Innovative Process Coupling Short Steam Blanching with Vacuum Flash-Expansion Produces in One Single Stage High-Quality Purple Passion Fruit Smoothies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vegetal Material
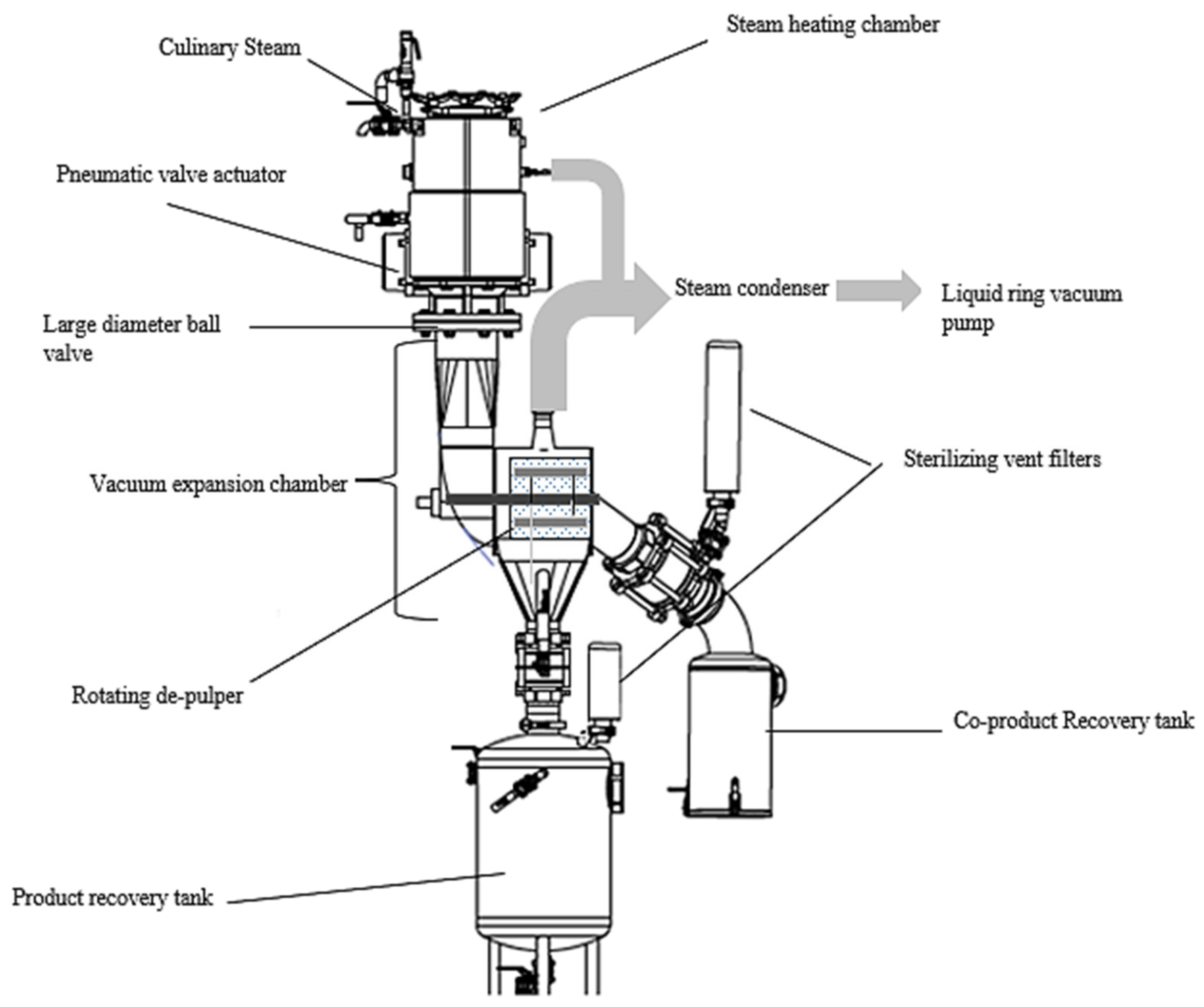
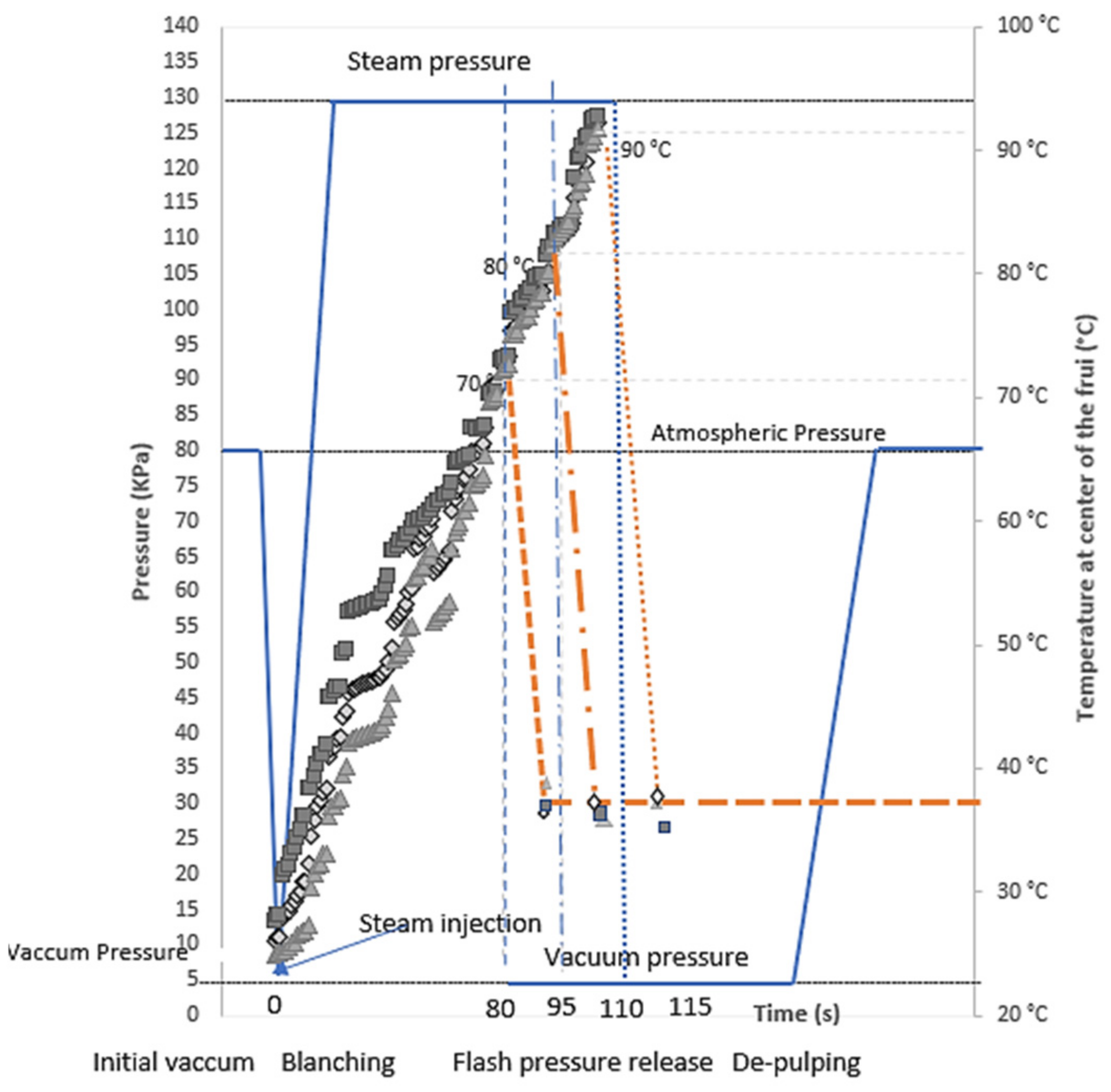
2.2. Flash-Vacuum Expansion Process (FVE)
2.3. Physicochemical Characterization
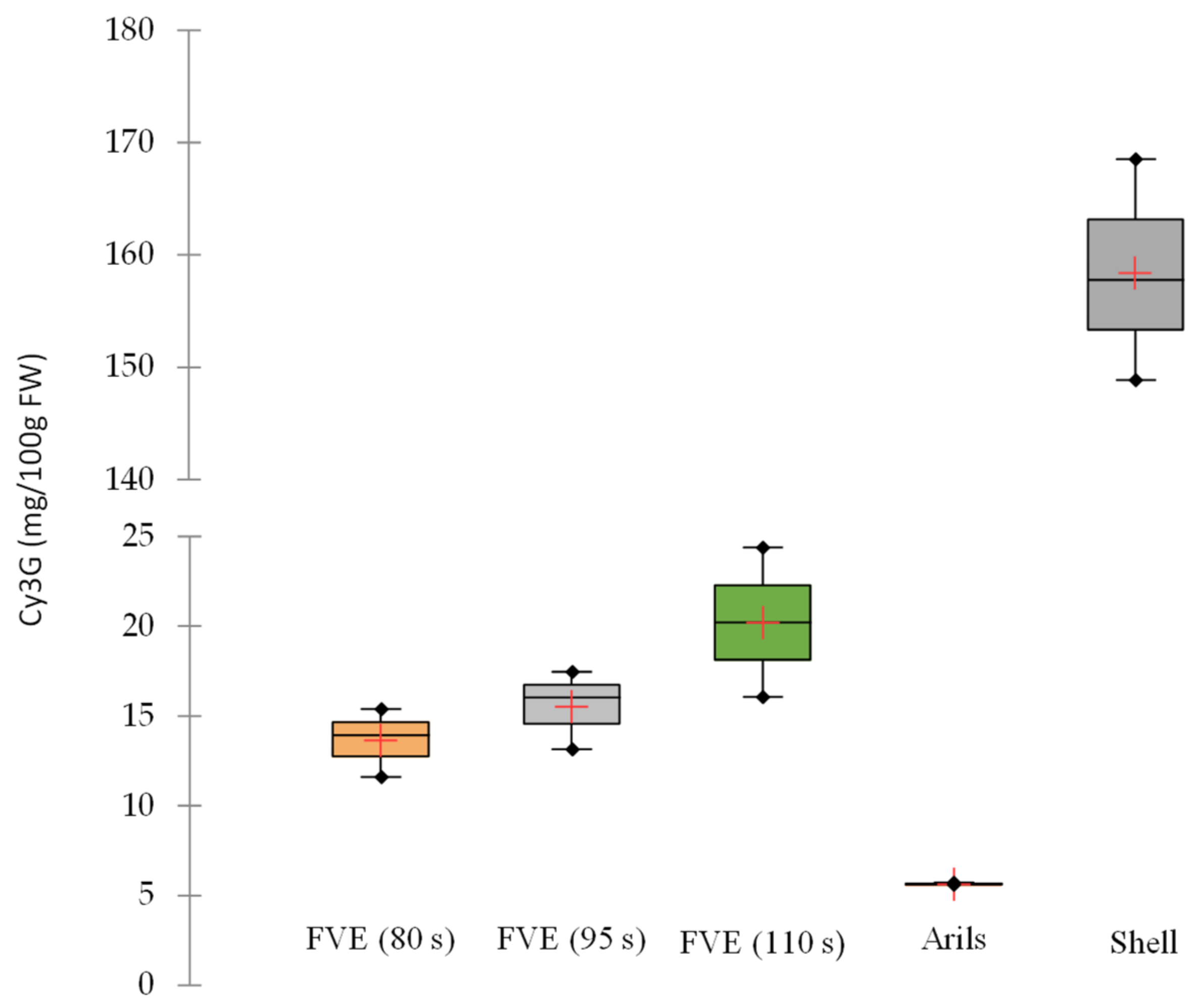
2.4. Determination of Anthocyanins
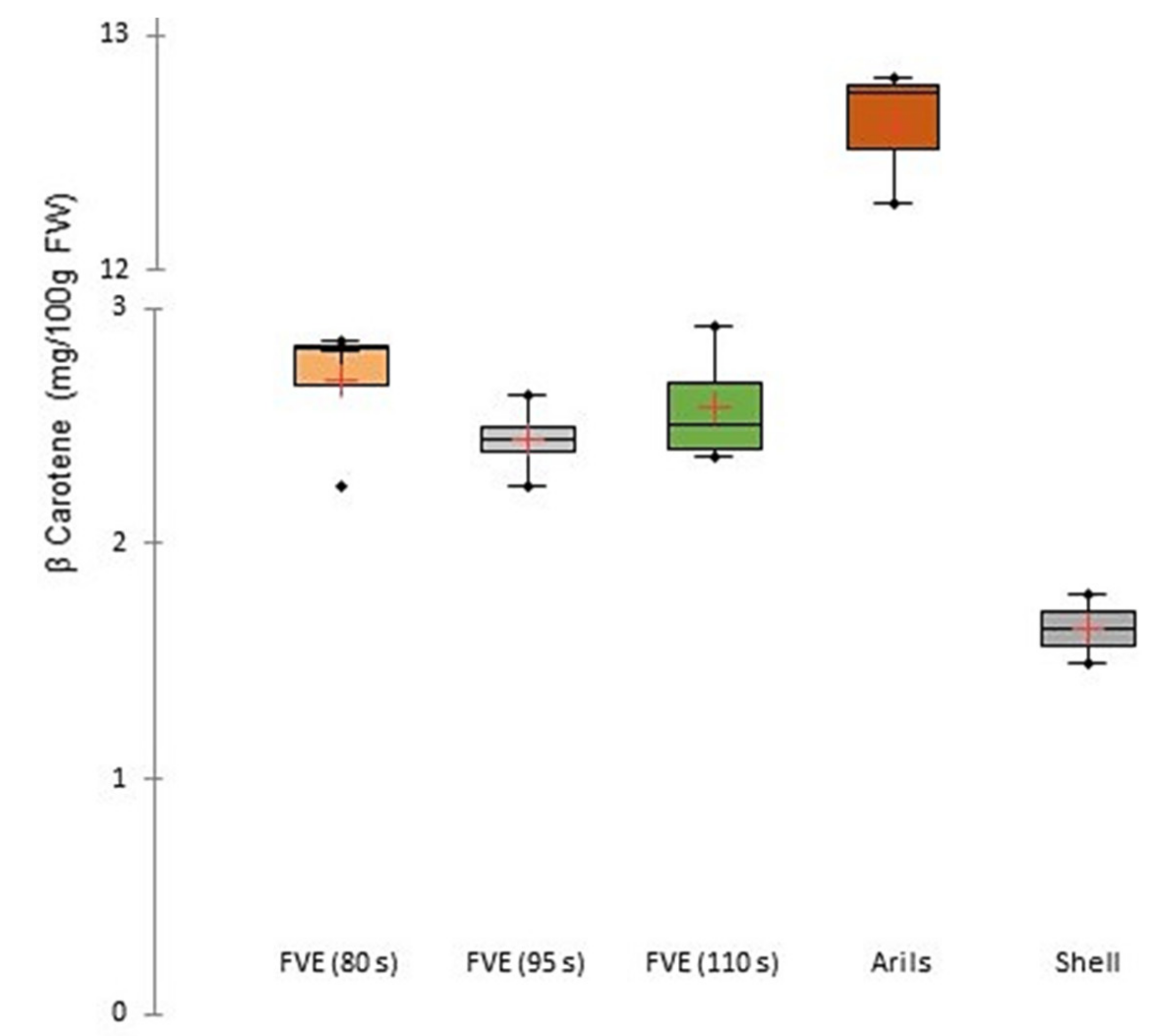
2.5. Determination of β-Carotene
2.6. Microbiological Analyses and Shelf Life
2.7. Rheology
2.8. Sensory Analysis
2.9. Statical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Fruit Purée
3.2. Rheology of the Sieved Fruit Purée Obtained
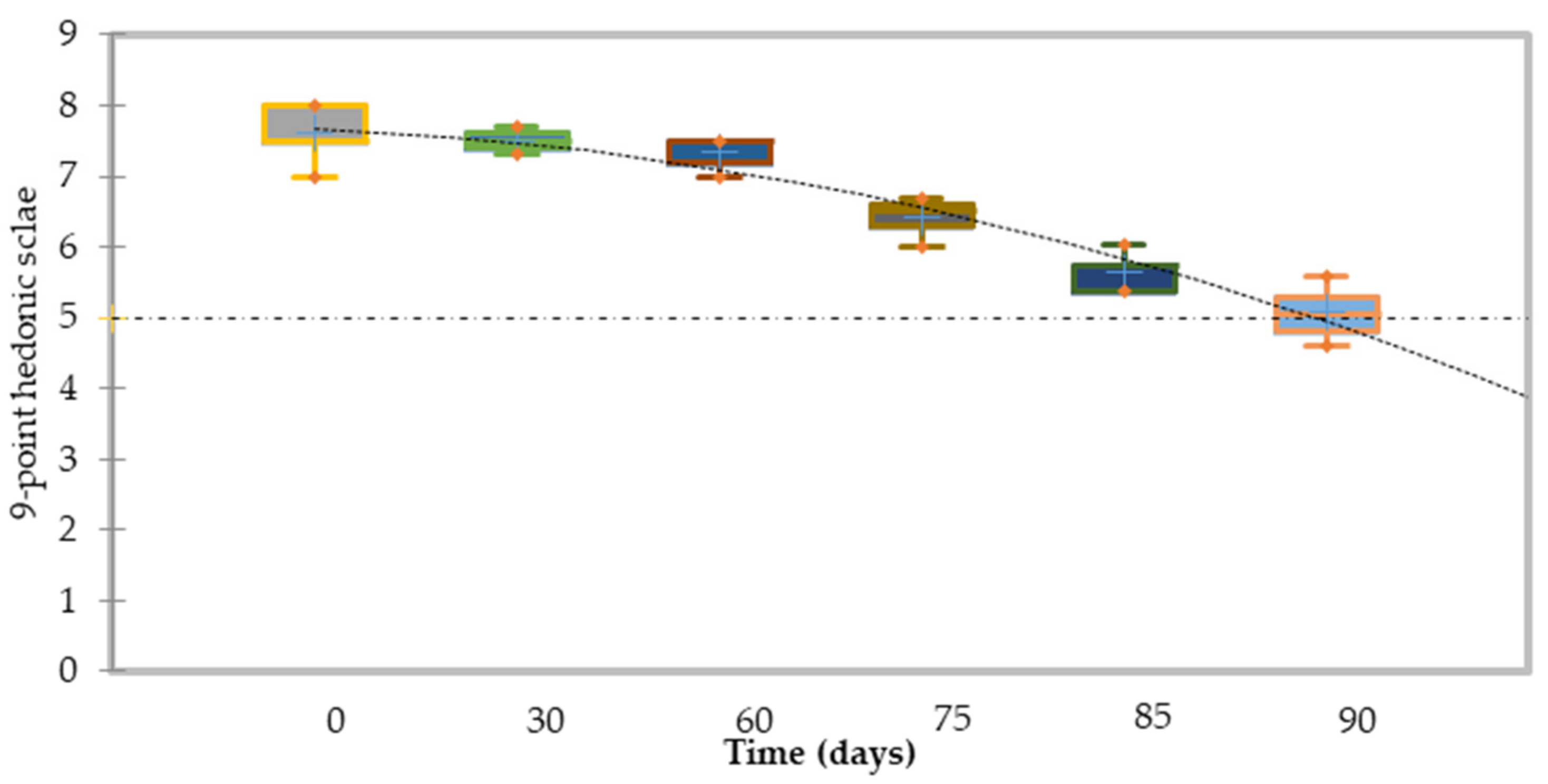
3.3. Microbiological Quality and Shelf-Life Stability
3.4. Considerations on Energy Saving and Investment Costs
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medina, S.; Collado-González, J.; Ferreres, F.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Guy, A.; Durand, T.; Galano, J.M.; Gil-Izquierdo, A. Quantification of phytoprostanes-bioactive oxylipins-and phenolic compounds of Passiflora edulis Sims shell using UHPLC-QqQ-MS/MS and LC-IT-DAD-MS/MS. Food Chem. 2017, 229, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, L.C.; Facco, E.M.; Salvador, M.; Flôres, S.H.; de Oliveira Rios, A. Antioxidant potential and physicochemical characterization of yellow, purple and orange passion fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Sonar, C.R.; Patel, J.; Albahr, Z.; Sablani, S.S. High pressure-assisted thermal sterilization of low-acid fruit and vegetable purees: Microbial safety, nutrient, quality, and packaging evaluation. Food Control 2020, 114, 107233. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Li, J.; Kang, J.; Zhang, Q. Application and research of new energy-efficiency technology for liquid ring vacuum pump based on turbulent drag reduction theory. Vacuum 2019, 172, 109076. [Google Scholar] [CrossRef]
- Téllez-Pérez, C.; Alonzo-Macías, M.; Mounir, S.; Besombes, C.; Allaf, T.; Amami, E.; Allaf, K. Instant Controlled Pressure-Drop DIC as a Strategic Technology for Different Types of Natural Functional Foods. In Funcional Food; Lagouri, V., Ed.; IntechOpen: London, UK, 2019; pp. 109–132. [Google Scholar]
- Allaf, T.; Allaf, K. Instant Controlled Pressure Drop (D.I.C.) in Food Processing. From Fundamental to Industrial Applications; Springer: New York, NY, USA, 2014; pp. 3–57. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Brito, B.; Rodríguez, M.; Samaniego, I.; Jaramillo, M.I.; Vaillant, F.E. Characterising polysaccharides in cherimoya (Annona cherimola Mill.) purée and their enzymatic liquefaction. Eur. Food Res. Technol. 2008, 226, 355–361. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Kroon, P.A.; Alasalvar, C.; Aaby, K.; Heinonen, M.; Voorspoels, S.; Tomás-Barberán, F. A validated method for the characterization and quantification of extractable and nonextractable ellagitannins after acid hydrolysis in pomegranate fruits, juices, and extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef]
- Mertz, C.; Cheynier, V.; Günata, Z.; Brat, P. Analysis of phenolic compounds in two blackberry species (Rubus glaucus and Rubus adenotrichus) by high-performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. [Google Scholar] [CrossRef]
- Franco, G.; Cartagena, J.R.; Correa, G.; Rojano, B.; Piedrahita, A.M. Antioxidant activity of passiflora edulis sims (Purple passion fruit) juice in the postharvest period. Rev. Cubana. Plantas Med. 2014, 19, 154–166. [Google Scholar]
- Zhu, B.; Liu, X.; Xie, C.; Liu, W.; Tang, C.; Lu, L. The flow behavior in as-extruded AZ31 magnesium alloy under impact loading. J. Magnes. Alloys 2018, 6, 180–188. [Google Scholar] [CrossRef]
- Mu, B.; Xu, H.; Li, W.; Xu, L.; Yang, Y. Spinnability and rheological properties of globular soy protein solution. Food Hydrocoll. 2019, 90, 443–451. [Google Scholar] [CrossRef]
- ISO 4121; Sensory Analysis—Guidelines for the Use of Quantitative Response Scales. 2nd ed. Technical Committee, ISO/TC 34/SC 12 Sensory Analysis. ISO: Geneva, Switzerland, 2003.
- ISO 8589:2007/AMD 1; Sensory Analysis—General Guidance for the Design of Test Rooms-Amendment 1. 2nd ed. Technical Committee, ISO/TC 34/SC 12 Sensory Analysis. ISO: Geneva, Switzerland, 2014.
- Brat, P.; Olle, D.; Reynes, M.; Cogat, P.O.; Brillouet, J.M. Preparation of passion fruit puree by flash vacuum-expansion. J. Food Sci. 2001, 66, 542–547. [Google Scholar] [CrossRef]
- Ghada, B.; Pereira, E.; Pinela, J.; Prieto, M.A.; Pereira, C.; Calhelha, R.C.; Stojkovic, D.; Sokóvic, M.; Zaghdoudi, K.; Barros, L.; et al. Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties. Molecules 2020, 25, 3203. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.M.; Sierra, C.A.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; Heredia, F.J.; Osorio, C. Physicochemical characterisation of gulupa (Passiflora edulis Sims. fo edulis) fruit from Colombia during the ripening. Food Res. Int. 2011, 44, 1912–1918. [Google Scholar] [CrossRef]
- Shi, M.; Ali, M.M.; He, Y.; Ma, S.; Rizwan, H.M.; Yang, Q.; Li, B.; Lin, Z.; Chen, F. Flavonoids accumulation in fruit peel and expression profiling of related genes in purple (Passiflora edulis f. edulis) and yellow (passiflora edulis f. flavicarpa) passion fruits. Plants 2021, 10, 2240. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Palma, A.C.; Chávez-Marín, J.; Oliveros-Gómez, D.F.; Murillo-Perea, E.; Méndez-Arteaga, J.J. Funcionalidades biológicas de Passiflora maliformis del sur macizo colombiano. Bioagro 2016, 28, 3–12. [Google Scholar]
- Vargas-Ortiz, M.; Rodríguez-Jimenes, G.; Salgado-Cervantes, M.; Pallet, D. Minimally Processed Avocado Through Flash Vacuum-Expansion: Its Effect in Major Physicochemical Aspects of the Puree and Stability on Storage. J. Food Process. Preserv. 2017, 41, e12988. [Google Scholar] [CrossRef]
- Martínez-Meza, Y.; Pérez-Jiménez, J.; Rocha-Guzmán, N.E.; Rodríguez-García, M.E.; Alonzo-Macías, M.; Reynoso-Camacho, R. Modification on the polyphenols and dietary fiber content of grape pomace by instant controlled pressure drop. Food Chem. 2021, 360, 130035. [Google Scholar] [CrossRef]
- Soukoulis, C.; Fisk, I.D.; Bohn, T. Ice cream as a vehicle for incorporating health-promoting ingredients: Conceptualization and overview of quality and storage stability. Compr. Rev. Food Sci. Food Saf. 2014, 13, 627–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, C.M.; Chan, H.T.; Nakayama, T.O.; Brekke, J.E. Passion Fruit Starch and Effect on Juice Viscosity. J. Food Sci. 1974, 39, 431–433. [Google Scholar] [CrossRef]
- Hamoud-Agha, M.M.; Allaf, K. Instant Controlled Pressure Drop (DIC) Technology in Food Preservation: Fundamental and Industrial Applications. In Food Preservation and Waste Exploitation; Socaci, S.A., Fărcaş, A.C., Laguerre, J.C., Aussenac, T., Eds.; IntechOpen: London, UK, 2020; pp. 5–24. [Google Scholar]
- Miladi, R.; Frikha, N.; Gabsi, S. Modeling and energy analysis of a solar thermal vacuum membrane distillation coupled with a liquid ring vacuum pump. Renew. Energy 2020, 164, 1395–1407. [Google Scholar] [CrossRef]

| Parameters 1 | Arils | Shell | Blanching Holding Time (Seconds) | ||
|---|---|---|---|---|---|
| 80 s | 95 s | 110 s | |||
| Yield (% w/w) | 27.401 ± 4.539 a | - | 46.205 ± 4.359 b | 47.656 ± 2.893 b | 47.295 ± 5.954 b |
| L* | 68.823 ± 0.116 b | 28.967 ± 0.373 a | 24.857 ± 1.979 a | 25.155 ± 0.838 a | 23.826 ± 2.236 a |
| a* | 15.180 ± 0.098 b | 6.900 ± 0.105 a | 33.838 ± 2.189 c | 33.323 ± 3.106 c | 30.630 ± 5.006 c |
| b* | 50.693 ± 0.144 c | 3.020 ± 0.234 a | 24.153 ± 3.938 b | 23.673 ± 4.999 b | 21.638 ± 5.663 b |
| SS 2 (gxL−1) | 15.667 ± 0.208 b | - | 10.125 ± 2.016 a | 10.875 ± 2.529 a | 10.425 ± 1.338 a |
| Acidity (% citric acid) | 3.025 ± 0.076 c | - | 2.483 ± 0.046 b | 1.624 ± 0.201 a | 1.690 ± 0.110 a |
| pH | 3.137 ± 0.047 a | - | 3.183 ± 0.128 a | 3.108 ± 0.061 a | 3.151 ± 0.044 a |
| SIS 3 (g/100 g FW) | 25.686 ± 1.508 a | - | 38.065 ± 1.687 b | 100 ± 0.000 c | 100 ± 0.000 c |
| AIR 4 (g/100 g FW) | 2.432 ± 0.380 a | - | 2.205 ± 0.075 b | 4.293 ± 0.054 c | 5.486 ± 0.078 d |
| WAIR 5 (g/100 g AIR) | 54.927 ± 1.514 a | - | 78.370 ± 0.253 b | 30.076 ± 5.774 c | 14.477 ± 1.612 d |
| Sample | n (-) | K (mPa·sn) | r2 | Viscosity to σ: 50 s−1 (mPa·s) |
|---|---|---|---|---|
| Juice arils | 0.692 ± 0.005 a | 0.057 ± 0.006 a | 0.994 ± 0.001 | 17.012 ± 1.409 a |
| 80 s only blanching | 0.874 ± 0.048 c | 0.003 ± 0.000 a | 0.960 ± 0.030 | 1.869 ± 0.345 a |
| 95 s only blanching | 0.734 ± 0.068 ab | 0.145 ± 0.004 a | 0.973 ± 0.018 | 51.859 ± 12.167 a |
| 110 s only blanching | 0.707 ± 0.068 ab | 1.560 ± 0.004 c | 0.963 ± 0.018 | 495.931 ± 12.167 c |
| 80 s with flash-expansion | 0.767 ± 0.019 b | 0.168 ± 0.074 ab | 0.981 ± 0.003 | 68.734 ± 34.975 ab |
| 95 s with flash-expansion | 0.720 ± 0.044 ab | 0.477 ± 0.053 b | 0.976 ± 0.002 | 158.934 ± 9.936 b |
| 110 s with flash-expansion | 0.581 ± 0.002 d | 10.698 ± 0.114 d | 0.926 ± 0.001 | 2078.199 ± 6.668 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, C.; Rodríguez, P.; Cortés, M.; Soto, I.; Quintero, J.; Vaillant, F. Innovative Process Coupling Short Steam Blanching with Vacuum Flash-Expansion Produces in One Single Stage High-Quality Purple Passion Fruit Smoothies. Foods 2022, 11, 832. https://doi.org/10.3390/foods11060832
Arias C, Rodríguez P, Cortés M, Soto I, Quintero J, Vaillant F. Innovative Process Coupling Short Steam Blanching with Vacuum Flash-Expansion Produces in One Single Stage High-Quality Purple Passion Fruit Smoothies. Foods. 2022; 11(6):832. https://doi.org/10.3390/foods11060832
Chicago/Turabian StyleArias, Claudia, Pablo Rodríguez, Misael Cortés, Iris Soto, Julián Quintero, and Fabrice Vaillant. 2022. "Innovative Process Coupling Short Steam Blanching with Vacuum Flash-Expansion Produces in One Single Stage High-Quality Purple Passion Fruit Smoothies" Foods 11, no. 6: 832. https://doi.org/10.3390/foods11060832







