Bioactive Compounds from Cocoa Husk: Extraction, Analysis and Applications in Food Production Chain
Abstract
:1. Introduction
2. Bioactive Compounds Extraction from Cocoa Bean Husk
2.1. Microwave-Assisted Extraction
2.2. Water Extraction
2.3. Extraction in Supercritical CO2
2.4. Subcritical Water Extraction
2.5. Ultrasound-Assisted Extraction
2.6. Conditions and Solvents Optimization
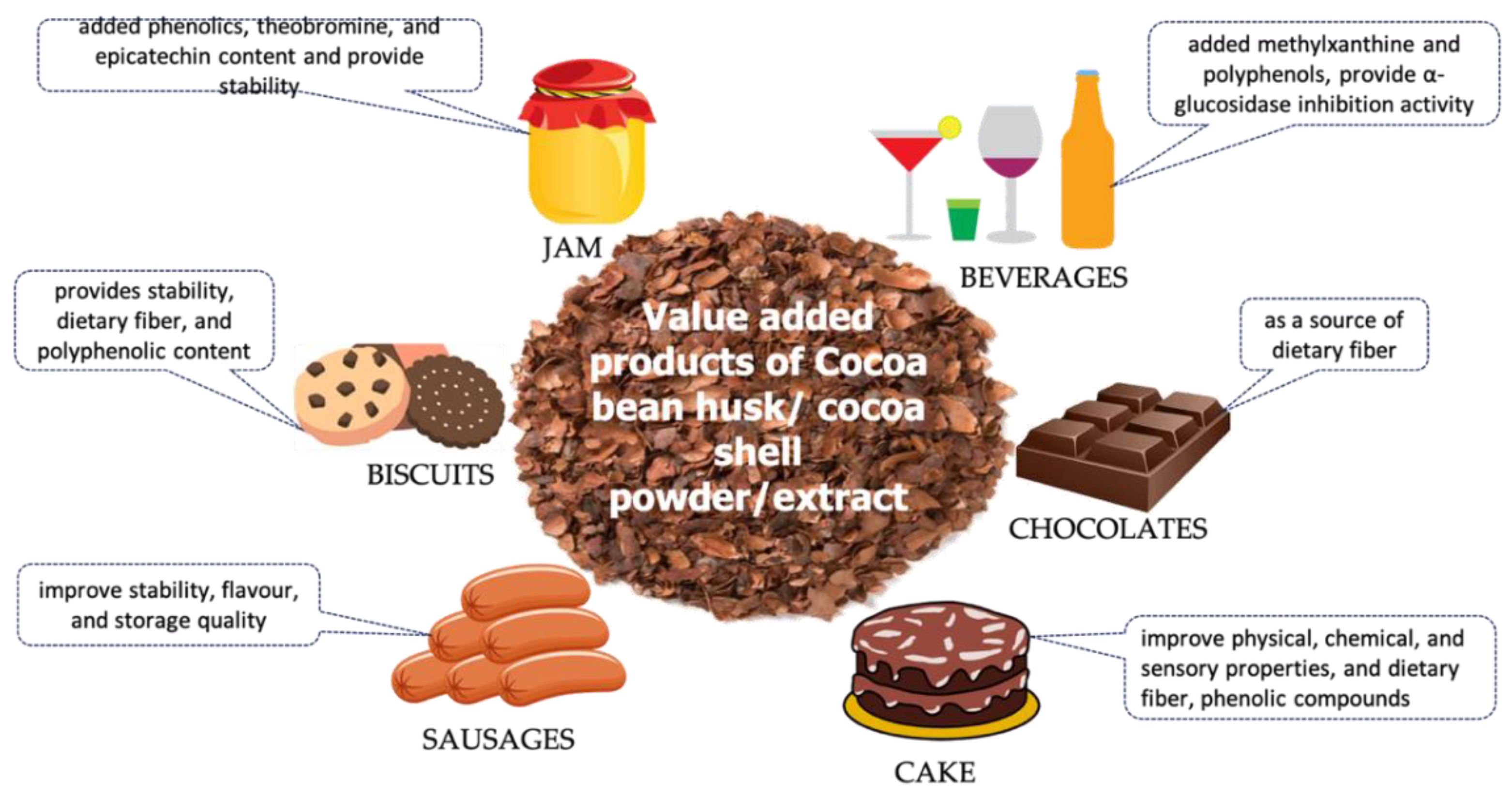
3. Functional Food Containing Cocoa Husk Powder/Extract
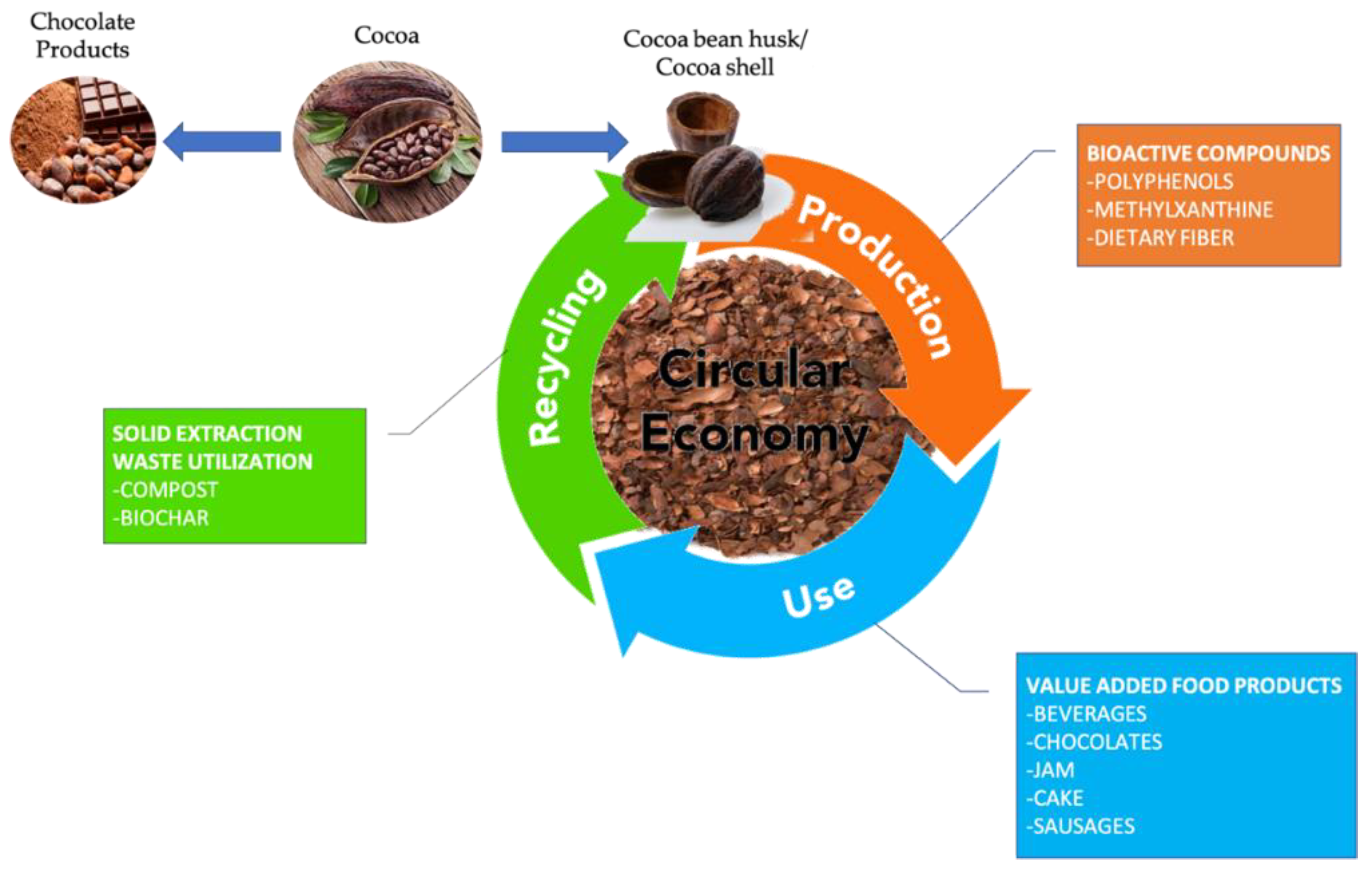
4. The Cocoa Pod Husk/Shell Solid Waste after Extraction: Circular Economy Concept?
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. Cocoa Production Worldwide from 1980/81 to 2020/21. 2021. Available online: https://www.statista.com/statistics/262620/global-cocoa-production/ (accessed on 12 December 2021).
- Handojo, L.; Triharyogi, H.; Indarto, A. Cocoa bean shell waste as potential raw material for dietary fiber powder. Int. J. Recycl. Org. Waste Agric. 2019, 8, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.; Vandenberghe, L.P.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.; Neto, A.G.; Soccol, C.R. Biotechnological approaches for cocoa waste management: A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Mariatti, F.; Gunjević, V.; Boffa, L.; Cravotto, G. Process intensification technologies for the recovery of valuable compounds from cocoa by-products. Innov. Food Sci. Emerg. Technol. 2021, 68, 102601. [Google Scholar] [CrossRef]
- Loullis, A.; Pinakoulaki, E. Carob as cocoa substitute: A review on composition, health benefits and food applications. Eur. Food Res. Technol. 2018, 244, 959–977. [Google Scholar] [CrossRef]
- Panak Balentić, J.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa shell: A by-product with great potential for wide application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef] [Green Version]
- Adi-Dako, O.; Ofori-Kwakye, K.; Manso, S.F.; Boakye-Gyasi, M.E.; Sasu, C.; Pobee, M. Physicochemical and antimicrobial properties of cocoa pod husk pectin intended as a versatile pharmaceutical excipient and nutraceutical. J. Pharm. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.Y.; Choo, W.S. Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 2013, 141, 3752–3758. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; Teófilo, R.F.; de Oliveira Petkowicz, C.L. Optimization of nitric acid-mediated extraction of pectin from cacao pod husks (Theobroma cacao L.) using response surface methodology. Carbohydr. Polym. 2011, 84, 1230–1236. [Google Scholar] [CrossRef] [Green Version]
- Pangestu, R.; Amanah, S.; Juanssilfero, A.B.; Perwitasari, U. Response surface methodology for microwave-assisted extraction of pectin from cocoa pod husk (Theobroma cacao) mediated by oxalic acid. J. Food Meas. Charact. 2020, 14, 2126–2133. [Google Scholar] [CrossRef]
- Priyangini, F.; Walde, S.G.; Chidambaram, R. Extraction optimization of pectin from cocoa pod husks (Theobroma cacao L.) with ascorbic acid using response surface methodology. Carbohydr. Polym. 2018, 202, 497–503. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, N.H. Proximate composition, extraction, and purification of theobromine from cacao pod husk (Theobroma cacao L.). Inf. Technol. J. 2017, 5, 14. [Google Scholar] [CrossRef]
- Pico Hernández, S.M.; Jaimes Estévez, J.; López Giraldo, L.J.; Murillo Méndez, C.J. Supercritical extraction of bioactive compounds from cocoa husk: Study of the main parameters. Rev. Fac. Ing. Univ. Antioq. 2019, 91, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Ruesgas-Ramón, M.; Suárez-Quiroz, M.L.; González-Ríos, O.; Baréa, B.; Cazals, G.; Figueroa-Espinoza, M.C.; Durand, E. Biomolecules extraction from coffee and cocoa by-and co-products using deep eutectic solvents. J. Sci. Food Agric. 2020, 100, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Almagro, N.; Valadez-Carmona, L.; Mendiola, J.A.; Ibáñez, E.; Villamiel, M. Structural characterisation of pectin obtained from cacao pod husk. Comparison of conventional and subcritical water extraction. Carbohydr. Polym. 2019, 217, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Segovia, Á.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Extraction of phenolic compounds from cocoa shell: Modeling using response surface methodology and artificial neural networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Rosyidi, D.; Thohari, I. Characteristics of catechin extracted from cocoa husks using microwave assisted extraction (MAE). Biodiversitas 2019, 20, 3626–3631. [Google Scholar]
- Okiyama, D.C.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties. Foods 2020, 9, 1572. [Google Scholar] [CrossRef]
- Rahmawati, I.; Fachri, B.A.; Manurung, Y.H.; Reza, M. Application of response surface methodology in optimization condition of anthocyanin extraction process of cocoa peel waste with Microwave Assisted Extraction Method (MAE). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: East Java, Indonesia, 2020; Volume 743, p. 01209. [Google Scholar]
- Ibrahim, N.H.; Mahmud, M.S.; Nurdin, S. Microwave-assisted extraction of β-sitosterol from cocoa shell waste. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Kuala Lumpur, Malaysia, 2020; Volume 991, p. 012106. [Google Scholar]
- Yusof, A.H.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E.; Zainudin, B.H. Optimization of an ultrasound-assisted extraction condition for flavonoid compounds from cocoa shells (Theobroma cacao) using response surface methodology. Molecules 2019, 24, 711. [Google Scholar] [CrossRef] [Green Version]
- González-Alejo, F.A.; Barajas-Fernández, J.; Olán-Acosta, M.D.L.Á.; Lagunes-Gálvez, L.M.; García-Alamilla, P. Supercritical Fluid Extraction of Fat and Caffeine with Theobromine Retention in the Cocoa Shell. Processes 2019, 7, 385. [Google Scholar] [CrossRef] [Green Version]
- Barišić, V.; Flanjak, I.; Kopjar, M.; Benšić, M.; Jozinović, A.; Babić, J.; Šubarić, D.; Miličević, B.; Doko, K.; Jašić, M.; et al. Does High Voltage Electrical Discharge Treatment Induce Changes in Tannin and Fiber Properties of Cocoa Shell? Foods 2020, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Jokić, S.; Gagić, T.; Knez, Ž.; Šubarić, D.; Škerget, M. Separation of active compounds from food by-product (cocoa shell) using subcritical water extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokić, S.; Nastić, N.; Vidović, S.; Flanjak, I.; Aladić, K.; Vladić, J. An approach to value cocoa bean by-product based on subcritical water extraction and spray drying using different carriers. Sustainability 2020, 12, 2174. [Google Scholar] [CrossRef] [Green Version]
- Pavlović, N.; Jokić, S.; Jakovljević, M.; Blažić, M.; Molnar, M. Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell. Foods 2020, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Botella-Martínez, C.; Lucas-Gonzalez, R.; Ballester-Costa, C.; Pérez-Álvarez, J.Á.; Fernández-López, J.; Delgado-Ospina, J.; Chaves-López, C.; Viuda-Martos, M. Ghanaian Cocoa (Theobroma cacao L.) Bean Shells Coproducts: Effect of Particle Size on Chemical Composition, Bioactive Compound Content and Antioxidant Activity. Agronomy 2021, 11, 401. [Google Scholar] [CrossRef]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Vanitha, T.; Khan, M. Role of pectin in food processing and food packaging. In Pectins-Extraction, Purification, Characterization and Applications; Masuelli, M., Ed.; Intech Open: London, UK, 2019. [Google Scholar]
- Cintas, P.; Calcio Gaudino, E.; Cravotto, G. Pharmaceutical and nutraceutical compounds from natural matrices. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., XII, Eds.; Series: Food Engineering Series; Springer Nature: New York, NY, USA, 2013; Volume 4, pp. 181–206. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Belwal, T.; Li, L.; Limwachiranon, J.; Liu, X.; Luo, Z. Phytosterols and their derivatives: Potential health-promoting uses against lipid metabolism and associated diseases, mechanism, and safety issues. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1243–1267. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadez-Carmona, L.; Ortiz-Moreno, A.; Ceballos-Reyes, G.; Mendiola, J.A.; Ibáñez, E. Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J. Supercrit. Fluids 2018, 131, 99–105. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.D.; Okiyama, D.C.G.; da Costa Rodrigues, C.E. Simultaneous green extraction of fat and bioactive compounds of cocoa shell and protein fraction functionalities evaluation. Food Res. Int. 2020, 137, 109622. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.D.L.L.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Guerrero-Muñoz, N.; Villegas-Aguilar, M.D.C.; Pimentel-Moral, S.; Ramos-Escudero, F.; Segura-Carretero, A. LC-MS and spectrophotometric approaches for evaluation of bioactive compounds from Peru cocoa by-products for commercial applications. Molecules 2020, 25, 3177. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J.; Bangoura, M.L.; Lagnika, C. Quantification of total polyphenolic content and antimicrobial activity of cocoa (Theobroma cacao L.) bean shells. Pak. J. Nutr 2012, 11, 672–677. [Google Scholar] [CrossRef] [Green Version]
- Braojos, C.; Benitez, V.; Rebollo-Hernanz, M.; Cañas, S.; Aguilera, Y.; Arribas, S.M.; Martin-Cabrejas, M.A. Evaluation of the Hypolipidemic Properties of Cocoa Shell after Simulated Digestion Using In Vitro Techniques and a Cell Culture Model of Non-Alcoholic Fatty Liver Disease. Proceedings 2021, 70, 58. [Google Scholar] [CrossRef]
- Barišić, V.; Stokanović, M.C.; Flanjak, I.; Doko, K.; Jozinović, A.; Babić, J.; Šubarić, D.; Miličević, B.; Cindrić, I.; Ačkar, Đ. Cocoa Shell as a Step Forward to Functional Chocolates—Bioactive Components in Chocolates with Different Composition. Molecules 2020, 25, 5470. [Google Scholar] [CrossRef]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Coïsson, J.D.; Arlorio, M. Cocoa hulls polyphenols stabilized by microencapsulation as functional ingredient for bakery applications. Food Res. Int. 2019, 115, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; El Khattabi, C.; Youl, E.N.; Bertolino, M.; Delporte, C.; Pochet, S.; Stévigny, C. Polyphenolic and methylxanthine bioaccessibility of cocoa bean shell functional biscuits: Metabolomics approach and intestinal permeability through caco-2 cell models. Antioxidants 2020, 9, 1164. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Ova, G. Evaluation of cocoa bean hulls as a fat replacer on functional cake production. Turk. J. Agric.-Food Sci. Technol. 2018, 6, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Kim, N.; Choi, H.Y.; Han, Y.S. Effect of cacao bean husk powder on the quality properties of pork sausages. Food Sci. Anim. Resour. 2019, 39, 742. [Google Scholar] [CrossRef]
- Choi, J.; Yang, C.; Lim, W.; Song, G.; Choi, H. Antioxidant and apoptotic activity of cocoa bean husk extract on prostate cancer cells. Mol. Cell Toxicol. 2021, 17, 1–11. [Google Scholar] [CrossRef]
- Irondi, A.E.; Olawuyi, A.D.; Lawal, B.S.; Boligon, A.A.; Olasupo, F.; Olalekan, S.I. Comparative inhibitory effects of cocoa bean and cocoa pod husk extracts on enzymes associated with hyperuricemia and hypertension in vitro. Int. Food Res. J. 2019, 26, 557–564. [Google Scholar]
- Karim, A.A.; Azlan, A.; Ismail, A.; Hashim, P.; Abd Gani, S.S.; Zainudin, B.H.; Abdullah, N.A. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement Altern. Med. 2014, 14, 1–13. [Google Scholar]
- Babu, N.V.; Vivek, D.K.; Ambika, G. Comparative evaluation of chlorhexidine mouthrinse versus cacao bean husk extract mouthrinse as antimicrobial agents in children. Eur. Arch. Paediatr. Dent. 2011, 12, 245–249. [Google Scholar] [CrossRef]
- Yuanita, T.; Oktavianti, R.A.; Suryani, D.F.; Rukmo, M.; Kunarti, S.; Kusuma, A.H. The Inhibitory Ability of Cocoa Pod Husk Extract on Enterococcus faecalis Glucosyltransferase Enzyme Activity. J. Contemp. Dent. 2020, 21, 271–276. [Google Scholar] [CrossRef]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Testa, G.; Sottero, B.; Poli, G.; Zeppa, G.; Biasi, F. A dietary mixture of oxysterols induces in vitro intestinal inflammation through TLR2/4 activation: The protective effect of cocoa bean shells. Antioxidants 2019, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Cantele, C.; Rojo-Poveda, O.; Bertolino, M.; Ghirardello, D.; Cardenia, V.; Barbosa-Pereira, L.; Zeppa, G. In vitro bioaccessibility and functional properties of phenolic compounds from enriched beverages based on cocoa bean shell. Foods 2020, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, C.; Morales-Sillero, A.; Fernández-Prior, M.Á.; Fernández-Bolaños, J.; de la Paz Aguilera-Herrera, M.; Rodríguez-Gutiérrez, G. Extra virgin olive oil jam enriched with cocoa bean husk extract rich in theobromine and phenols. LWT 2019, 111, 278–283. [Google Scholar] [CrossRef]
- Ogidi, C.O.; Abioye, S.A.; Akinyemi, D.D.; Fadairo, F.B.; Bolaniran, T.; Akinyele, B.J. Bioactivity assessment of ethanolic extracts from Theobroma cacao and Cola spp. wastes after solid state fermentation by Pleurotus ostreatus and Calocybe indica. Adv. Tradit. Med. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Lessa, O.A.; dos Santos Reis, N.; Leite, S.G.F.; Gutarra, M.L.E.; Souza, A.O.; Gualberto, S.A.; de Oliveira, J.R.; Aguiar-Oliveira, E.; Franco, M. Effect of the solid-state fermentation of cocoa shell on the secondary metabolites, antioxidant activity, and fatty acids. Food Sci. Biotechnol. 2018, 27, 107–113. [Google Scholar] [CrossRef]
- Munongo, M.E.; Nkeng, G.E.; Njukeng, J.N. Production and characterization of compost manure and biochar from cocoa pod husks. Int. J. Adv. Sci. Res. Manag. 2017, 2, 26–31. [Google Scholar]
- Tsai, C.H.; Tsai, W.T.; Liu, S.C.; Lin, Y.Q. Thermochemical characterization of biochar from cocoa pod husk prepared at low pyrolysis temperature. Biomass Convers. Biorefin. 2018, 8, 237–243. [Google Scholar] [CrossRef]
- Tsai, W.T.; Hsu, C.H.; Lin, Y.Q.; Tsai, C.H.; Chen, W.S.; Chang, Y.T. Enhancing the pore properties and adsorption performance of cocoa pod husk (CPH)-Derived biochars via post-acid treatment. Processes 2020, 8, 144. [Google Scholar] [CrossRef] [Green Version]
- Kayode, C.O.; Adeoye, G.O.; Ezekiel-Adewoyin, D.T.; AyanfeOluwa, O.E.; Ogunleti, D.O.; Adekunle, A.F. Influence of cocoa pod husk-based compost on nutrient uptake of okra (Abelmoschus esculentus (L.) MOENCH) and soil properties on an Alfisol. Commun. Soil Sci. Plant Anal. 2018, 49, 2113–2122. [Google Scholar] [CrossRef]
- Bahrun, A.; Fahimuddin, M.Y.; Rakian, T.C. Cocoa Pod Husk Biochar Reduce Watering Frequency and Increase Cocoa Seedlings Growth. Int. J. Agric. Environ. Biotechnol. 2018, 3, 1635–1639. [Google Scholar] [CrossRef]
- Córdova, B.M.; Santa Cruz, J.P.; Huamani-Palomino, R.G.; Baena-Moncada, A.M. Simultaneous adsorption of a ternary mixture of brilliant green, rhodamine B and methyl orange as artificial wastewater onto biochar from cocoa pod husk waste. Quantification of dyes using the derivative spectrophotometry method. New J. Chem. 2020, 44, 8303–8316. [Google Scholar] [CrossRef]
- Yong, S.K.; Leyom, J.; Tay, C.C.; Talib, S.A. Sorption of lead from aqueous system using cocoa pod husk biochar: Kinetic and isotherm studies. Int. J. Eng. Technol. 2018, 7, 241–244. [Google Scholar] [CrossRef]
- Acciardo, E.; Tabasso, S.; Cravotto, G.; Bensaid, S. Process intensification for lignin valorization. Chem. Eng. Proc. Process Intensif. 2022, 171, 108732. [Google Scholar] [CrossRef]

| Compounds | Extraction Method | Yield (w/w) | References |
|---|---|---|---|
| Cocoa pod husk | |||
| Pectin | Aqueous citric acid (4% w/v) followed by precipitation of extract using ethanol | 23.3% | [7] |
| Pectin | Water, citric acid (2.5, 4 pH), and hydrochloric acid (2.5, 4 pH) | 7.62% | [8] |
| Pectin | Nitric acid, pH 1.5, 100 °C of extraction temperature, and 30 min of extraction time | 9.0% | [9] |
| Pectin | Oxalic acid + microwave radiation condition at pH 1.16, L/S = 25.0 and 15 min. of irradiation time | 9.64% | [10] |
| Pectin | Ascorbic acid-based extraction, pH 2.5, 95 °C, for 45 min | 4.2% | [11] |
| Theobromine rich extract | 70% ethanol, extraction time of 90 min, temperature of 80 °C, and 1 cycle of extraction | Theobromine yield (6.79 mg/100 g) | [12] |
| TPC, total flavon-3-oles, and total carotenoids content | Supercritical fluid extraction, particle size less than 0.26 mm, extraction time of 147 min, extraction temperature of 308.15 K, pressure of 20 MPa, and 20% ethanol | TPC (35.11 EAG mg/g), a total flavan-3-oles content (12.89 EEP mg/g) and total carotenoids content (64.35 EBC mg/g) | [13] |
| phenolics and alkaloids | Heat-stirring assisted extraction (HSE) or ultrasound probe assisted extraction was used along with deep eutectic solvents | ultrasound (3 min, 200 W) Des (lactic acid:ChCl) was found superior in extracting the compounds (chlorogenic acid, caffeine, and theobromine) compared to HSE | [14] |
| Pectin | Subcritical water extraction | 121 °C, 103.4 bar, and 30 min | [15] |
| Total phenolic compounds, total flavonoids, total flavanols, total phenolic acids, total proanthrocyanidins, total ortho-diphenols, and antioxidant activity | Heat-assisted extraction, 100 °C, 90 min, 0% citric acid, and 0.02 g cocoa shell/mL of water | UPLC-ESI-MS/MS revealed the presence of 15 phenolic compounds, being protocatechuic acid, procyanidin B2, (−)-epicatechin, and (+)-catechin, the major ones | [16] |
| Total phenolic content and total catechin content | MAE, absolute ethanol, 70 °C, 3:100 g/mL, 8 and 10 min | Total phenol content (TPC) and total catechin content (TCC) | [17] |
| Cocoa bean shell | |||
| Flavonoids and alkaloids | Pressurized liquid extraction | Lyophilized extract showed higher flavonoids (catechin, epicatechin, procyanidin B2) and alkaloid (theobromine, caffeine) content as compared to the dried cocoa shell powder extract | [18] |
| Polyphenols and polysaccharides- pectin-based films | Microwave-assisted extraction (MAE) | obtained biofilm prepared by pectin-cocoa bean shell extract-ZnO/Zn nanoparticle showed greater UV and oxygen barrier properties | [19] |
| Anthocyanin | MAE, particle size (60 mesh), sample to solvent ratio (0.0625 w/v), extraction time (10 min), and microwave power (450 W) | 1.435 mM | [20] |
| β-sitosterol | MAE, absolute ethanol, 70 °C, 500 W, and 10 min | 3546.1 mg/100 g | [21] |
| Flavonoids | Ultrasound-assisted extraction under 80% ethanol, 55 °C, for 45 min | TFC = 7.47 mg RE/g dw | [22] |
| Protein, polysaccharide, and polyphenols | MAE, 5 min of extraction time, pH of 12, 97 °C of temperature, and sample to solvent ratio of 0.04 g/L | Pectin-based films | [19] |
| Fat and methylxanthines (theobromine and caffeine) | Supercritical CO2, 6000 psi, 313 K, 90 min | 94.73% (which is most effective extraction), while for caffeine the extraction yield is about 90% | [23] |
| Dietary fiber | High-voltage electric discharge | Increased fiber content | [24] |
| Polyphenols and methylxanthines | Subcritical water extraction, temperature 170 °C, time 75 min, sample to solvent ratio 1:20 | theobromine, caffeine, theophylline, gallic acid, epicatechin, catechin, chlorogenic acid, and total phenols | [25] |
| Polyphenols and methylxanthines | Subcritical water extraction, 150 °C with extraction pressure of 30 bar for 15 min | that whey protein protects the phenolic content resulted in higher content of gallic acid, caffeine, and theobromine as compared to maltodextrin | [26] |
| Alkaloids | MAE was performed using DES | Theobromine (2.502–5.004 mg/g) and caffeine (0.778–1.599 mg/g) | [27] |
| Dietary fiber, polyphenolic compounds, and methylxanthine | Particle sizes were considered, i.e., high (Dp > 701 um), intermediate (417 um < Dp < 701 um) and lowest (Dp < 417 um) | Dietary fiber (65.58 g/100 g), polyphenolic compounds (epicatechin, 6.33 mg/g; catechin, 4.58 mg/g), and methylxanthine (theobromine, 12.77 mg/g; caffeine, 6.13 mg/g) | [28] |
| Phenolics | Combined effect of supercritical fluid extraction and pressurized liquid extraction | TPC values from 35 to 51 mg GAE/g and EC50 values from 115 to 177 µg/mL | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belwal, T.; Cravotto, C.; Ramola, S.; Thakur, M.; Chemat, F.; Cravotto, G. Bioactive Compounds from Cocoa Husk: Extraction, Analysis and Applications in Food Production Chain. Foods 2022, 11, 798. https://doi.org/10.3390/foods11060798
Belwal T, Cravotto C, Ramola S, Thakur M, Chemat F, Cravotto G. Bioactive Compounds from Cocoa Husk: Extraction, Analysis and Applications in Food Production Chain. Foods. 2022; 11(6):798. https://doi.org/10.3390/foods11060798
Chicago/Turabian StyleBelwal, Tarun, Christian Cravotto, Sudipta Ramola, Monika Thakur, Farid Chemat, and Giancarlo Cravotto. 2022. "Bioactive Compounds from Cocoa Husk: Extraction, Analysis and Applications in Food Production Chain" Foods 11, no. 6: 798. https://doi.org/10.3390/foods11060798









