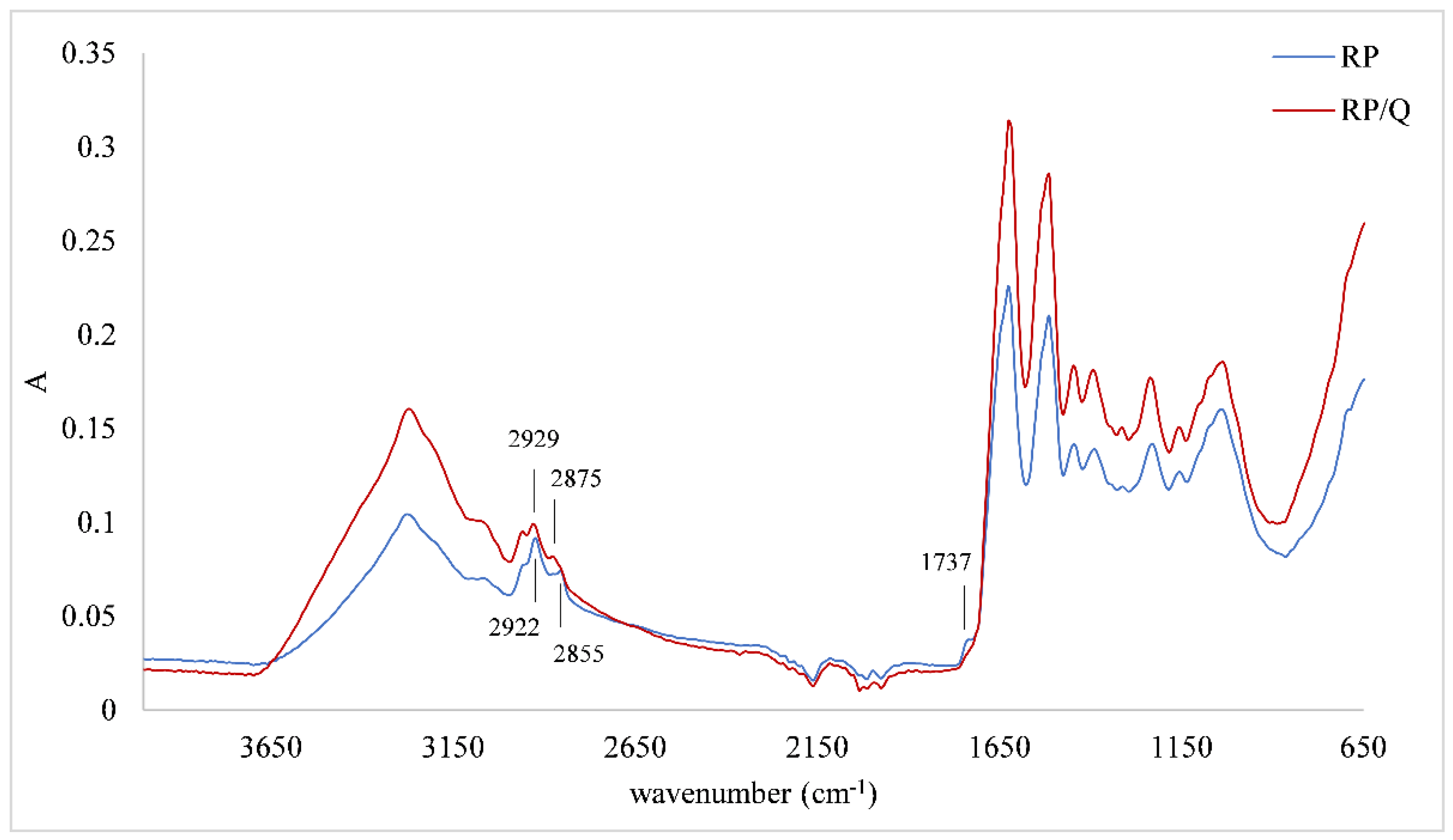
3.3. FTIR-ATR Spectra of Protein Matrices and Protein/Quercetin Microparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kardum, N.; Glibetic, M. Polyphenols and their interactions with other dietary compounds: Implications for human health. Adv. Food Nutr. Res. 2018, 84, 103–144. [Google Scholar] [PubMed]
- Corredig, M.; Young, N.; Dalsgaard, T.K. Food proteins: Processing solutions and challenges. Curr. Opin. Food Sci. 2020, 35, 49–53. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A mini review of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Vijayalakshmi, S.; Nadanasabapathi, S. Health benefits of quercetin. Def. Life Sci. J. 2017, 2, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Mumtaz, S.U.; Chaudhry, M.M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential clinical benefits of quercetin in the early stage of COVID-19: Results of a second, pilot, randomized, controlled and open-label clinical trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- McClements, D.J. Delivery by Design (DbD): A standardized approach to the development of efficacious nanoparticle- and microparticle-based delivery systems. Compr. Rev. Food Sci. Food Saf. 2018, 17, 200–219. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Diaz, J.T.; Foegeding, E.A.; Lila, M.A. Formulation of protein–polyphenol particles for applications in food systems. Food Funct. 2020, 11, 5091–5104. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, L.; Wen, Y.; Qin, W.; Wu, D.; Chen, H.; Zhang, Q.; Zhang, Q. Comparison of apple polyphenol-gelatin binary complex and apple polyphenol-gelatin-pectin ternary complex: Antioxidant and structural characterization. LWT 2021, 148, 111740. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, J.; Liu, X.; Ding, Q.; Jiang, L.; Guo, H.; Ren, F. Milk protein and fat play different roles in affecting the bioavailability and the antioxidant activity of jujube juice phenolics in rats. Mol. Nutr. Food Res. 2012, 56, 1511–1519. [Google Scholar] [CrossRef]
- Rohn, S.; Rawel, H.M.; Kroll, J. Antioxidant activity of protein-bound quercetin. J. Agric. Food Chem. 2004, 52, 4725–4729. [Google Scholar] [CrossRef]
- Ali, M. Chemical, structural and functional properties of whey proteins covalently modified with phytochemical compounds. J. Food Meas. Charact. 2019, 13, 2970–2979. [Google Scholar] [CrossRef]
- Grace, M.H.; Truong, A.N.; Truong, V.D.; Raskin, I.; Lila, M.A. Novel value-added uses for sweet potato juice and flour in polyphenol-and protein-enriched functional food ingredients. Food Sci. Nutr. 2015, 3, 415–424. [Google Scholar] [CrossRef]
- Ting, L.; Li, W.; Zhengxing, C.; Xinxia, Z.; Ziying, Z. Functional properties and structural changes of rice proteins with anthocyanins complexation. Food Chem. 2020, 331, 127336. [Google Scholar]
- Lila, M.A.; Schneider, M.; Devlin, A.; Plundrich, N.; Lasterc, S.; Foegeding, E.A. Polyphenol-enriched berry extracts naturally modulate reactive proteins in model foods. Food Funct. 2017, 8, 4760–4767. [Google Scholar] [CrossRef]
- Bohin, M.C.; Vincken, J.-P.; van der Hijden, H.T.W.M.; Gruppen, H. Efficacy of food proteins as carriers for flavonoids. J. Agric. Food Chem. 2012, 60, 4136–4143. [Google Scholar] [CrossRef]
- Roopchand, D.; Grace, M.H.; Kuhen, P.; Cheng, D.; Plundrich, N.; Pouleva, A.; Lila, M.A. Efficient sorption of polyphenols to soybean four enables natural fortification of foods. Food Chem. 2012, 131, 1193–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Plundrich, N.J.; Kulis, M.; White, B.L.; Grace, M.H.; Guo, R.; Burks, W.; Davis, J.P.; Lila, M.A. Novel strategy to create hypoallergenic peanut protein−polyphenol edible matrices for oral immunotherapy. J. Agric. Food Chem. 2014, 62, 7010–7021. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Yao, X.; Hu, H.; Yun, D.; Xiao, L. Recent advances in phenolic–protein conjugates: Synthesis, characterization, biological activities and potential applications. RSC Adv. 2019, 9, 35825. [Google Scholar] [CrossRef] [Green Version]
- Quiroz, J.Q.; Velazquez, V.; Corrales-Garcia, L.L.; Torres, J.D.; Delgado, E.; Ciro, G.; Rojas, J. Use of plant proteins as microencapsulating agents of bioactive compounds extracted from annatto seeds (Bixa orellana L.). Antioxidants 2020, 9, 310. [Google Scholar] [CrossRef]
- Kelemen, V.; Pichler, A.; Ivić, I.; Buljeta, I.; Šimunović, J.; Kopjar, M. Brown rice proteins as delivery system of phenolic and volatile compounds of raspberry juice. Int. J. Food Sci. Technol. 2021, 1–9. [Google Scholar] [CrossRef]
- Kopjar, M.; Buljeta, I.; Jelić, I.; Kelemen, V.; Šimunović, J.; Pichler, A. Encapsulation of cinnamic acid on plant-based proteins: Evaluation by HPLC, DSC and FTIR-ATR. Plants 2021, 10, 2158. [Google Scholar] [CrossRef]
- Kopjar, M.; Buljeta, I.; Ćorković, I.; Kelemen, V.; Šimunović, J.; Pichler, A. Plant-based proteins as encapsulating materials for glucosyl-hesperidin. Int. J. Food Sci. Technol. 2022, 57, 728–737. [Google Scholar] [CrossRef]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polyphenols and antioxidant activity of citrus fiber/blackberry juice complexes. Molecules 2021, 26, 4400. [Google Scholar] [CrossRef]
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein–phenolic compound interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Rawel, H.M.; Czajka, D.; Rohn, S.; Kroll, J. Interactions of different phenolic acids and flavonoids with soy proteins. Int. J. Biol. Macromol. 2002, 30, 137–150. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein-phenolic interactions as a factor affecting the physicochemical properties of white bean proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef]
- Yildirim-Elikoglu, S.; Erdem, K.E. Interactions between milk proteins and polyphenols: Binding mechanisms, related changes and the future trends in dairy industry. Food Rev. Int. 2018, 34, 665–697. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Interaction of whey proteins with phenolic derivatives under neutral and acidic pH conditions. J. Food Sci. 2017, 82, 409–419. [Google Scholar] [CrossRef]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assay. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Clifford, M.; Howell, N.K. The effect of instant green tea on the foaming and rheological properties of egg albumen proteins. J. Sci. Food Agric. 2007, 87, 1810–1819. [Google Scholar] [CrossRef]
- Von Staszewskia, M.; Jaraa, F.L.; Ruizb, A.L.T.G.; Jagusa, R.J.; Carvalhob, J.E.; Pilosof, A.M.R. Nanocomplex formation between β-lactoglobulin or caseinomacropeptide and green tea polyphenols: Impact on protein gelation and polyphenols antiproliferative activity. J. Funct. Foods 2012, 4, 800–8009. [Google Scholar] [CrossRef]
- Prigent, S.V.E.; Gruppen, H.; Visser, A.J.W.G.; van Koningsveld, G.A.; de Jong, D.A.H.; Voragen, A.G.J. Effects of non-covalent interactions with 5-O-caffeoylquinic acid (chlorogenic acid) on the heat denaturation and solubility of globular proteins. J. Agric. Food Chem. 2003, 51, 5088–5095. [Google Scholar] [CrossRef]
- Prigent, S.V.E.; Voragen, A.G.J.; Visser, A.J.W.G.; van Koningsveld, G.A.; Gruppen, H. Covalent interactions between proteins and oxidation products of caffeoylquinic acid (chlorogenic acid). J. Sci. Food Agric. 2007, 87, 2502–2510. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.-A. Interaction of milk α- and β-caseins with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
| Microparticles | Q Amount | DPPH | CUPRAC | FRAP |
|---|---|---|---|---|
| Almond protein matrix | ||||
| AP/Q_1 | 60.18 ± 0.17 a | 29.38 ± 0.95 a | 106.39 ± 0.93 b | 0.66 ± 0.04 a |
| AP/Q_2 | 98.38 ± 1.21 b | 30.63 ± 0.21 a | 125.42 ± 1.91 c | 1.02 ± 0.01 b |
| AP/Q_5 | 196.34 ± 1.45 d | 32.18 ± 0.00 b | 192.75 ± 1.14 e | 2.34 ± 0.08 c |
| Brown rice protein matrix | ||||
| RP/Q_1 | 108.24 ± 1.75 c | 31.12 ± 0.47 a,b | 84.38 ± 1.91 a | 1.20 ± 0.02 b |
| RP/Q_2 | 226.50 ± 3.17 e | 34.22 ± 0.12 c | 161.59 ± 1.19 d | 2.78 ± 0.04 c |
| RP/Q_5 | 506.98 ± 0.42 f | 40.40 ± 0.52 d | 414.52 ± 1.03 f | 8.35 ± 0.19 d |
| Samples | Td (°C) |
|---|---|
| Almond protein matrix | |
| 100% | 85.24 ± 0.07 b |
| AP/Q_1 | 83.71 ± 0.28 a |
| AP/Q_2 | 83.27 ± 0.25 a |
| AP/Q_5 | 83.24 ± 0.29 a |
| Brown rice protein matrix | |
| 100% | 85.26 ± 0.05 b |
| RP/Q_1 | 85.78 ± 0.12 c |
| RP/Q_2 | 86.70 ± 0.14 d |
| RP/Q_5 | 86.72 ± 0.22 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopjar, M.; Buljeta, I.; Ćorković, I.; Pichler, A.; Šimunović, J. Adsorption of Quercetin on Brown Rice and Almond Protein Matrices: Effect of Quercetin Concentration. Foods 2022, 11, 793. https://doi.org/10.3390/foods11060793
Kopjar M, Buljeta I, Ćorković I, Pichler A, Šimunović J. Adsorption of Quercetin on Brown Rice and Almond Protein Matrices: Effect of Quercetin Concentration. Foods. 2022; 11(6):793. https://doi.org/10.3390/foods11060793
Chicago/Turabian StyleKopjar, Mirela, Ivana Buljeta, Ina Ćorković, Anita Pichler, and Josip Šimunović. 2022. "Adsorption of Quercetin on Brown Rice and Almond Protein Matrices: Effect of Quercetin Concentration" Foods 11, no. 6: 793. https://doi.org/10.3390/foods11060793
APA StyleKopjar, M., Buljeta, I., Ćorković, I., Pichler, A., & Šimunović, J. (2022). Adsorption of Quercetin on Brown Rice and Almond Protein Matrices: Effect of Quercetin Concentration. Foods, 11(6), 793. https://doi.org/10.3390/foods11060793







