Effects of Tea Powder on the Cooking Properties, Antioxidative Potential and Volatile Profiles of Dried Noodles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Composition of Tea Powder and Wheat Flour
2.3. Dried Tea Noodle Production
2.4. Cooking Properties
2.5. Color Analysis
2.6. Antioxidant Activity
2.7. Determination of Volatile Compounds
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of Tea Powder and Wheat Flour
3.2. Cooking Properties
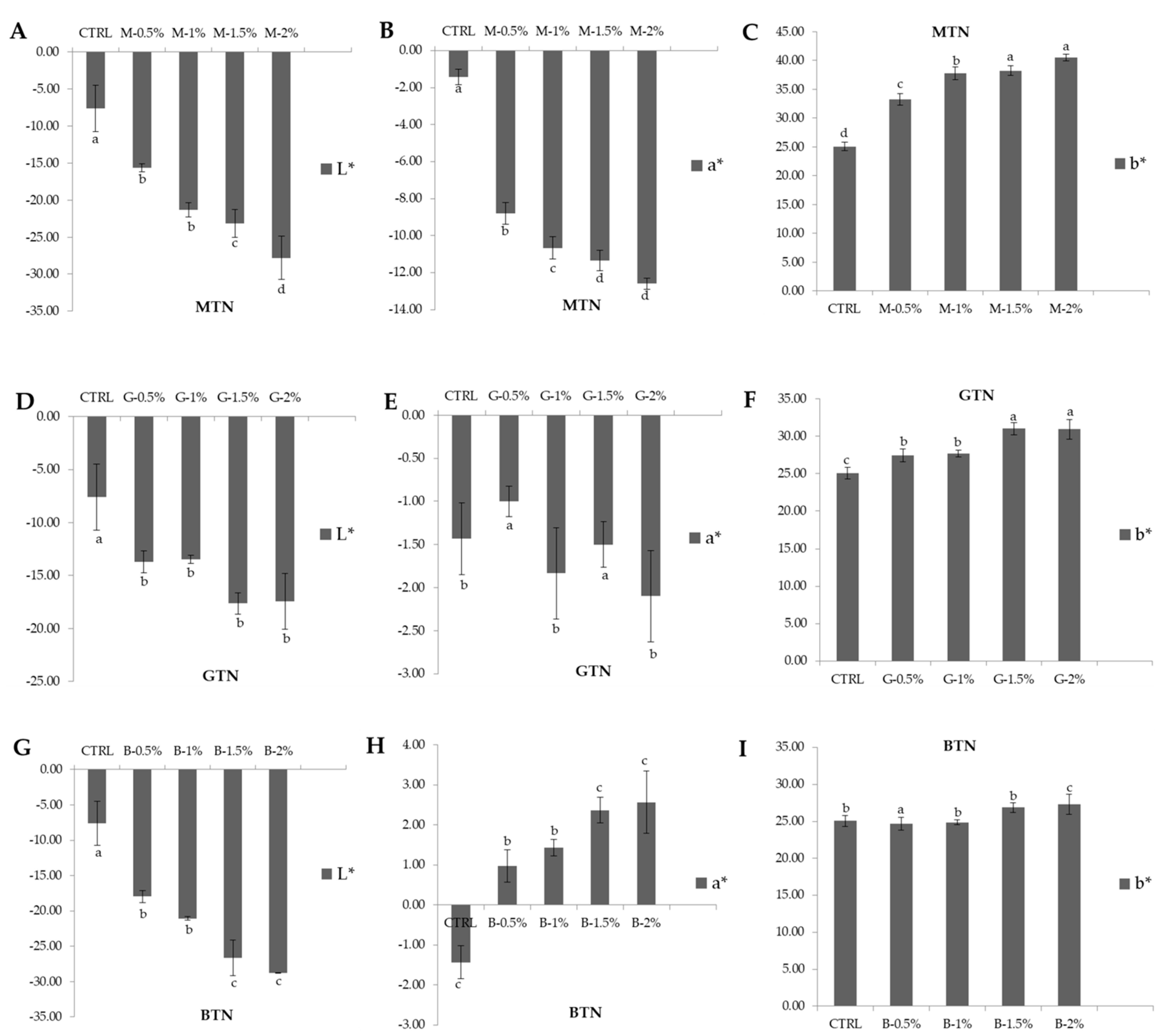
3.3. Color Analysis
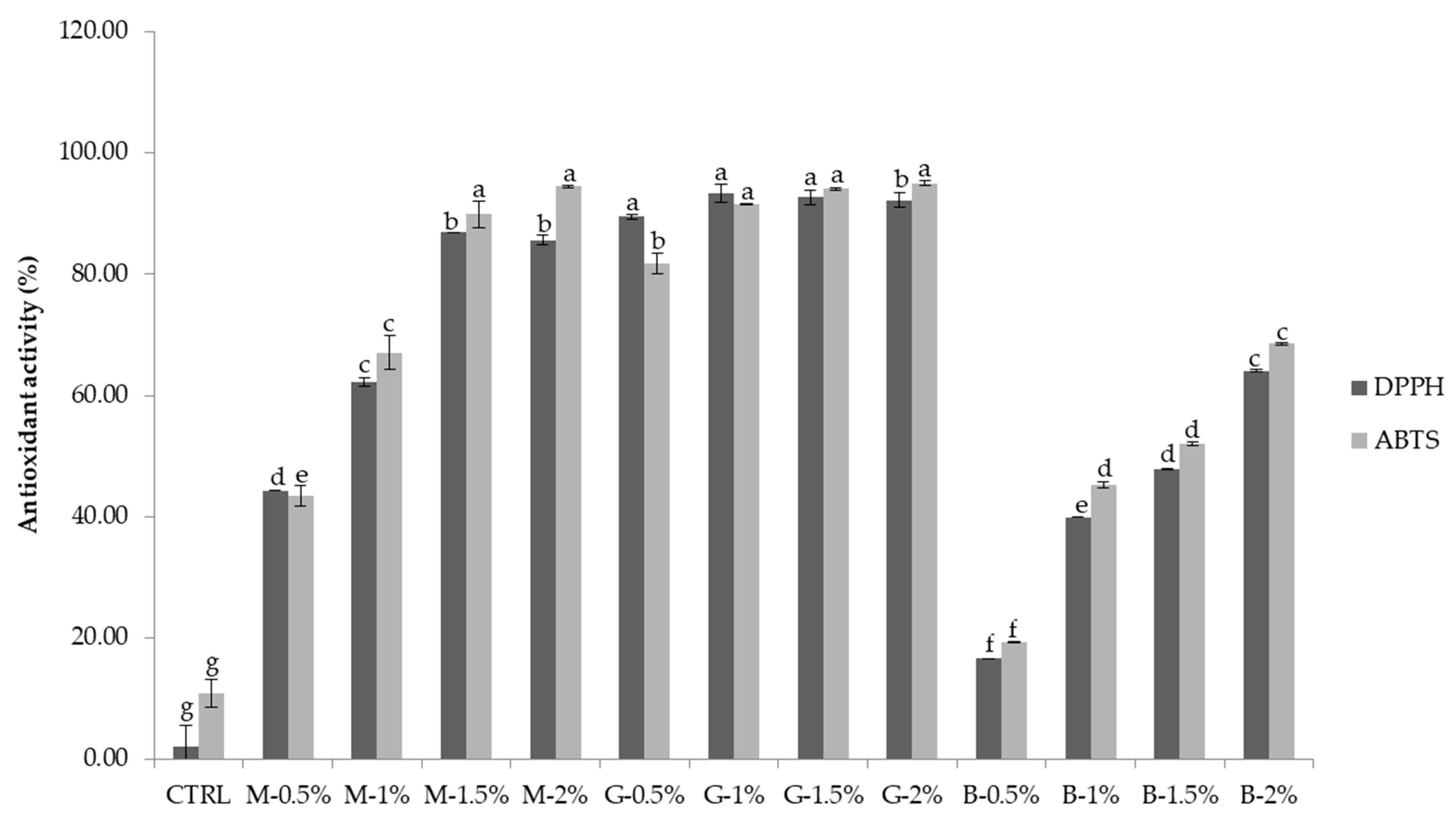
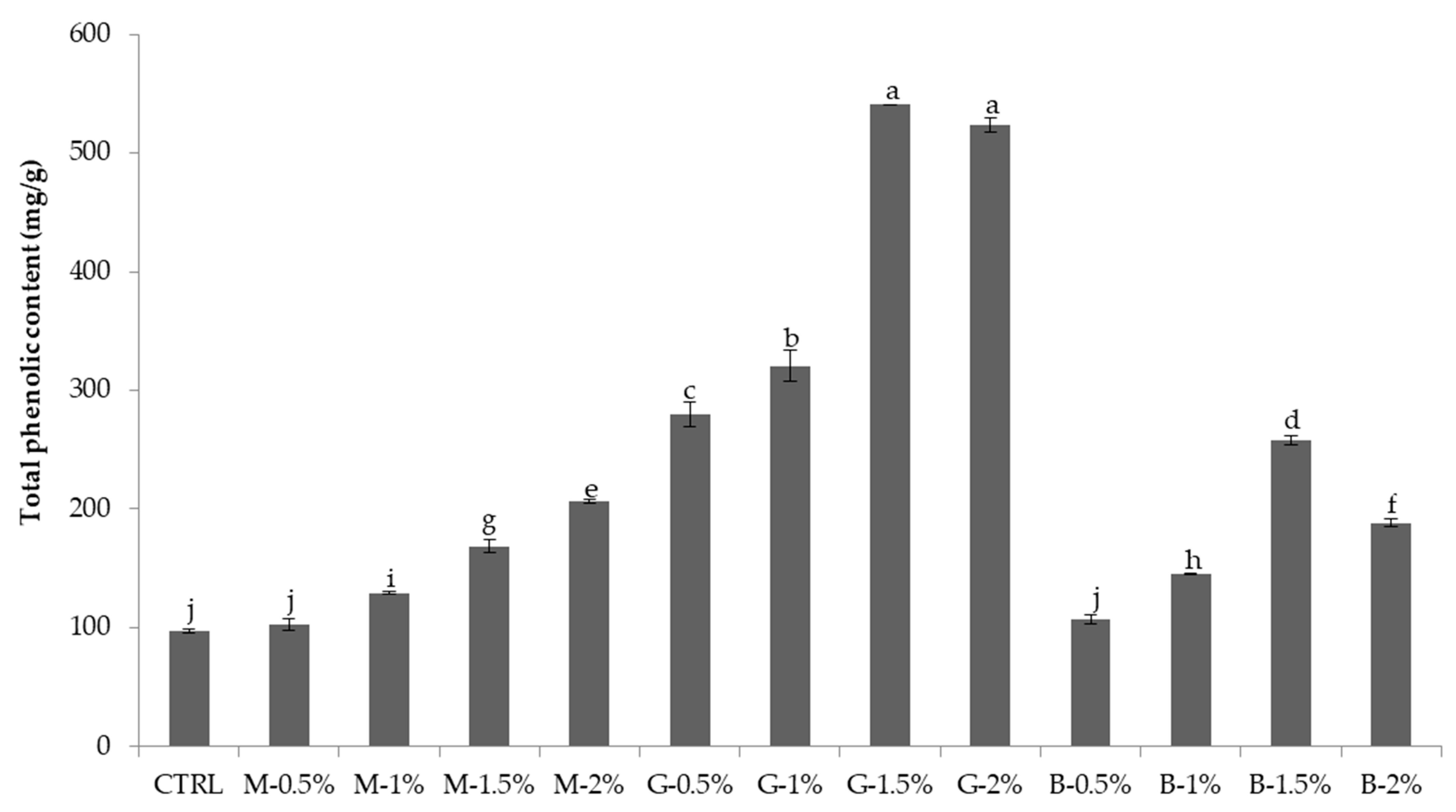
3.4. Antioxidant Activity
3.5. Determination of Volatile Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrivastava, R.R.S.R.R.; Pateriya, P.P.P.; Singh, M.S.M. Green tea-A short review. Int. J. Indig. Herbs Drugs 2018, 3, 12–21. [Google Scholar]
- Ruxton, C. Black tea and health. Nutr. Bull. 2008, 33, 91–101. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Ramirez-Mares, M.V.; Puangpraphant, S. Bioactive components of tea: Cancer, inflammation and behavior. Brain Behav. Immun. 2009, 23, 721–731. [Google Scholar] [CrossRef]
- Yang, T.; Koo, M. Inhibitory effect of Chinese green tea on endothelial cell-induced LDL oxidation. Atherosclerosis 2000, 148, 67–73. [Google Scholar] [CrossRef]
- Kumar, R.S.S.; Murugesan, S.; Kottur, G.; Gyamfi, D. Black tea: The plants, processing/manufacturing and production. Tea Health Dis. Prev. 2013, 5, 41–57. [Google Scholar]
- Butt, M.S.; Imran, A.; Sharif, M.K.; Ahmad, R.S.; Xiao, H.; Imran, M.H.A.R.; Rsool, H.A. Black tea polyphenols: A mechanistic treatise. Crit. Rev. Food Sci. Nutr. 2014, 54, 1002–1011. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, H.; Xu, P.; Yao, L.; Xie, Q.; Mao, L.; Wang, Y. Matcha green tea prevents obesity-induced hypothalamic inflammation via suppressing the JAK2/STAT3 signaling pathway. Food Funct. 2020, 11, 8987–8995. [Google Scholar] [CrossRef]
- Hou, G.; Kruk, M.; Center, W.M. Asian noodle technology. Tech. Bull. 1998, 20, 1–10. [Google Scholar]
- Marconi, E.; Carcea, M. Pasta from nontraditional raw materials. Cereal Foods World 2001, 46, 522–530. [Google Scholar]
- Xu, M.; Wu, Y.; Hou, G.G.; Du, X. Evaluation of different tea extracts on dough, textural, and functional properties of dry Chinese white salted noodle. LWT 2019, 101, 456–462. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, H.M.; Zhu, K.X.; Guo, X.N.; Peng, W. Water cooking stability of dried noodles enriched with different particle size and concentration green tea powders. Foods 2020, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Ying, L.; Kong, D.D.; Gao, Y.Y.; Yan, F.; Wang, Y.F.; Xu, P. In vitro antioxidant activity of phenolic-enriched extracts from Zhangping Narcissus tea cake and their inhibition on growth and metastatic capacity of 4T1 murine breast cancer cells. J. Zhejiang Univ.-SCIENCE B 2018, 19, 199–210. [Google Scholar] [CrossRef]
- Xu, P.; Chen, L.; Wang, Y. Effect of storage time on antioxidant activity and inhibition on α-Amylase and α-Glucosidase of white tea. Food Sci. Nutr. 2019, 7, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Morris, D.L. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Parvin, R.; Farzana, T.; Mohajan, S.; Rahman, H.; Rahman, S.S. Quality improvement of noodles with mushroom fortified and its comparison with local branded noodles. NFS J. 2020, 20, 37–42. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the Association of Cereal Chemists, 10th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Sirichokworrakit, S.; Phetkhut, J.; Khommoon, A. Effect of partial substitution of wheat flour with riceberry flour on quality of noodles. Procedia-Soc. Behav. Sci. 2015, 197, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Kim, J.H.; Jeong, S.M.; Kim, D.R.; Ha, J.U.; Nam, K.C.; Ahn, D.U. Effect of far-infrared radiation on the antioxidant activity of rice hulls. J. Agric. Food Chem. 2003, 5, 4400–4403. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Antoniewska, A.; Rutkowska, J.; Pineda, M.M. Antioxidative, sensory and volatile profiles of cookies enriched with freeze-dried Japanese quince (Chaenomeles japonica) fruits. Food Chem. 2019, 286, 376–387. [Google Scholar] [CrossRef] [Green Version]
- Nishitani, E.; Sagesaka, Y.M. Simultaneous determination of catechins, caffeine and other phenolic compounds in tea using new HPLC method. J. Food Compos. Anal. 2004, 17, 675–685. [Google Scholar] [CrossRef]
- Lin, J.K.; Lin, C.L.; Liang, Y.C.; Lin-Shiau, S.Y.; Juan, I.M. Survey of catechins, gallic acid, and methylxanthines in green, oolong, pu-erh, and black teas. J. Agric. Food Chem. 1998, 46, 3635–3642. [Google Scholar] [CrossRef]
- Unno, K.; Furushima, D.; Hamamoto, S.; Iguchi, K.; Yamada, H.; Morita, A.; Pervin, M.; Nakamura, Y. Stress-reducing effect of cookies containing matcha green tea: Essential ratio among theanine, arginine, caffeine and epigallocatechin gallate. Heliyon 2019, 5, e01653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.S.; Lee, C.H. Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J. Agric. Food Chem. 2010, 58, 418–426. [Google Scholar] [CrossRef]
- Finnie, S.; Atwell, W.A. Wheat Flour, 2nd ed.; AACC International, Inc.: St. Paul, MN, USA, 2016. [Google Scholar]
- Rekha, M.N.; Chauhan, A.S.; Prabhasankar, P.; Ramteke, R.S.; Rao, G.V. Influence of vegetable purees on quality attributes of pastas made from bread wheat (T. aestivum). CyTA-J. Food 2013, 11, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Chillo, S.; Laverse, J.; Falcone, P.M.; Protopapa, A.; Del Nobile, M.A. Influence of the addition of buckwheat flour and durum wheat bran on spaghetti quality. J. Cereal Sci. 2008, 47, 144–152. [Google Scholar] [CrossRef]
- Petitot, M.; Boyer, L.; Minier, C.; Micard, V. Fortification of pasta with split pea and faba bean flours: Pasta processing and quality evaluation. Food Res. Int. 2010, 43, 634–641. [Google Scholar] [CrossRef]
- Kushtova, V.; Kohajdova, Z.; Karovicova, J.; Mesterova, E. Use of pumpkin fiber for the preparation of pasta. Chem. Listy 2016, 110, 808–811. [Google Scholar]
- Padalino, L.; Conte, A.; Lecce, L.; Likyova, D.; Sicari, V.; Pellicanò, T.M.; Poiana, M.; Del Nobile, M.A. Functional pasta with tomato by-product as a source of antioxidant compounds and dietary fibre. Czech J. Food Sci. 2017, 35, 48–56. [Google Scholar]
- Ahmad, M.; Baba, W.N.; Wani, T.A.; Gani, A.; Gani, A.; Shah, U.; Wani, S.M.; Masoodi, F.A. Effect of green tea powder on thermal, rheological & functional properties of wheat flour and physical, nutraceutical & sensory analysis of cookies. J. Food Sci. Technol. 2015, 52, 5799–5807. [Google Scholar]
- Takata, K.; Yanaka, M.; Fujita, Y.; Ishikawa, N. Evaluation of the grain and flour quality in near-isogenic wheat lines with waxy and double-null Wx proteins. Breed. Sci. 2007, 57, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Grassi, D.; Desideri, G.; Di Giosia, P.; De Feo, M.; Fellini, E.; Cheli, P.; Ferri, L.; Ferri, C. Tea, flavonoids, and cardiovascular health: Endothelial protection. Am. J. Clin. Nutr. 2013, 98, 1660S–1666S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butkhup, L.; Jeenphakdee, M.; Jorjong, S.; Samappito, S.; Samappito, W.; Chowtivannakul, S. HS-SPME-GC-MS analysis of volatile aromatic compounds in alcohol related beverages made with mulberry fruits. Food Sci. Biotechnol. 2011, 20, 1021–1032. [Google Scholar] [CrossRef]
- Agca, A.C.; Vural, N.; Sarer, E. Determination of volatile compounds in green tea and black tea from Turkey by using HS-SPME and GC-MS. J. Fac. Pharm. Istanb. Univ. 2020, 50, 111–116. [Google Scholar]
- Lee, J.; Chambers, D.H.; Chambers, E.; Adhikari, K.; Yoon, Y. Volatile aroma compounds in various brewed green teas. Molecules 2013, 18, 10024–10041. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Yin, H.X.; Yuan, H.B.; Jiang, Y.W.; Dong, C.W.; Deng, Y.L. Characterization of the volatile components in green tea by IRAE-HS-SPME/GC-MS combined with multivariate analysis. PLoS ONE 2018, 13, e0193393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Liang, S.; Zheng, Y.; Zhang, M. Volatile Compounds of Different Fresh Wet Noodle Cultivars Evaluated by Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry. An. Acad. Bras. Ciências 2020, 92, e20190063. [Google Scholar] [CrossRef]
- Min, D.B.; Lee, H.O. Flavor Chem; Springer: Boston, MA, USA, 1999; pp. 175–187. [Google Scholar]
- Sayaslan, A.; Chung, O.K.; Seib, P.A.; Seitz, L.M. Volatile compounds in five starches. Cereal Chem. 2000, 77, 248–253. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Enguix, L.; Verdú, A.; García-García, E.; Carbonell-Barrachina, A.A. Investigation of aromatic compounds in toasted almonds used for the manufacture of turrón. Eur. Food Res. Technol. 2008, 227, 243–254. [Google Scholar] [CrossRef]
- Chen, Y.J.; Kuo, P.C.; Yang, M.L.; Li, F.Y.; Tzen, J.T. Effects of baking and aging on the changes of phenolic and volatile compounds in the preparation of old Tieguanyin oolong teas. Food Res. Int. 2013, 53, 732–743. [Google Scholar] [CrossRef]
| MTP | GTP | BTP | |
|---|---|---|---|
| Amino acids (%) | 9.30 ± 0.22 a | 2.40 ± 0.35 b | 1.50 ± 0.06 b |
| Tea polyphenols (%) | 10.45 ± 1.35 b | 14.10 ± 0.58 a | 7.40 ± 0.32 c |
| Soluble sugar (%) | 1.75 ± 0.05 a | 1.98 ± 0.27 a | 1.99 ± 0.04 a |
| Caffeine (%) | 2.18 ± 0.13 a | 2.32 ± 0.54 a | 2.10 ± 0.07 a |
| Moisture (%) | 5.00 ± 0.02 a | 4.00 ± 0.19 b | 6.00 ± 1.03 a |
| Compounds (mg/g) | MTP | GTP | BTP |
|---|---|---|---|
| GC | 13.12 ± 0.32 c | 44.80 ± 0.79 b | 51.85 ± 0.24 a |
| EGC | 14.92 ± 0.24 a | 7.68 ± 0.02 b | 2.74 ± 0.01 c |
| C | 0.70 ± 0.01 b | 61.87 ± 0.33 a | 0.89 ± 0.00 b |
| EC | 3.60 ± 0.21 b | 27.30 ± 0.04 a | 1.61 ± 0.23 b |
| EGCG | 66.18 ± 0.12 b | 75.84 ± 0.05 a | 3.65 ± 0.46 c |
| GCG | 2.91 ± 0.06 b | 6.97 ± 0.23 a | 0.54 ± 0.11 c |
| ECG | 1.10 ± 0.09 b | 2.17 ± 0.05 a | 0.56 ± 0.02 c |
| CG | 10.75 ± 0.12 b | 58.86 ± 0.23 a | 1.70 ± 0.00 c |
| Moisture (%) | Ash (%) | Protein (%) | Gluten (%) | Fat (%) | |
|---|---|---|---|---|---|
| Wheat flour | 13.91 ± 0.01 | 0.50 ± 0.06 | 11.99 ± 0.06 | 30.24 ± 0.26 | 1.38 ± 0.07 |
| Sample | CT (min) | CL (%) | WAC (%) | MC (%) |
|---|---|---|---|---|
| CTRL | 12.75 ± 0.25 a | 7.00 ± 4.24 a | 146.50 ± 2.12 a | 10.14 ± 0.04 d |
| M-0.5% | 11.50 ± 0.50 a | 6.00 ± 5.65 a | 83.50 ± 16.26 e | 11.67 ± 0.05 c |
| M-1.0% | 11.25 ± 0.25 b | 6.00 ± 2.82 a | 105.25 ± 19.44 c | 11.77 ± 0.07 a |
| M-1.5% | 10.50 ± 0.00 b | 5.00 ± 4.24 a | 113.65 ± 16.05 a | 11.84 ± 0.02 b |
| M-2.0% | 10.25 ± 0.25 c | 6.00 ± 2.82 a | 138.70 ± 5.23 b | 11.85 ± 0.04 b |
| G-0.5% | 11.50 ± 0.00 b | 5.00 ± 1.41 a | 111.00 ± 1.41 d | 11.65 ± 0.04 c |
| G-1.0% | 11.00 ± 0.00 c | 6.00 ± 2.82 a | 120.00 ± 1.41 c | 11.75 ± 0.08 b |
| G-1.5% | 10.25 ± 0.25 d | 5.00 ± 1.41 a | 128.50 ± 2.12 a | 11.91 ± 0.01 a |
| G-2.0% | 9.25 ± 0.25 e | 6.00 ± 2.82 a | 141.50 ± 0.70 b | 11.96 ± 0.01 a |
| B-0.5% | 11.25 ± 0.25 c | 5.00 ± 4.24 a | 76.50 ± 2.12 f | 11.50 ± 0.07 c |
| B-1.0% | 10.75 ± 0.25 d | 5.00 ± 1.41 a | 102.00 ± 1.41 e | 11.64 ± 0.09 b |
| B-1.5% | 10.00 ± 0.00 e | 6.00 ± 2.82 a | 110.50 ± 2.12 b | 11.72 ± 0.09 c |
| B-2.0% | 9.75 ± 0.25 e | 5.00 ± 1.41 a | 135.00 ± 2.82 d | 11.95 ± 0.04 a |
| Volatile Compound (%) | Cc | DTN (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTRL | M-0.5% | M-1% | M-1.5% | M-2% | G-0.5% | G-1% | G-1.5% | G-2% | B-0.5% | B-1% | B-1.5% | B-2% | ||
| 1-heptanol | A | 0.95 ± 0.00 | 1.70 ± 0.52 | 1.41 ± 0.21 | 1.45 ± 0.21 | 1.69 ± 0.67 | 1.27 ± 0.16 | 1.35 ± 0.13 | 1.36 ± 0.13 | 1.17 ± 0.31 | 1.43 ± 0.63 | 1.01 ± 0.43 | 1.09 ± 0.14 | 1.93 ± 0.35 |
| Hexanol | A | 5.37 ± 0.03 | 6.67 ± 0.77 | 6.52 ± 0.79 | 7.48 ± 1.02 | 9.38 ± 2.35 | 8.05 ± 1.91 | 8.71 ± 0.81 | 7.65 ± 0.40 | 6.45 ± 0.04 | 9.40 ± 1.15 | 8.51 ± 0.58 | 7.70 ± 1.39 | 8.09 ± 0.16 |
| Isooctyl alcohol | A | 1.78 ± 0.56 | 1.66 ± 0.20 | 1.85 ± 0.07 | 1.66 ± 0.50 | 2.39 ± 0.09 | n.d | 1.76 ± 0.06 | 1.84 ± 0.03 | 1.66 ± 0.05 | 2.08 ± 0.03 | 1.36 ± 0.03 | 1.47 ± 0.09 | 2.10 ± 0.00 |
| 1-Octen-3-ol | A | 3.02 ± 0.01 | n.d | 11.09 ± 0.05 | 12.31 ± 1.41 | 8.91 ± 0.24 | 7.73 ± 1.76 | 13.26 ± 2.23 | 14.77 ± 0.02 | 17.74 ± 1.45 | 4.86 ± 0.40 | 3.49 ± 0.33 | 3.41 ± 0.59 | 4.73 ± 0.94 |
| 1-pentanol | A | 3.23 ± 1.50 | 3.86 ± 0.23 | 4.94 ± 0.64 | 4.27 ± 1.32 | 7.74 ± 1.35 | 3.97 ± 0.56 | 4.83 ± 1.05 | 4.24 ± 0.05 | 4.63 ± 0.02 | 4.00 ± 0.64 | 3.61 ± 0.63 | 2.95 ± 0.35 | 4.17 ± 1.09 |
| (Z)-3-hexen-1-ol | A | n.d | 1.84 ± 0.37 | 3.42 ± 0.21 | 4.46 ± 1.28 | 8.09 ± 1.78 | 1.46 ± 0.23 | 1.44 ± 0.04 | 1.27 ± 0.33 | 1.64 ± 0.12 | 1.50 ± 0.02 | 1.79 ± 0.01 | 2.29 ± 0.25 | 3.11 ± 0.39 |
| 3-Octen-2-ol, (Z)- | A | 1.09 ± 0.09 | n.d | 1.57 ± 0.29 | n.d | 1.98 ± 0.27 | 1.38 ± 0.56 | 1.48 ± 0.21 | 1.41 ± 0.02 | 1.21 ± 0.45 | 1.39 ± 0.67 | 1.13 ± 0.69 | n.d | 2.24 ± 0.70 |
| (18S,19S)-18,19-Dihydroxy-1,4,7,10,13,16-hexaoxocycloencosane | A | 1.21 ± 0.78 | 1.69 ± 0.28 | 3.60 ± 0.28 | n.d | 3.21 ± 0.72 | n.d | n.d | 1.44 ± 0.27 | 1.87 ± 0.25 | n.d | n.d | 3.19 ± 0.16 | n.d |
| Linalool | A | n.d | n.d | n.d | n.d | n.d | 1.51 ± 0.36 | 1.59 ± 0.09 | 1.37 ± 0.13 | 2.52 ± 0.06 | 1.05 ± 0.01 | 1.38 ± 0.24 | 1.98 ± 0.20 | 2.77 ± 0.11 |
| (E)-2-Heptanal | Ald | n.d | 4.35 ± 0.15 | 11.41 ± 0.10 | 10.63 ± 2.09 | 3.13 ± 0.07 | 3.52 ± 0.27 | n.d | 14.40 ± 0.06 | 17.65 ± 0.92 | 2.72 ± 0.20 | 1.01 ± 0.05 | n.d | 1.87 ± 0.25 |
| Trans-2-nonenal | Ald | n.d | 0.70 ± 0.13 | 0.75 ± 0.06 | 0.91 ± 0.18 | n.d | n.d | n.d | n.d | 0.58 ± 0.13 | 0.79 ± 0.08 | n.d | n.d | 0.82 ± 0.03 |
| Benzaldehyde | Ald | 6.14 ± 0.09 | 2.27 ± 0.29 | 2.39 ± 0.31 | 1.92 ± 0.48 | 2.93 ± 0.31 | 2.08 ± 0.70 | 1.67 ± 0.08 | 1.88 ± 0.16 | 1.63 ± 0.04 | 2.86 ± 0.22 | 2.82 ± 0.78 | 2.28 ± 0.16 | 3.87 ± 0.53 |
| Phenylacetaldehyde | Ald | 0.55 ± 0.04 | n.d | n.d | n.d | n.d | n.d | n.d | 0.77 ± 0.01 | 0.94 ± 0.13 | 1.29 ± 0.04 | 1.99 ± 0.66 | 1.57 ± 0.06 | 1.86 ± 0.18 |
| Decanal | Ald | n.d | 1.50 ± 0.20 | 2.09 ± 0.31 | 2.18 ± 0.65 | 1.80 ± 0.31 | n.d | 1.22 ± 0.04 | 1.66 ± 0.13 | 1.51 ± 0.01 | 1.75 ± 0.01 | 1.14 ± 0.38 | 0.65 ± 0.16 | 2.22 ± 0.22 |
| Furfural | Ald | 1.46 ± 0.01 | 2.37 ± 0.23 | 1.06 ± 0.30 | n.d | 1.52 ± 0.57 | 1.70 ± 0.54 | n.d | 0.60 ± 0.32 | n.d | 1.40 ± 0.27 | 2.93 ± 0.02 | 2.11 ± 0.08 | 3.01 ± 0.02 |
| Heptanal | Ald | 5.68 ± 0.11 | 4.98 ± 0.81 | 4.28 ± 0.24 | n.d | 2.89 ± 1.90 | 5.70 ± 0.37 | n.d | 2.62 ± 0.27 | n.d | n.d | n.d | n.d | 4.38 ± 1.93 |
| Hexanal | Ald | 38.6 ± 2.38 | 19.6 ± 2.17 | 6.15 ± 0.98 | 12.3± 2.24 | 11.5 ± 0.28 | 15.2 ± 2.44 | 13.0 ± 2.58 | 12.97 ± 1.35 | 7.23 ± 0.58 | 23.5 ± 0.02 | 29.16 ± 0.38 | 30.9 ± 3.53 | 20.8 ± 1.07 |
| 1,4,7,10,13,16-hexaoxacyclooctadecane | Et | 3.84 ± 1.40 | 6.62 ± 0.33 | 5.53 ± 0.27 | 5.22 ± 0.03 | 6.57 ± 0.78 | 7.01 ± 0.24 | 5.61 ± 1.11 | 2.89 ± 0.23 | 6.29 ± 1.67 | 4.01 ± 0.87 | 1.76 ± 0.66 | 4.59 ± 0.74 | 7.48 ± 2.47 |
| 15-crown-5 | Et | 4.05 ± 1.96 | n.d | n.d | n.d | n.d | n.d | n.d | 1.59 ± 0.46 | 1.51 ± 0.61 | 3.35 ± 1.61 | 3.04 ± 0.80 | 1.53 ± 0.04 | 1.62 ± 0.52 |
| 18,18’-Bi-1,4,7,10,13,16-hexaoxacyclononadecane | Et | 1.64 ± 0.15 | 5.53 ± 0.12 | n.d | n.d | 2.02 ± 0.07 | n.d | n.d | n.d | 1.38 ± 0.25 | n.d | 2.43 ± 1.45 | n.d | 2.38 ± 0.21 |
| 3,6,9,12-Tetraoxatetradecan-1-ol | Et | 2.08 ± 0.83 | n.d | 2.89 ± 0.15 | n.d | n.d | n.d | n.d | 0.55 ± 0.20 | 2.83 ± 0.22 | n.d | 1.14 ± 0.03 | n.d | n.d |
| Methoxybenzoxime | Et | 2.49 ± 0.05 | 2.57 ± 0.58 | 1.52 ± 0.18 | 3.39 ± 1.13 | 3.54 ± 0.73 | 3.94 ± 0.18 | 3.54 ± 1.17 | 2.44 ± 0.11 | 2.33 ± 0.61 | 2.71 ± 0.38 | 3.49 ± 1.70 | 4.11 ± 0.01 | 2.79 ± 1.22 |
| Tetraethylene glycol diethyl ether | Et | n.d | 1.53 ± 0.05 | 1.35 ± 0.03 | n.d | n.d | n.d | n.d | n.d | 2.73 ± 0.53 | 1.57 ± 0.02 | n.d | n.d | 3.24 ± 0.48 |
| Conjugated (10E, 12Z)-linoleic acid | Fc | n.d | n.d | 0.76 ± 0.12 | 1.61 ± 0.08 | n.d | 1.65 ± 0.55 | 3.19 ± 0.54 | n.d | 2.07 ± 0.05 | 1.39 ± 0.03 | n.d | n.d | 1.57 ± 0.05 |
| (2S,2’S)-2,2’-Bis[1,4,7,10,13-pentaoxacyclopentadecane] | E | 1.73 ± 0.52 | 2.20 ± 0.21 | n.d | 3.32 ± 0.45 | 2.38 ± 0.88 | n.d | n.d | n.d | n.d | n.d | 4.17 ± 0.15 | 2.35 ± 0.22 | n.d |
| 3-ethyl-2-methyl-1,3-hexadiene | Hc | 1.50 ± 0.34 | 0.79 ± 0.16 | 1.04 ± 0.01 | n.d | n.d | n.d | 1.44 ± 0.31 | 1.03 ± 0.33 | 1.02 ± 0.03 | 1.22 ± 0.51 | n.d | n.d | n.d |
| 6-Methyl-5-hepten-2-one | K | 0.87 ± 0.21 | 1.66 ± 0.49 | 2.37 ± 0.37 | 2.24 ± 0.39 | 2.16 ± 0.02 | 2.13 ± 0.15 | 1.72 ± 0.03 | 2.14 ± 0.04 | 2.23 ± 0.18 | 1.75 ± 0.01 | 1.41 ± 0.23 | 1.50 ± 0.16 | 1.23 ± 0.46 |
| 2-pentyl-furan | Ar | 2.85 ±0.43 | n.d | n.d | n.d | 0.98 ± 0.01 | 2.47 ± 0.36 | n.d | n.d | n.d | 4.38 ± 0.39 | 4.85 ± 2.01 | 5.11 ± 0.41 | 1.81 ± 0.31 |
| (2S,13S)-12,13-Dihydroxy-1,4,7,10-tetraoxacyclotetradecane | 0.58 ± 0.05 | n.d | 2.00 ± 0.15 | 3.58 ± 0.02 | n.d | n.d | n.d | n.d | n.d | 1.70 ± 0.37 | 5.65 ± 2.33 | n.d | n.d | |
| 2- [2- [2- [2- [2- [2- [2-(2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy Base] ethanol | 2.77 ± 0.28 | 7.44 ± 0.22 | 5.96 ± 0.53 | 3.47 ± 0.15 | 7.45 ± 2.37 | 3.72 ± 0.22 | 4.07 ± 1.24 | 2.95 ± 0.11 | 2.37 ± 0.87 | n.d | 2.59 ± 1.22 | 1.89 ± 0.07 | n.d | |
| 2- [2- [2- [2- [2- [2- [2- [2- [2- [2-(2-Methoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy Ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol | n.d | n.d | 6.00 ± 1.33 | n.d | 2.19 ± 0.15 | 6.10 ± 1.25 | 1.06 ± 0.76 | 0.91 ± 0.22 | 1.85 ± 0.77 | n.d | n.d | n.d | n.d | |
| 2- [2- [2- [2- [2- [2- [2- [2- [2- [2- [2- [2- [2- [2-(Trimethylsilyloxy)ethoxy Ethoxy]ethoxy]ethoxy]ethoxy | 0.91± 0.02 | 4.66 ± 1.88 | n.d | n.d | 1.27 ± 0.11 | 5.51 ± 0.03 | 3.75 ± 1.27 | 4.26 ± 0.75 | 1.41 ± 0.47 | 2.02 ± 0.33 | n.d | 1.99 ± 0.05 | n.d | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayama, K.; Wei, R.; Zhang, Y.; Wu, F.; Su, Z.; Dong, J.; Liu, X. Effects of Tea Powder on the Cooking Properties, Antioxidative Potential and Volatile Profiles of Dried Noodles. Foods 2022, 11, 858. https://doi.org/10.3390/foods11060858
Kayama K, Wei R, Zhang Y, Wu F, Su Z, Dong J, Liu X. Effects of Tea Powder on the Cooking Properties, Antioxidative Potential and Volatile Profiles of Dried Noodles. Foods. 2022; 11(6):858. https://doi.org/10.3390/foods11060858
Chicago/Turabian StyleKayama, Kayama, Ran Wei, Yuanping Zhang, Fenghua Wu, Zhucheng Su, Junjie Dong, and Xingquan Liu. 2022. "Effects of Tea Powder on the Cooking Properties, Antioxidative Potential and Volatile Profiles of Dried Noodles" Foods 11, no. 6: 858. https://doi.org/10.3390/foods11060858
APA StyleKayama, K., Wei, R., Zhang, Y., Wu, F., Su, Z., Dong, J., & Liu, X. (2022). Effects of Tea Powder on the Cooking Properties, Antioxidative Potential and Volatile Profiles of Dried Noodles. Foods, 11(6), 858. https://doi.org/10.3390/foods11060858








