Abstract
Coffee is a worldwide beverage of increasing consumption, owing to its unique flavor and several health benefits. Metabolites of coffee are numerous and could be classified on various bases, of which some are endogenous to coffee seeds, i.e., alkaloids, diterpenes, sugars, and amino acids, while others are generated during coffee processing, for example during roasting and brewing, such as furans, pyrazines, and melanoidins. As a beverage, it provides various distinct flavors, i.e., sourness, bitterness, and an astringent taste attributed to the presence of carboxylic acids, alkaloids, and chlorogenic acids. To resolve such a complex chemical makeup and to relate chemical composition to coffee effects, large-scale metabolomics technologies are being increasingly reported in the literature for proof of coffee quality and efficacy. This review summarizes the applications of various mass spectrometry (MS)- and nuclear magnetic resonance (NMR)-based metabolomics technologies in determining the impact of coffee breeding, origin, roasting, and brewing on coffee chemical composition, and considers this in relation to quality control (QC) determination, for example, by classifying defected and non-defected seeds or detecting the adulteration of raw materials. Resolving the coffee metabolome can aid future attempts to yield coffee seeds of desirable traits and best flavor types.
1. Introduction
Coffee is a major commodity traded worldwide that contributes to the economy of several countries [1]. In some regions, it is recognized as the second most valuable naturally traded product after oil [2], with an estimate of more than 1.6 billion cups of coffee consumed on a daily basis [3]. Coffee production reached 10,314,000 tons in 2020, with coffee consumption reaching 9,997,000 tons in 2020/2021. This production is contributed by ca. 60 tropical and subtropical countries, as reported by the International Coffee Organization [4,5]. The global coffee industry yield is expressed economically to around USD 200 billion annually [6]. Additionally, recent statistics in the Middle East region show that Saudi Arabia and Egypt account for 75,000 and 77,000 tons of coffee consumption, respectively, which accounts for 1.5% of global coffee consumption [7].
Coffee habitual consumption is attributed mostly for its central nervous system (CNS) stimulant effect, specifically for its rich caffeine content, in addition to its characteristic aroma and taste. There are more than 120 species of Coffea, and coffee is brewed mainly from the seeds of Coffea arabica L. and C. canephora L. var. robusta or C. robusta. Therefore, these species are the most important commercial sources in coffee production [8]. Arabica coffee is favored by most consumers because of its richer aroma and flavor compared to robusta [9]. C. arabica accounts for 60% of global coffee production, while C. robusta accounts for the remaining 40% [7]. Further analysis of the global market revealed that Latin America produces about 60% and 80% of the world’s coffee supply and arabica coffee, respectively [10]. Particularly, Brazilian coffee is historically a premium coffee that accounts for one third (33.3%) of the worldwide production, and about 24.4% of total coffee exported worldwide [11,12].
Further evaluation of coffee import worldwide revealed that coffee is typically imported in the form of green coffee seeds [13], reflecting customers’ special patterns of consumption based on domestic roasting and different blends favored in each region, e.g., cardamom addition in the Middle East, adding further complexity to the coffee metabolome [14,15]. Synergism among several metabolites has been reported for natural products bioactivity, including the antioxidant activity as in the case of coffee constituents [16,17]. Variations within the different species, complexity of the coffee’s chemistry, and low levels of most secondary metabolites through being active or contributing to coffee organoleptic characters warrant for the development of sensitive analytical techniques to monitor all these differences [18]. In addition, one of the main concerns in coffee production lies in the adulteration of roasted coffee to gain economic benefit either by blending low-quality coffee seeds or adding other ingredients, i.e., brown sugar, coffee husks, maize, soybean, etc. [19], or mixing robusta of low quality to premium coffee arabica. Toci et al. have recently reviewed the adulteration practices that necessitate an authenticity quality control protocol to determine coffee origin [20]. For all the aforementioned reasons, in-depth phytochemical analysis of natural metabolites with advanced analytical and evaluation techniques of metabolomics is warranted in coffee for proof of quality and efficacy.
A typical platform for plant extract profiling includes chromatographic techniques such as ultra-high-performance liquid chromatography coupled to mass spectrometry (UPLC/MS), which presents an excellent combination of selectivity and sensitivity allowing for the separation of a large number of components with different modes of ionization, i.e., negative versus positive ESI to increase the identified metabolite scores or coverage [21]. UPLC/MS is especially suited for the profiling of medium polar and large molecular weight bioactives, e.g., chlorogenic acids in coffee, whereas gas chromatography–mass spectrometry is more suitable for aroma profiling in coffee targeting low molecular weight and other volatile compounds or their derivatives, e.g., furans and pyrazines [22].
Recently, LC/MS-based metabolomics techniques have delivered an extraordinary combination of selectivity and sensitivity, which can be employed as an effective platform for metabolite profiling. Different MS techniques provide different ionization modes to gain a wide range of identified metabolites [19]. Moreover, UPLC/MS or fluorescence detection (HPLC/FLD) and UV (HPLC/UV) are well suited for secondary metabolite fingerprinting as a powerful analytical technique for natural plant characterization and classification [21].
Compared to chromatographic techniques most commonly employed in metabolomics setups, the direct spectroscopic measurement employed in fingerprinting approaches provides a more robust approach with less run time, including ultraviolet (UV), infrared (IR), and nuclear magnetic resonance (NMR), but it is less able to identify a large pool of compounds [23,24,25]. The UV-VIS technique has been previously applied for coffee fingerprinting, where UV spectral bands provided semi qualitative and quantitative analytical information about selected bioactive components, i.e., phenolic acids, methylxanthines, chlorogenic acids, and pentacyclic alcohols. Moreover, as a cheaper, simpler, and non-destructive technique, UV fingerprinting can be considered an alternative tool to UPLC/MS for coffee sample quality control analyses [26,27]. Similarly, infrared Fourier transform (FTIR) spectroscopy is recognized as a direct spectroscopic technique for discrimination between defective and non-defective roasted coffee seeds [23]. It is also noteworthy to find that NMR has been increasingly employed in food omics studies in the last few years [25,28]. Particularly, 1H-NMR has been proven as a potential candidate for the authentication of the commercial Brazilian arabica blends composed of roasted coffee [29]. The common factor in all previous techniques lies in the generation of huge datasets which warrant for the application of statistical modelling tools, including principal component analysis (PCA) and orthogonal projections to latent structure discriminant analysis (OPLS-DA). Conventional analytical methods are challenged with several variables such as huge data sets, geographical location, harvesting time, and chemotypes. These models provide an indicator for method reliability and insights into separations between the investigated sample groups as typical in nutraceutical analyses [30].
Since coffee seeds undergo various post-harvesting steps, volatile and non-volatile metabolites are affected, and are consequently adapted to (regional) consumer preferences. In this review, we shed light on the application of different metabolomics approaches based on different analytical platforms, and specifically those coupled with chemometric data processing. The review aims to unravel methods for identifying metabolic profiles and highlight markers responsible for the unique flavor, aroma, and taste of each coffee type in relation to coffee processing and brewing methods. We present the advantages and limitations of each platform in the context of different post-harvesting methods of coffee, i.e., processing, roasting, and brewing methods, and discuss metabolic profiling-based quality attributes compared to conventional analysis where possible.
2. Metabolomics Applications in Coffee Breeding and Origin Determination
A metabolomics-based approach was applied in the classification of coffee seeds derived from different biological origins, i.e., arabica and robusta coffees [22], and conventionally vs. organically grown coffees [31]. Metabolomics has been employed to assess the main chemical differences in these types of coffee, and thus, assign metabolites to the best coffee characteristics. Approaches shall be explained in detail in the next subsections based on the different analytical techniques used.
2.1. Gas Chromatography–Mass Spectrophotometry (GC/MS)
GC/MS was employed by Anagbogu et al. [32] to study the different varieties of C. robusta in order to determine which genotype should be used in coffee breeding programs to improve its quality. GC–MS analysis revealed 340 metabolites, among which 66 showed differences between genotypes, mainly attributed to sugar derivatives, while the others were organic acids, amino acids and nitrogenous compounds. The study also assessed sucrose to caffeine ratio among genotypes as being indicative of a low cup quality. The germplasm of the ‘Niaouli’ group with a high sucrose/caffeine ratio was recommended for further breeding.
In another study, a similar metabolomics approach was employed to differentiate between the volatile metabolites of arabica and robusta coffee seeds from different geographical origins in the Philippines in two forms: standard and civet (an animal) eaten forms. PCA was used to model the dataset accounting for 31% of the sample variance, and this identified that arabica samples passed through the civets intestine were enriched in acetic acid, furfural, 2-acetylfuran, 5-methylfurfural, furfuryl alcohol, 3-methylcyclopentane-1,2-dione, maltol, and 2-formylpyrrole, while robusta showed higher levels of 3-ethyl-2,5-dimethylpyrazine, 2-ethyl-3,5-dimethylpyrazine, guaiacol, phenol, 4-ethylguaiacol, and 3-acetylanisole. Likewise, similar metabolites were identified in arabica and robusta coffee seeds when compared to the metabolic profiles of other coffee grown from regions outside the Philippines, mostly attributed to Maillard products, i.e., pyrazines and furans [33].
Similarly, a non-targeted GC/MS metabolite profiling was performed on coffee seeds of C. arabica and C. robusta from different geographical origins within Indonesia. PCA analysis explained 52.9% of the samples’ variance. This is higher than that found in previous studies and revealed that arabica samples contained higher levels of malic acid, whereas robusta showed higher levels of caffeine, while 16-methyl cafestol was suggested as a discriminant metabolite exclusively present in C. robusta.
Moreover, the assessment of geographical origin effects on metabolic profiles was investigated for Indonesian coffees derived from different species and geographical origins using a non-targeted approach. It was found that Sulawesi, Papua, Flores and Sumatra coffee samples showed higher levels of glycerol, glucuno-1,5-lactone, gluconic acid and sorbitol. Galactinol and galactitol were indicated as metabolite markers to discriminate Sumatra, Bali and East Java from the Eastern parts of Indonesia, with the latter showing higher galactitol levels [34]. Galactitol is a sugar alcohol and its accumulation in the human body has yet to be investigated.
In another study, GC-Q/MS coupled to multivariate data analysis was employed to determine metabolite markers to differentiate C. arabica samples grown in Brazilian coffee producing municipalities, namely, Lavras, Santo Antônio do Amparo (SAA), and São Sebastião da Grama (SSG). PCA explained 51.6% of the samples’ variance, with SAA samples to show higher levels of organic acids, i.e., oxalic acid, malic acid, and sugars, i.e., glucose, fructose, sorbitol, and galactinol. In contrast, quinic acid, caffeine, and 5-caffeoylquinic acid (5-CQA) showed higher levels in Lavras samples. Citric and glutamic acids had higher levels in SSG samples [35]. Independently, blood pressure lowering effects have been implied for glutamic acid-rich foods [36].
2.2. LC/MS
As previously mentioned, coffee taste and flavor are important criteria in determining quality. They are strongly influenced by coffee genotype and geographic origin. In a study by Choi et al. [37], an integrated metabolomics approach was performed on coffee samples of different origins representing three continents (Asia, South America, and Africa) using LC/MS along with total proteins, total carbohydrates, and total sugars quantification. Multivariate data analysis showed that monosaccharides, proteins, volatile components and to a lesser extent disaccharide were the most important discriminant factors of the coffee samples and accounted for ca. 65% of metabolite variations. Though, sugars as primary metabolites are not strong markers as they are related to several other factors, e.g., weather. Likewise, Gamboa-Becerra et al. [38] used a metabolome-wide association study (MWAS) employing ultra-performance liquid chromatography–ion trap–mass spectrometry (UPLC–IT–MS) for the analysis of 40 varieties of C. robusta, comprising a total of 120 coffee plants. About 91 metabolites were identified as major contributors in determining coffee flavor and taste, which were classified in 11 chemical groups including alkaloids, carbohydrates, carotenoids, chlorogenic acids, fatty acids, flavonoids, lipids, organic acids and terpenoids. Interestingly, quercetin-4-glucoside was found to exert a positive correlation with acidity and sourness and a negative correlation with flavor and aroma, while 52 lipids were found to have a positive correlation with coffee flavor, color, and foam. Foam is a desired feature in most coffee brews and attempts to improve constituents contributing to that factor ought to be further investigated.
2.3. Direct Spectroscopic Techniques, i.e., NMR
NMR fingerprinting represents a valuable tool in the classification of the different coffee samples based on their origin. Quantitative 1H-NMR (qNMR) methodology was proven efficient for discriminating between numerous arabica samples collected from different regions of Brazil, including São Paulo (North), Minas Gerais (South), Paraná (Tomazina-North), Bahia (Vitória da Conquista-South), and Paraná (Ribeirão Claro-North). Catechol, trigonelline, caffeine, and N-methylpyridine are important markers in differentiation [39].
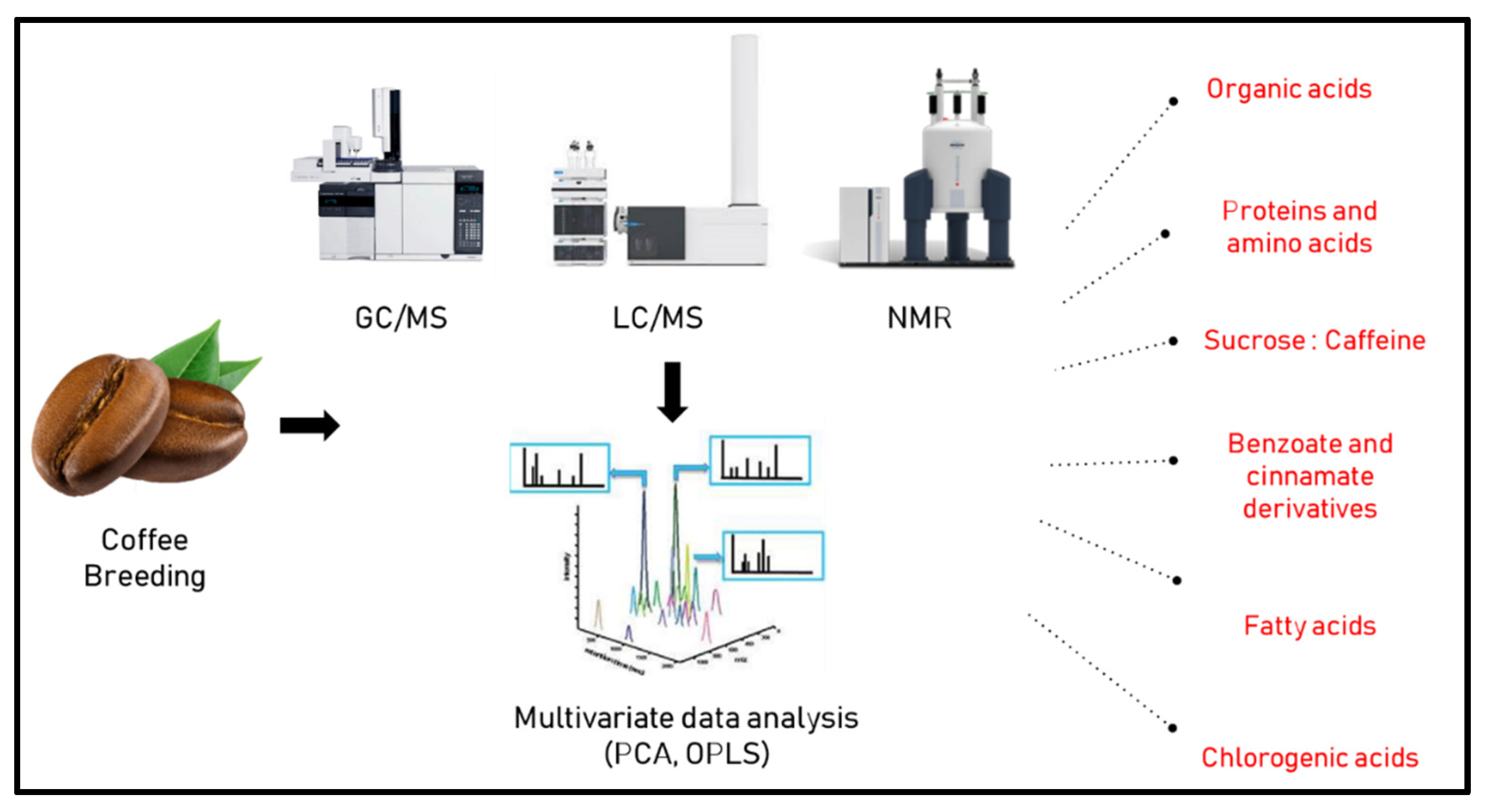
In another study by Arana et al. [40] 1H-NMR fingerprinting was used to discriminate between 192 samples from different origins, i.e., Asia, Africa and America, belonging to arabica and robusta species in comparison to Colombian counterparts with more than 300 spectra collected. Partial least square discriminant analysis (PLS-DA) was then employed to classify samples, with discrimination among samples attributed largely to fatty acids, acetate and caffeine levels. A similar approach was used by Choi et al. [41] using direct NMR spectroscopy for the classification of coffee samples from different origins revealing that chlorogenic acid, caffeine, citrate, and sucrose were the most discriminant metabolites showing higher levels in Colombian samples in comparison to other samples from around the world. These metabolites in turn were suggested by the authors as factors for determining the impact of plant regions on metabolite concentrations. Figure 1 lists various factors and metabolite classes which are likely to affect the choice of coffee species for breeding, as revealed using different metabolomics approaches. We would like to point out that contents of primary metabolites such as sugars, but also some secondary metabolites, strongly depend on weather which may differ between regions and year to year, and thus for reasonable comparisons of different regions several seasons should be sampled, ideally.
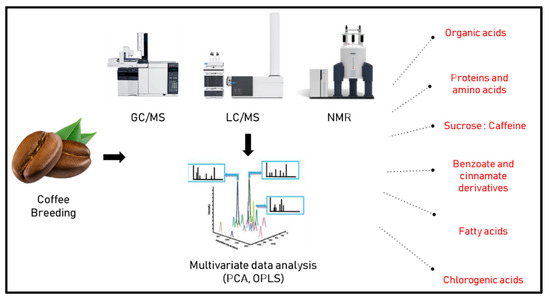
Figure 1.
Metabolic class determinants affected by coffee breeding can be determined by different metabolomics approaches.
3. Metabolomics Applications in Coffee Roasting
Upon roasting, the chemical composition of raw coffee seeds undergoes massive transformation processes such as esterification, thermal isomerization, acyl migration, dehydration, and lactonization (epimerization) [42]. Furthermore, in the roasting process part of the carbohydrates get degraded to mono and oligosaccharides (low molecular weight compounds) and interact with amino acids yielding Maillard reaction products such as melanoidins and pyrazines to affect coffee color, flavor, and aroma significantly [14,43].
Further processing of ground, roasted coffee by water-extraction and spray-drying or freeze-drying produces instant coffee products. Such treatment is often associated with a reduction in certain (volatile) compounds such as 2-furylmethanol and low molecular weight organic acids, i.e., acetic, tartaric, malic, formic, and 2-oxo-butyric acid. In contrast, sugars (i.e., sucrose, ribose, and myo-inositol), in addition to acids (i.e., fumaric, propionic, glycolic, and malonic acids) were found to be more enriched in instant coffee compared to ground, roasted coffee products [44]. These variations in acid composition were in part attributed to the processing method of coffee.
3.1. GC/MS
In one study, the metabolite profiles of various robusta coffee seeds from Vietnam and Indonesia with different roasting degrees were analyzed using GC/MS and followed by multivariate data analysis. PCA modelling of the acquired dataset explained 75% of the sample variations. Metabolites such as 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, 2-ethylpyrazine, 2-ethyl-6-methylyrazine, 2-ethyl-5-methylpyrazine, dihydro-2-methyl-3-(2H)-furanone, and 5-methyl-2-furancarboxaldehyde decreased with roasting degree. In contrast, phenol derivatives (e.g., 2-methoxyphenol), phenol, and 4-ethyl-2-methoxypheno, as well as 2-hydroxy-3-methyl-2-cyclopenten-1-one, and 2,2′-oxybis(methylene)bisfuran exhibited increased levels upon roasting [45].
3.2. LC/MS
Chlorogenic acids were annotated in various green coffee specimens based on LC-MS4 patterns of fragmentation, including derivatives of dimethoxycinnamoylquinic acids, caffeoyl-dimethoxycinnamoylquinic acids, and diferuloylquinic acids [46], in addition to hydroxycinnamoyl amides [47]. Additionally, roasting changes the coffee chemical profile and increases its complexity, due to the formation of quinides, chlorogenic derivatives, and shikimates. The application of LC in roasting detection in either arabica and robusta roasted coffee via UHPLC-ESI-qTOF-MS/MS mostly targets the visualization of changes incurred by phenolic acids, which are not detected using GC/MS. These include caffeoyl, feruloyl, and diferuloyl quinides described as chlorogenic lactones (CGLs). They are formed by roasting through an esterification reaction on all hydroxyl groups, including C1, followed by dehydration and the formation of lactones which contribute to coffee flavor [48]. These are associated with health effects. Compared to chlorogenic acids, CGLs exert lower antioxidant effects as determined using in vitro DPPH and FRAP assays, although this has yet to be confirmed using in vivo models [15].
Likewise, during coffee roasting, shikimate and lactone derivatives of feruloylquinic acids are also formed. Jaiswal et al. investigated commercial roasted robusta coffee samples using LC/MS and could discriminate between them based on their CGLs and hydroxycinnamoyl shikimates composition [49]. Chemical analysis revealed the presence of various hydroxycinnamoyl shikimates such as p-coumaroylshikimic acids, feruloylshikimic acids and caffeoylshikimic acids as characteristic metabolites for roasted coffee seeds.
Pérez-Míguez et al. reported on a non-targeted metabolomics approach based on LC/MS for the identification of metabolites in arabica coffee seeds roasted at different degrees. Chemical compounds that could distinguish between the different degrees of roasting in coffee included caffeoylquinic acid, chlorogenic acid lactones, and N-caffeoyl tryptophan which showed a marked increase. The opposite trend was seen for caffeoyl feruloyl quinic acid, mozambioside, coumaroyl quinic acid, and dicaffeoylquinic acid [50].
Metabolomic analysis has revealed that caffeoylquinic acid isomers are the major chlorogenic acids; mainly 5-CQA is naturally present in coffee and accounts for its antioxidant and slimming effect. It shows a 33% decline upon roasting, concurrent with an increase in 3-and 4-caffeoyl isomers, though with 5-CQA still being the major isomer. HPLC analysis along with antioxidant activity was performed to evaluate the effect of roasting on the antioxidant activity of caffeoylquinic acid, revealing that antioxidant activity of medium-roasted samples was doubled compared to green coffee, likely attributed to melanoidins generated during roasting and to overcome the loss in chlorogenic acid’s antioxidant action. During roasting, a portion of chlorogenic acid undergoes hydrolysis to form lactones and Maillard reaction products which contribute to the seed antioxidant activity [51]. Melanoidins are a major product of roasting and warrant deeper investigations as scarce information exists on structural changes and technical effects of roasting. Detection and identification of melanoidin levels using LC/MS should aid the optimization of the roasting process of coffee, and likewise for other thermal-processed products, e.g., nuts etc.
3.3. Direct Spectroscopic Techniques, i.e., NMR, UV, and IR
To provide a fingerprint of the extract, direct spectroscopic devices are typically used, including UV, IR, and NMR, though with the latter being most powerful in structural elucidation, especially if two-dimensional spectra are used which allow deep amalyses even from crude extracts [23,29,52]. The application of IR spectral analysis in quality control and the metabolite fingerprinting of food products such as coffee seeds has been widely used in determining certain attributes. These methods meet the required criteria for food analysis as they are accurate, non-destructive, rapid, reliable and relatively inexpensive [53]. Previous fingerprinting approaches in coffee have included the discrimination between defective and non-defective roasted arabica coffee seeds employing diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), where spectral regions 1700–1500 and 970–600 cm−1 were reported to show higher absorbance intensity in non-defective compared to defective coffee roasted seeds [23]. Moreover, another application of metabolomic fingerprinting in coffee analysis included phenolic compounds and alkaloid analysis in Indonesian arabica and robusta coffee extracts based on FTIR. The study assigned bands at 1280 and 1605 cm−1 for chlorogenic acids and 3123, 3011, 1703 and 1653 cm−1 for caffeine [54].
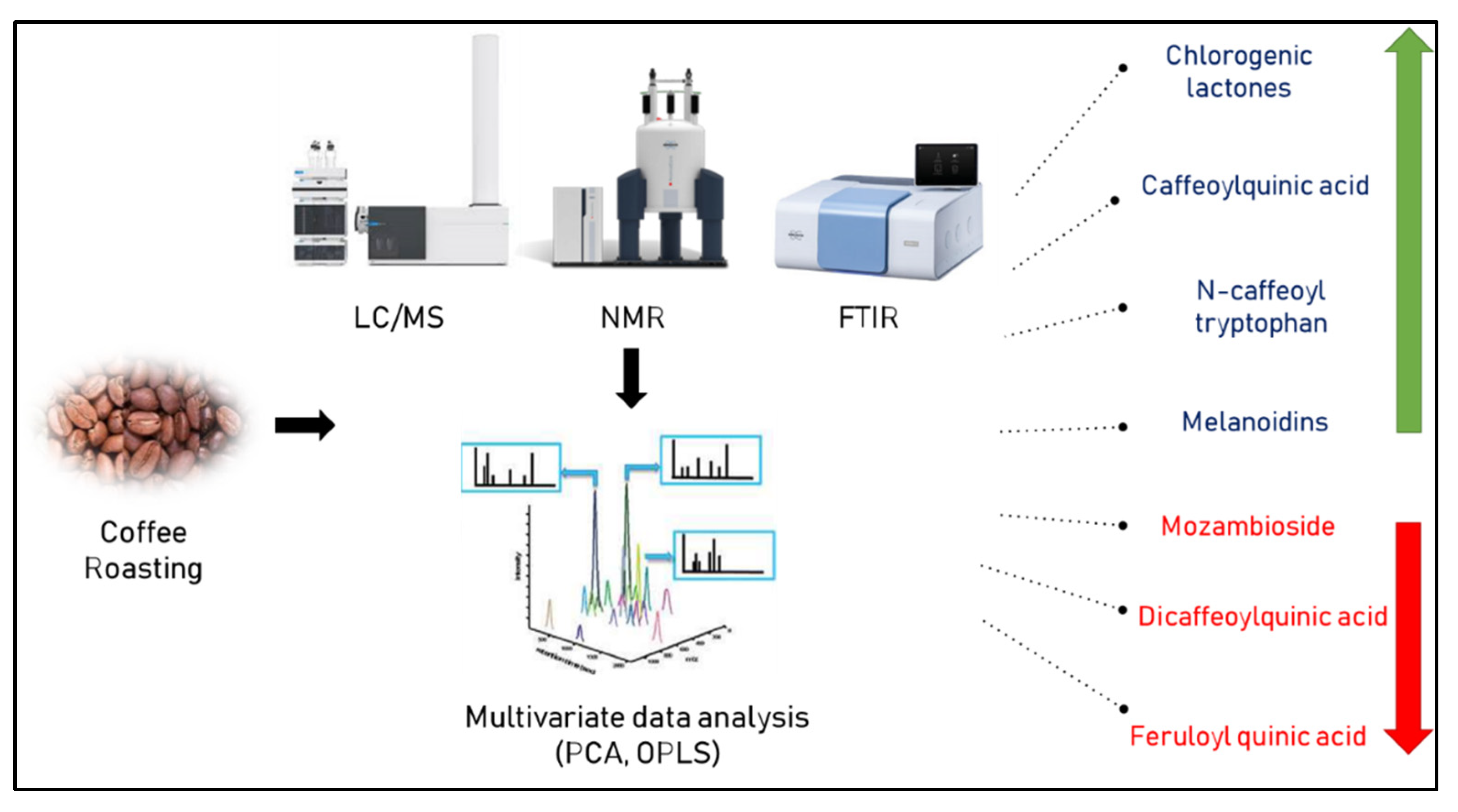
Furthermore, the authentication of 31 commercial Brazilian arabica blends composed of roasted coffee seeds was successfully performed by 1H-NMR based on the signals, particularly between δH 5.1 and 9.5 ppm, specific to carbohydrates of some cereals used for coffee adulteration [29]. Figure 2 lists the various metabolite markers for assessing the degree of coffee roasting as determined by various metabolomic approaches.
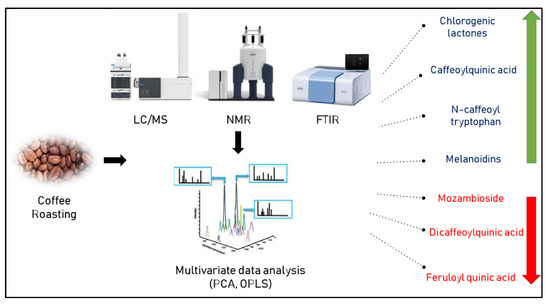
Figure 2.
Metabolic markers indicative of roasting degree of coffee as determined by different metabolomic approaches. Green and red arrows indicate increase or decrease upon roasting, respectively.
4. Metabolomics Applications in Coffee Brewing
Coffee brewing is a historical coffee preparation method employed using various extraction methods, including drip, French press, boiled brew, pressurized extraction (espresso), and others [54,55]. These forms can be performed by different devices ranging from traditional brewing pots to automatized coffee makers resulting in various coffee beverages according to several factors, mainly related to consumers’ preferences and acceptability for each region or country [56,57,58]. Cold brewing has gained consumers’ preference recently, whereby coffee is extracted at 20 to 25 °C or colder and steeped for a much longer time ranging from 8 to 24 h, compared to the classical hot brewing methods performed on a minute scale [59]. Beside coffee origin and post-harvest processing, coffee brewing is recognized as a critical step to affect coffee sensory attributes, including flavor and taste [54,60,61]. For instance, Nariño cold brew shows unique sweetness and fruity and floral flavors, in addition to medium bitterness and acidity, with a creamy consistency [62]. Consequently, several studies have attempted to investigate the sensory profiles associated with different brewing methods [63,64], which are yet to be correlated with chemical profiles to be conclusive.
Both volatile and non-volatile metabolites are typical markers contributing to common coffee characteristics, i.e., aroma perception, astringency, and bitter taste [56,65]. This asks for the employment of more than one analytical technique to holistically assess coffee flavor makeup. Furthermore, the preparation of different blends unique to world regions can also contribute to coffee sensory properties during the brewing process and add to its chemical complexity. For instance, blends containing some herbal spices such as cardamom seeds and clove buds are frequently consumed in the Middle East, masking the smoky odor of roasting products, including 5-(hydroxymethyl) furfural (5-HMF) and pyrazines [14]. The brewing method also affects non-volatile bioactives levels in coffee, including caffeine and phenolic acids to account for claimed health benefits of the seeds, i.e., antioxidant and anti-tumor properties [55,66,67,68,69]. Examples include hot extraction methods found to affect the polyphenol and caffeine contents, while further analysis of cold brewing methods, i.e., dripping and steeping, resulted in changes of chlorogenic acid and trigonelline levels [62].
Additionally, extraction conditions in cold brews affect Maillard reaction products such as acrylamide and furans. Hence, the optimization of acrylamide and furan levels is a major goal in coffee brewing to ensure healthier coffee beverages with less hazards. Han et al., showed that coffee brewed for 3 h contained the lowest acrylamide levels, while steeping and dripping featured the lowest furan levels after 24 h and 12 h, respectively [64]. The effect of coffee brewing on microelement composition appears conflicting though, and likely is not significantly influenced by the brewing method, warranting further studies [70]. Janda et al. have reported recently that coffee beverages prepared by simple infusion and AeroPress were rich in magnesium (116.3 mg/L), manganese (0.6 mg/L), chromium (0.03 mg/L), cobalt (0.01 mg/L), and potassium (1540.7 mg/L), while the drip brew contained a valuable silicon content (3.4 mg/L) [71].
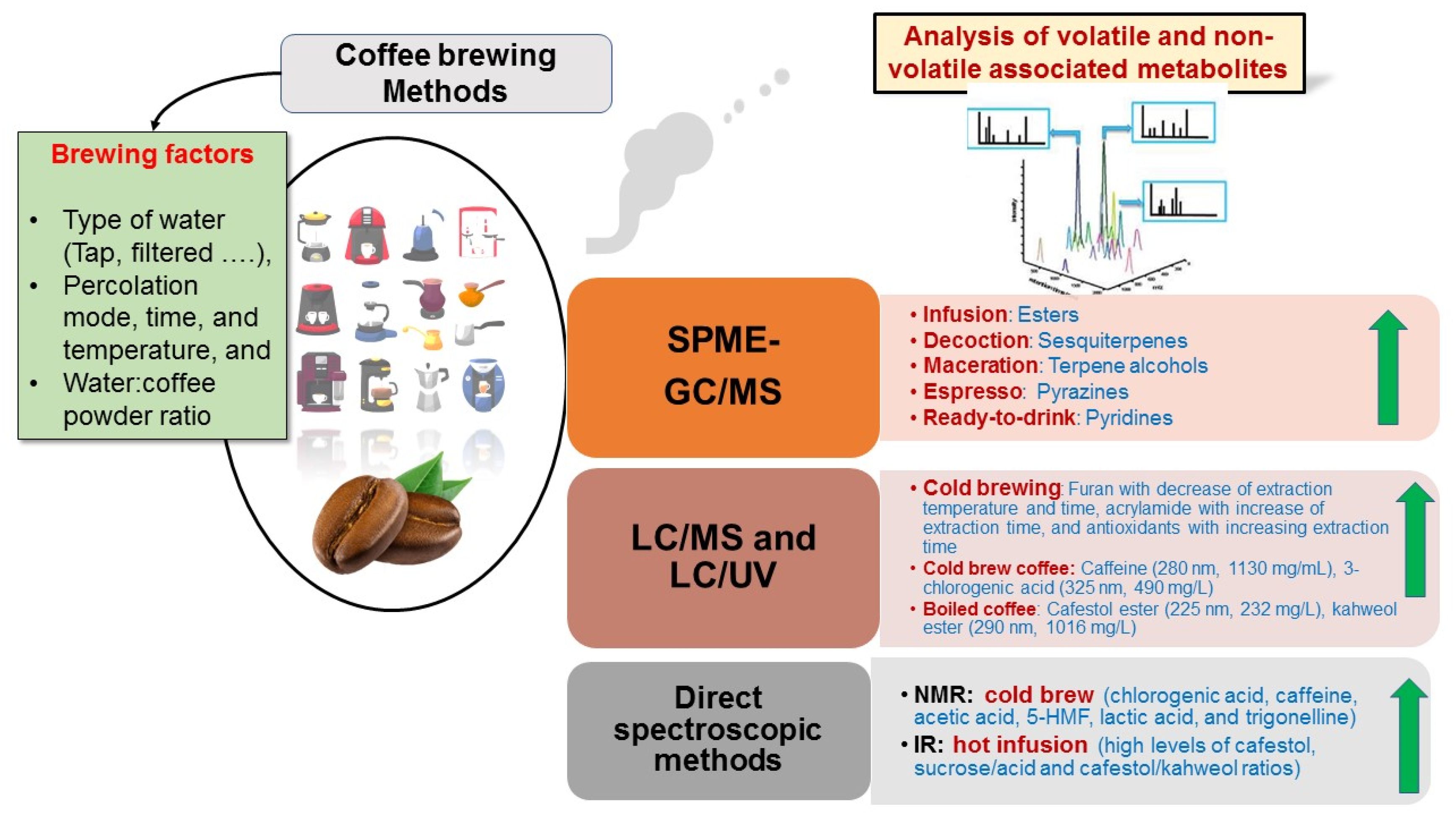
Different variables have been determined in previous coffee brewing studies to ensure the best products, including device used such as coffee maker or traditional coffee pot, temperatures, times of percolation, water/coffee ratio, and water type [54,72]. Some studies have targeted few metabolites, such as caffeine, furan, and 5-HMF [73], while metabolomics-based approaches using different platforms, i.e., GC/MS, LC/MS, and NMR could provide better readouts of brewing impact on coffee. GC/MS targets low molecular weight aroma compounds, e.g., terpenes and polar primary metabolites post-derivatization, i.e., fatty acids, sugars, and amino acids. LC/MS and NMR were more effective to detect and identify potential secondary bioactive compounds, including diterpenoids, chlorogenic acid derivatives, and alkaloids [22]. In the context of coffee brewing, various factors and their different platforms are illustrated in Figure 3 and shall be covered in the following subsections highlighting the effects of different brewing methods on coffee metabolomes using different analytical techniques. In addition, the different metabolites that markedly increased with the main brewing method are summarized in Figure 3, along with the platform which could detect and quantify them.
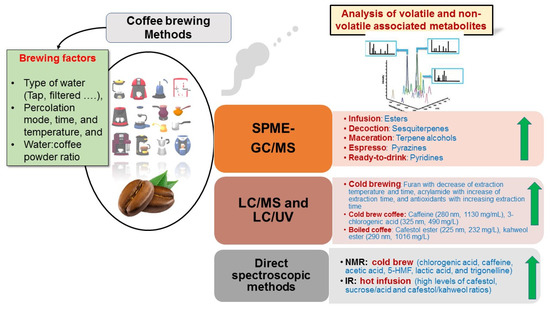
Figure 3.
List of the different brewing factors contributing to volatile and non-volatile metabolic profiles of coffee brews. The metabolites markedly increased with specific brewing methods are mentioned based on the detection method used.
4.1. GC/MS
Mostly, headspace solid-phase microextraction (HS-SPME) coupled with GC/MS was employed for investigating volatile constituents or volatile organic compounds (VOCs) associated with coffee brewing. In addition, gas chromatography/olfactometry (GC/O, CharmAnalysis) is sometimes used [74,75,76]. HS-SPME coupled to GC/MS was employed to assess the different brewing methods, including hot infusion, maceration, and decoction, typically reported in the Middle East region and analyzed using chemometric tools to identify markers for each brewing method. HS-SPME improved volatile detection with a total of 102 VOCs. Among VOCs, esters of mainly octyl acetate and terpinyl acetate, sesquiterpenes (e.g., α-curcumene, bergamotene, and β-caryophyllene), and terpene alcohols (e.g., terpineol, linalool and octanol) were found to predominate coffee infusion, decoction, and maceration, respectively [14].
Using a similar volatile collection setup, Yu et al. assessed the type of water, i.e., filtered, tap, mineral, and bottled, used in the brewing process for C. arabica of different roasting degrees by an espresso coffee machine versus the cold brew method. SPME-GC/MS results showed that brewing with so-called “filtered” water in an espresso coffee machine increased pyrazines, i.e., 2-methylpyrazine, 2,5-dimethylpyrazine, and phenols, (i.e., 2-methoxy-4-vinylphenol levels) compared with tap and bottled water. Compared to hot espresso-brewed coffee, cold brew increased the contents of 2-methylpyrazine, 1-methylpyrrole, and 2-acetylfuran, possessing a sweet, nutty, and fruity odor [77]. However, health perspectives have not been investigated based on such chemical makeup.
Moreover, chemometric tools have been applied to comprehensively assess the aroma profile of capsule-brewed espresso coffees derived from diverse Italian brands. Partial least squares discriminant analysis (PLS-DA) revealed that pyrazines (i.e., 2-methylpyrazine, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, and 2,6-diethyl-pyrazine) are significant coffee markers imparting a characteristic and intense aroma [78]. Heo et al. monitored VOCs derived from different volatile compounds in cold brew coffee samples, including cold brew from a coffee shop, ready-to-drink coffee, and brewed coffee from a coffee maker by HS-SPME/GC–MS. The results demonstrated a total of 36 volatile compounds with higher levels in coffee shop vs. ready-to-drink coffee samples, especially with pyridines imparting bitter, burnt, roasted, and astringent characteristics, such as N-acetyl-4-(H)-pyridine. Pyridine and its derivatives are likely to be products of trigonelline degradation, especially for seeds roasted strongly at high temperatures [79,80]. In addition, simple phenolics, i.e., guaiacol and its congers, were among the potential markers that characterized coffee shop samples, whereas coffee from a coffee maker showed higher amounts of maltol, which is responsible for the caramel-like odor and chocolate flavor in coffee and other confectionaries [80].
Moreover, GC/O of freshly brewed drip coffee (Ethiopian arabica coffee, roast degree: L value; 23, fiber type: divinylbenzene/carboxen/polydimethylsiloxane adsorbant) showed different VOCs, possessing a nutty–roast odor and a raspberry ketone sweet–fruity odor, represented by 1-(3,4-dihydro-2H-pyrrol-2-yl)-ethanone and 4-(4′-hydroxyphenyl)-2-butanone, respectively [74]. PCA was applied for the dissipation of VOC profiles to discriminate between various arabica coffee extracts collected from three production countries, Ethiopia, Tanzania, and Guatemala, with different roasting degrees. The results showed that the Ethiopian coffee extract was characteristic by its high amount of 4-(4′-hydroxyphenyl)-2-butanone [81].
4.2. LC/MS and LC/UV
LC/MS has been applied for studying the non-volatile metabolites associated with coffee brewing, including polyphenolics, alkaloids, and diterpenes. Compared to roasting effects, the effects of brewing on phytochemical composition, particularly chlorogenic acid and hydroxycinnamoyl amide derivatives, has not been extensively reported [46,47]. LC/MS showed potential in detecting health-related metabolites, either undesirable as acrylamide and furans, or desirable as the antioxidant phenolics, providing a good readout of coffee safety and efficacy of coffee prepared by cold methods [64,82]. The International Agency for Research on Cancer (IARC) classify acrylamide and furan among probable carcinogens (Group 2A) and possible human carcinogens (Group 2B), respectively [83,84]. Therefore, levels of acrylamide should be accurately determined by sensitive analytical techniques, including LC–MS/MS [82] and they are typically in the range of 5.9–38.8 ng/mL in ready-to-drink (brewed) coffees [83]. Moreover, furan levels in brewed coffee should be near or below 120 μg/L [84]. The effect of cold brewing on health hazard-related metabolites showed that acrylamide levels increased with increasing extraction time (5.5 ± 0.4 ng/mL for steeping after 24 h at 20 °C and 5.2 ± 0.4 ng/mL for dripping after 12 h at 20 °C), whereas a decrease in furan levels was observed with increasing extraction temperature and time (10.1 ± 0.2 ng/mL for steeping after 24 h at 20 °C and 12.3 ± 0.3 ng/mL for dripping after 12 h at 20 °C). Total phenolic content increased with increasing extraction time and independently from brewing method, especially with samples prepared for 24 h steeping at 20 °C, 18.9 ± 0.3 µmol GAE/mL [64,82].
In addition, HPLC coupled to a diode array detector (HPLC-DAD) at 280 nm and 325 nm was used to target key coffee constituents, i.e., caffeine and 3-chlorogenic acid (3-CGA), respectively, in the context of the different extraction parameters, including time, roasting temperature, and grind size, in cold and hot brews [85]. After 400 min of brewing time, results revealed that 3-CGA was more abundant in cold brew coffee made with medium roast coffees, detected at a concentration of 490 ± 30 mg/L. Moreover, caffeine showed higher levels in cold brew of coarse grind samples (1130 ± 50 mg/L) than in their hot counterparts [86]. HPLC–DAD combined with spectral deconvolution showed potential for the quantitation of different coffee brews’ diterpenes, i.e., cafestol and kahweol as esters of linoleate, oleate, palmitate and stearate [87,88], adjusted at 225 and 290 nm for cafestol esters and kahweol esters, respectively. Results demonstrated that boiled coffee showed highest diterpene ester levels (232 mg/L and 1016 mg/L for total cafestol and kahweol esters, respectively), whereas instant brews showed lowest levels at 1.3 mg/L and 2.0 mg/L for total cafestol esters and kahweol esters in instant espresso, respectively [87]. Previous reports showed that cafestol and kahweol are typically found in the range of 182–1308 and 0–1265 mg/100 g, respectively [89]. Diverse health-promoting benefits of coffee, including anti-inflammatory, immunomodulatory, anti-tumor, anti-diabetic, hepato-, cardio-, and neuroprotective effects are attributed to such diterpene content [90].
4.3. Direct Spectroscopy Techniques, i.e., IR and NMR
Compared to hyphenated chromatographic techniques, i.e., GC/MS and LC/MS, NMR is not commonly applied in coffee brewing studies. However, NMR allowed the successful differentiation between diverse brewing methods, including cold and hot brew, aided by PCA modelling of the full NMR spectra [91]. The result of this study revealed that the levels of chlorogenic acid, caffeine, acetic acid, 5-HMF, lactic acid, and trigonelline were increased with of ultrasonication assisted extraction by 71%, 26%, 21%, 16%, 81%, and 19%, respectively, after a one-hour cold brew extraction compared to an extraction without agitation.
IR was also applied in a few studies on coffee brewing in order to aid the sensory quality evaluation of Brazilian arabica coffee brews [92]. The various coffee brews were prepared by hot infusion and the results showed that green coffee brews with high quality scores were associated with low levels of caffeine and protein chlorogenic acids, in addition to high levels of cafestol, sucrose/acid and cafestol/kahweol ratios [92].
5. Coffee Authentication and Adulteration Detection
Metabolomics approaches are increasingly reported for herbal drug authentication and quality control [93,94]. According to the reports of the International Coffee Organization (ICO, London, UK), coffee adulteration either intentionally or accidentally is a serious issue threatening the coffee market [95], mainly through the replacement of coffee powder with other, cheaper products [31]. More than adulteration detection, the development of methods to detect and identify coffee substitutes is of equal value, as is the discrimination between natural coffee and substitutes, including cereal grains (e.g., corn, barley, soy, oat, and rice) and legumes (e.g., lupin seeds and nuts) or chicory root [96,97]. Practically, authentication approaches are always based on either targeting specific markers of adulterants, or through the non-targeted fingerprinting approach [98]. Different metabolomics-based platforms were applied, including direct NMR spectroscopy, infrared/Raman spectroscopy, and UV-Vis versus hyphenated chromatographic technique coupled to mass spectrometry (i.e., GC/MS and LC/MS) [99], as highlighted in the next subsections.
5.1. GC/MS
Static headspace GC–MS (SHS–GC/MS) combined with chemometric tools was applied for the authentication of elephant dung coffee (Black Ivory Coffee) via its volatile profile [100]. Elephant dung coffee is a unique non-bitter Thai coffee derived from C. arabica collected from feces after consumption by Asian elephants to improve its organoleptic properties via fermentation in the animal gut [101]. Among 78 identified VOCs, 3-methyl-1-butanol, 2-methyl-1-butanol, 2-furfurylfuran, and 3-penten-2-one have been recognized as potential discriminant markers for elephant dung coffee [100].
Authentication and quality control through the non-volatile constituents can be also carried out by GC/MS, but after derivatization. Considering the premium quality of Arabic versus robusta coffee, the identification of markers for each type is warranted for QC purposes. GC/MS post silylation profiling, targeting nutrients in arabica and robusta, led to the detection of 143 metabolites, with arabica seeds found to be more enriched in fatty and organic acids, i.e., palmitic acid and acetic acid, respectively. In contrast, robusta was more rich in cyclitol sugars, i.e., myo-inositol and sorbitol [22]. However, these markers need to be confirmed by analyzing several other specimens of other origins, considering that macronutrients are highly affected by plant agricultural conditions and ecological backgrounds, as pointed out earlier.
5.2. LC/MS and LC/UV
LC-based quality control of coffee products has been comprehensively reviewed previously, and it is considered a targeted analytical platform using carbohydrates and other phenolic compounds as markers [99]. Moreover, flavoromics-based untargeted multidimensional preparative LC/MS analysis, with the aid of 1D- and 2D-NMR, could reveal different metabolites that positively and negatively affect coffee quality based on cup score. Three compounds were reported to be associated with an increase in coffee quality, including 3-O-caffeoyl-4-O-3-methylbutanoylquinic acid, 3-O-caffeoyl-4-O-3-methylbutanoyl-1,5-quinide, and an unknown phenolic derivative containing a 3-methylbutanoyl moiety [102]. In contrast, six metabolites were associated with low cup score, including ent-kaurane diterpenes, i.e., 16α,17-dihydroxy-ent-kauran-19-oic acid, 16α,17-dihydroxy-ent-kauran-19-diglycoside, 16α,17,18-trihydroxy-ent-kauran-19-oic acid, and 16α-hydroxy-17-ent-kauren-19-oic acid reported from green seeds. These findings were observed from the analysis of C. arabica cultivated in different countries worldwide [103]. Furthermore, Gao et al. attempted to modify the bitter flavor of coffee brew through the identification of the bitter modulators with the aid of untargeted LC/MS profiling combined with descriptive sensory analysis and modelling using OPLS. 4-CQA, 5-CQA, and 2-O-β-d-glucopyranosyl-atractyligenin were verified to suppress the bitterness perception [104], in agreement with Blumberg et al.’s findings [105].
Moreover, the antioxidant power of coffee seeds has been successfully related to metabolites tentatively identified by UHPLC-ESI-HRMS. PLS-DA showed that caffeoylquinic acids and caffeine are mostly related to the antioxidant effects of unroasted coffee, while dicaffeoyl quinolactone and melanoidins are responsible in roasted coffee [15].
Furthermore, UPLC-HRMS was used for coffee adulteration detection based on the oligosaccharide profiling of common adulterants, i.e., soybeans and rice, in ground coffee as low as 5% [96]. About 17 oligosaccharides were identified and calculated by Glycoworkbench as potential candidates, including Hex5-Hex14 and Hex2Pen4&Hex5AcHex of molecular masses in the range of 0.9–2.0 kDa.
Untargeted high-performance liquid chromatography coupled to ultraviolet (HPLC–UV) fingerprinting analyzed using PLS-DA led to the detection and estimation of coffee frauds by mixing coffees of different geographical origin. The results showed a detection and quantitation of the adulterant levels down to 15%. Moreover, the validation parameters, including calibration, cross-validation, and prediction errors which were determined to be below 2.9%, 6.5%, and 8.9%, respectively [19], were suggestive of no model overfit. Moreover, HPLC coupled to ultraviolet and fluorescence detection (HPLC–UV–FLD) was employed for the detection of three common adulterants, including chicory, barley, and flours. The proposed methodology could assess coffee authenticity and quantify adulteration levels down to 15% [106].
5.3. Direct Spectroscopy, i.e., NMR, UV, and IR
Despite NMR’s potential as a metabolomic tool for marker identification and strong structural elucidation, it is less used compared to GC/MS and LC/MS [107]. qNMR could discriminate between arabica vs robusta, in addition to the prediction of robusta percentile in coffee blends based on the quantification of alkaloids (e.g., caffeine, trigonelline), caffeoylquinic acids (e.g., 3- and 5-CQA), diterpenes (e.g., cafestol, kahweol, and 16-O-methylcafestol (16-OMC), and organic acids (E.G., acetic acid). The results showed that diterpenes are potential discriminators; 100% of arabica seeds had low 16-OMC, while robusta had low kahweol content [108]. Furthermore, β-ethanolamine has been reported as roasting marker in arabica and robusta, as revealed by NMR aided with multivariate data analyses [22].
Increasing robusta level in coffee mixture has been found to lower its quality compared to pure arabica coffee. Compared to arabica seeds, robusta is more abundant in caffeine and myo-inositol [22] and can rationalize the enrichment of caffeine in instant coffee products being produced mostly from robusta seeds [69]. Interestingly, modelling of the coffee NMR dataset resulted in a stronger mathematical model, evident from variance coverage and prediction power in comparison to GC/MS data, applied in the discrimination between instant coffee from other roasted products [22]. In addition, authentication of commercial Brazilian arabica blends composed of roasted coffee seeds was effectively based on 1H-NMR through the identification and quantification of common coffee adulterants, including corn, coffee husks, barley, and soybean. The results revealed that the spectral region δH 5.1 to 9.5 ppm provided potential signals that can be used for differentiation between coffee samples and the adulterants, based mostly on the anomeric proton signals of carbohydrates [29].
Moreover, UV fingerprinting with the aid of multivariate data analysis models demonstrated high absorption values, particularly at 350–450 nm, assigned to melanoidin content in roasted seeds and blended coffee products as often consumed in the Middle East region [15]. Moreover, UV-based metabolomics has been proven as a potential alternative for LC/MS and a non-destructive analytical tool for the identification of the roasting-induced toxins, especially acrylamide. Its detection was confirmed by the spiking method, recording an increased optical density at 273 nm, in agreement with Alfarhani [15,109].
Furthermore, previous reports have reported the use of IR for the detection of adulteration in coffee products. For instance, Winkler-Moser et al. authenticated the Brazilian roasted coffee via near infrared (NIR) in the presence of roasted, ground corn based on their tocopherol content in a sensitivity as low as 5% [110]. Moreover, the detection of multiple adulterants, including corn, barley, soybean, rice, and even coffee husks and robusta coffee by NIR in roasted and ground arabica coffee was possible, derived from different geographic origins. The developed method was able to detect adulterants in concentrations ≥10% [111]. In addition, mid-infrared (MIR) Fourier transform spectroscopy (FT-MIR) coupled with chemometrics showed its identifying and quantifying power for arabica coffee adulterants (e.g., 1–30% of corn, barley, soy, oat, rice and coffee husks). The established chemometric models exhibited influential validation parameters of R2c: ≥0.99, in addition to a standard error of calibration (SEC) and standard error of prediction (SEP) of 0.39–0.82 and 0.45–0.94, respectively [97]. Another study used attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR) of the mid region for the detection of four adulterants, i.e., spent coffee grounds, roasted coffee husks, roasted corn, and roasted barley, simultaneously. The validation parameters were improved based on two approaches, including hierarchical models (HM) and data fusion (DF) reaching to 0% misclassified samples in the second level models [112].
6. Conclusions
Coffee brewing is the last step of coffee post-harvest processing to affect aroma and flavor. Various techniques are used, based mostly on consumer and cultural preferences. SPME coupled with GC/MS is the most commonly used platform to unravel volatiles behind the unique aroma, which are mainly generated during roasting processing. Other analytical platforms, including NMR and LC/MS proved suitable for profiling non-volatiles in coffee, particularly cinnamoylquinic and feruloylquinic acid derivatives, warranting the employment of comparative metabolomics approaches to assess coffee composition. Metabolomics-based authentications of coffee products are relatively novel techniques depending on non-targeted analytics combined with chemometric tools. Compared to authenticated coffee seeds of different botanical and geographic origins, roasting degrees, production methods, and blends with other aromatic herbs, it allows the detection of governing molecular components and marker compounds. UV-based metabolomics has gained a particular interest as an alternative tool to the powerful LC/MS for potential marker and toxin identification, including the identification of the carcinogenic roasting metabolite acrylamide. Therefore, metabolomics investigation could successfully and comprehensively assess coffee products for quality control purposes, although it has yet to be applied at commercial levels. Compared to hyphenated techniques, direct spectroscopic measurement (especially simple and cheap IR) appears more suitable for local industrial applications monitoring consistency among batches, similar to methods routinely performed in drug analyses aided by chemometric tools.
Author Contributions
Conceptualization, M.A.F. and L.A.W.; formal analysis, A.Z.; investigation, I.E.S.; resources, M.A.F.; data curation, A.A.-W.; writing—original draft preparation, M.A.F., A.Z., I.E.S. and A.A.-W.; writing—review and editing, M.A.F., A.Z., I.E.S. and L.A.W.; supervision, M.A.F. and L.A.W.; project administration, M.A.F.; funding acquisition, M.A.F., L.A.W. and A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the BMBF-IB through project WTZ Colombia CG-HighValBio 01DN18048 (L.A.W.), Alexander von Humboldt Foundation, Germany (M.A.F.), and “Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)-Project-ID 172116086-SFB 926”. The APC was funded by Mohamed A. Farag and Ludger A. Wessjohann.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and application of coffee and its industrial residues. Food Bioproc. Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.-H.; Park, J.-H.; Im, S.-S.; Song, D.-K. Coffee and health. Integr. Med. Res. 2014, 3, 189–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappelletti, S.; Piacentino, D.; Sani, G.; Aromatario, M. Caffeine: Cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, D.; Abreu, J.; Jordão, N.; Rosa, J.S.; da Freitas-Silva, O.; Teodoro, A. Effect of roasting levels and drying process of Coffea canephora on the quality of bioactive compounds and cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreola, F.; Borghi, A.; Pedrazzi, S.; Allesina, G.; Tartarini, P.; Lancellotti, I.; Barbieri, L. Spent coffee Grounds in the production of lightweight clay ceramic aggregates in view of urban and agricultural sustainable development. Materials 2019, 12, 3581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmud, M.M.C.; Shellie, R.A.; Keast, R. Unravelling the relationship between aroma compounds and consumer acceptance: Coffee as an example. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2380–2420. [Google Scholar] [CrossRef] [PubMed]
- International Coffee Organization. Available online: http://www.ico.org/ (accessed on 24 January 2022).
- Shokouh, P.; Jeppesen, P.B.; Christiansen, C.B.; Mellbye, F.B.; Hermansen, K.; Gregersen, S. Efficacy of arabica versus robusta coffee in improving weight, insulin resistance, and liver steatosis in a rat model of type-2 diabetes. Nutrients 2019, 11, 2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rosso, M.; Colomban, S.; Flamini, R.; Navarini, L. UHPLC-ESI-QqTOF-MS/MS characterization of minor chlorogenic acids in roasted Coffea arabica from different geographical origin. J. Mass Spectrom. 2018, 53, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.A.; Pritts, A.A.; Zwetsloot, M.J.; Jansen, K.; Pulleman, M.M.; Armbrecht, I.; Avelino, J.; Barrera, J.F.; Bunn, C.; García, J.H.; et al. Transformation of coffee-growing landscapes across Latin America. A review. Agron. Sustain. Dev. 2021, 41, 62. [Google Scholar] [CrossRef]
- Mendes, G.F.; Reis, C.E.; Nakano, E.Y.; da Costa, T.H.; Saunders, B.; Zandonadi, R.P. Translation and validation of the caffeine expectancy questionnaire in Brazil (CaffEQ-BR). Nutrients 2020, 12, 2248. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdulkader, A.M.; Al-Namazi, A.A.; AlTurki, T.A.; Al-Khuraish, M.M.; Al-Dakhil, A.I. Optimizing coffee cultivation and its impact on economic growth and export earnings of the producing countries: The case of Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 776–782. [Google Scholar] [CrossRef] [PubMed]
- ESS. FAO Coffee Pocketbook; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; Available online: http://www.fao.org/documents/card/en/c/f0dd1f3c-fddd-48de-b6a6-7b5840ea8d85/ (accessed on 24 January 2022).
- Abdelwareth, A.; Zayed, A.; Farag, M.A. Chemometrics-based aroma profiling for revealing origin, roasting indices, and brewing method in coffee seeds and its commercial blends in the Middle East. Food Chem. 2021, 349, 129162. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, E.A.; Zayed, A.; Laub, A.; Modolo, L.V.; Wessjohann, L.; Farag, M.A. How does LC/MS compare to UV in coffee authentication and determination of antioxidant effects? Brazilian and Middle Eastern coffee as case studies. Antioxidants 2022, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Sonam, K.S.; Guleria, S. Synergistic antioxidant activity of natural products. Ann. Pharmacol. Pharm. 2017, 2, 1086. [Google Scholar]
- Yashin, A.; Yashin, Y.; Wang, J.Y.; Nemzer, B. Antioxidant and antiradical activity of coffee. Antioxidants 2013, 2, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Otify, A.M.; El-Sayed, A.M.; Michel, C.G.; Farag, M.A. Metabolites profiling of date palm (Phoenix dactylifera L.) commercial by-products (pits and pollen) in relation to its antioxidant effect: A multiplex approach of MS and NMR metabolomics. Metabolomics 2019, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Núñez, N.; Collado, X.; Martínez, C.; Saurina, J.; Núñez, O. Authentication of the origin, variety and roasting degree of coffee samples by non-targeted HPLC-UV fingerprinting and chemometrics. Application to the detection and quantitation of adulterated coffee samples. Foods 2020, 9, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toci, A.T.; Farah, A.; Pezza, H.R.; Pezza, L. Coffee adulteration: More than two decades of research. Crit. Rev. Anal. Chem. 2016, 46, 83–92. [Google Scholar] [CrossRef]
- Nunez, N.; Martinez, C.; Saurina, J.; Nunez, O. High-performance liquid chromatography with fluorescence detection fingerprints as chemical descriptors to authenticate the origin, variety and roasting degree of coffee by multivariate chemometric methods. J. Sci. Food Agric. 2020, 101, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Abdelwareth, A.; Mohamed, T.A.; Fahmy, H.A.; Porzel, A.; Wessjohann, L.A.; Farag, M.A. Dissecting coffee seeds metabolome in context of genotype, roasting degree, and blending in the Middle East using NMR and GC/MS techniques. Food Chem. 2022, 373, 131452. [Google Scholar] [CrossRef]
- Craig, A.P.; Franca, A.S.; Oliveira, L.S. Discrimination between defective and non-defective roasted coffees by diffuse reflectance infrared Fourier transform spectroscopy. LWT 2012, 47, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Hegazi, N.M.; Khattab, A.R.; Frolov, A.; Wessjohann, L.A.; Farag, M.A. Authentication of saffron spice accessions from its common substitutes via a multiplex approach of UV/VIS fingerprints and UPLC/MS using molecular networking and chemometrics. Food Chem. 2022, 367, 130739. [Google Scholar] [CrossRef] [PubMed]
- van der Hooft, J.J.J.; Rankin, N. Metabolite Identification in Complex Mixtures Using Nuclear Magnetic Resonance Spectroscopy. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1309–1341. [Google Scholar]
- Terrile, A.E.; Marcheafave, G.G.; Oliveira, G.S.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S. Chemometric analysis of UV characteristic profile and infrared fingerprint variations of Coffea arabica green beans under different space management treatments. J. Braz. Chem. Soc. 2016, 27, 1254–1263. [Google Scholar]
- Bobková, A.; Hudáček, M.; Jakabová, S.; Belej, Ľ.; Capcarová, M.; Čurlej, J.; Bobko, M.; Árvay, J.; Jakab, I.; Čapla, J.; et al. The effect of roasting on the total polyphenols and antioxidant activity of coffee. J. Environ. Sci. Health B 2020, 55, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Unraveling the active hypoglycemic agent trigonelline in Balanites aegyptiaca date fruit using metabolite fingerprinting by NMR. J. Pharm. Biomed. 2015, 115, 383–387. [Google Scholar] [CrossRef] [PubMed]
- de Moura Ribeiro, M.V.; Boralle, N.; Redigolo Pezza, H.; Pezza, L.; Toci, A.T. Authenticity of roasted coffee using 1H NMR spectroscopy. J. Food Compost. Anal. 2017, 57, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, P.I.; Santos, J.S.; Rodionova, O.Y.; Pomerantsev, A.; Chaves, E.S.; Rosso, N.D.; Granato, D. Chemometric Authentication of Brazilian Coffees Based on Chemical Profiling. J. Food Sci. 2019, 84, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Anagbogu, C.F.; Ilori, C.O.; Bhattacharjee, R.; Olaniyi, O.O.; Beckles, D.M. Gas chromatography-mass spectrometry and single nucleotide polymorphism-genotype-by-sequencing analyses reveal the bean chemical profiles and relatedness of Coffea canephora genotypes in Nigeria. Plants 2019, 8, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ongo, E.A.; Montevecchi, G.; Antonelli, A.; Sberveglieri, V.; Sevilla, F., III. Metabolomics fingerprint of Philippine coffee by SPME-GC-MS for geographical and varietal classification. Food Res. Int. 2020, 134, 109227. [Google Scholar] [CrossRef]
- Putri, S.; Irifune, T.; Yusianto; Fukusaki, E. GC/MS based metabolite profiling of Indonesian specialty coffee from different species and geographical origin. Metabolomics 2019, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- da Silva Taveira, J.H.; Borém, F.M.; Figueiredo, L.P.; Reis, N.; Franca, A.S.; Harding, S.A.; Tsai, C.-J. Potential markers of coffee genotypes grown in different Brazilian regions: A metabolomics approach. Food Res. Int. 2014, 61, 75–82. [Google Scholar] [CrossRef]
- Stamler, J.; Brown, I.J.; Daviglus, M.L.; Chan, Q.; Kesteloot, H.; Ueshima, H.; Zhao, L.; Elliott, P.; Group, I.R. Glutamic acid, the main dietary amino acid, and blood pressure: The INTERMAP Study (International Collaborative Study of Macronutrients, Micronutrients and Blood Pressure). Circulation 2009, 120, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.-Y.; Choi, W.; Park, J.H.; Lim, J.; Kwon, S.W. Determination of coffee origins by integrated metabolomic approach of combining multiple analytical data. Food Chem. 2010, 121, 1260–1268. [Google Scholar] [CrossRef]
- Gamboa-Becerra, R.; Hernández-Hernández, M.C.; González-Ríos, Ó.; Suárez-Quiroz, M.L.; Gálvez-Ponce, E.; Ordaz-Ortiz, J.J.; Winkler, R. Metabolomic markers for the early selection of Coffea canephora plants with desirable cup quality traits. Metabolites 2019, 9, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toci, A.T.; de Moura Ribeiro, M.V.; de Toledo, P.R.A.B.; Boralle, N.; Pezza, H.R.; Pezza, L. Fingerprint and authenticity roasted coffees by 1H-NMR: The Brazilian coffee case. Food Sci. Biotechnol. 2018, 27, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Arana, V.A.; Medina, J.; Alarcon, R.; Moreno, E.; Heintz, L.; Schäfer, H.; Wist, J. Coffee’s country of origin determined by NMR: The Colombian case. Food Chem. 2015, 175, 500–506. [Google Scholar] [CrossRef]
- Choi, W.S.; In, Y.W.; Kim, H.H.; Hyun, J.-S.; Park, S.J. Metabolic features of coffee beans depending on planted areas. J. Korean Magn. Reson. Soc. 2017, 21, 44–49. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [Green Version]
- El-Hawary, S.S.; El-Kammar, H.A.; Farag, M.A.; Saleh, D.O.; El Dine, R.S. Metabolomic profiling of five Agave leaf taxa via UHPLC/PDA/ESI-MS in relation to their anti-inflammatory, immunomodulatory and ulceroprotective activities. Steroids 2020, 160, 108648. [Google Scholar] [CrossRef]
- Villalón-López, N.; Serrano-Contreras, J.I.; Téllez-Medina, D.I.; Gerardo Zepeda, L. An 1H NMR-based metabolomic approach to compare the chemical profiling of retail samples of ground roasted and instant coffees. Food Res. Int. 2018, 106, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Murakami, K.; Ikeda, M.; Iwatsuki, K.; Kokubo, S.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H.; Tanaka, K. Characterization of flavor compounds released during grinding of roasted robusta coffee beans. Food Sci. Technol. Res. 2005, 11, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; Knight, S.; Surucu, B.; Kuhnert, N. Characterization by LC-MS(n) of four new classes of chlorogenic acids in green coffee beans: Dimethoxycinnamoylquinic acids, diferuloylquinic acids, caffeoyl-dimethoxycinnamoylquinic acids, and feruloyl-dimethoxycinnamoylquinic acids. J. Agric. Food Chem. 2006, 54, 1957–1969. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Navarini, L.; Colomban, S.; Forzato, C. Hydroxycinnamoyl amino acids conjugates: A chiral pool to distinguish commercially exploited Coffea spp. Molecules 2020, 25, 1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalmach, A.; Mullen, W.; Nagai, C.; Crozier, A. On-line HPLC analysis of the antioxidant activity of phenolic compounds in brewed, paper-filtered coffee. Braz. J. Plant Physiol. 2006, 18, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, R.; Matei, M.F.; Subedi, P.; Kuhnert, N. Does roasted coffee contain chlorogenic acid lactones or/and cinnamoylshikimate esters? Food Res. Int. 2014, 61, 214–227. [Google Scholar] [CrossRef]
- Pérez-Míguez, R.; Sánchez-López, E.; Plaza, M.; Castro-Puyana, M.; Marina, M.L. A non-targeted metabolomic approach based on reversed-phase liquid chromatography–mass spectrometry to evaluate coffee roasting process. Anal. Bioanal. Chem. 2018, 410, 7859–7870. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, J.Á.; Leake, D.S.; Ames, J.M. In Vitro Antioxidant Activity of Coffee Compounds and Their Metabolites. J. Agric. Food Chem. 2007, 55, 6962–6969. [Google Scholar] [CrossRef]
- Mahrous, E.A.; Farag, M.A. Two dimensional NMR spectroscopic approaches for exploring plant metabolome: A review. J. Adv. Res. 2015, 6, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Barbin, D.F.; de Souza Madureira Felicio, A.L.; Sun, D.-W.; Nixdorf, S.L.; Hirooka, E.Y. Application of infrared spectral techniques on quality and compositional attributes of coffee: An overview. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Stanek, N.; Zarębska, M.; Biłos, Ł.; Barabosz, K.; Nowakowska-Bogdan, E.; Semeniuk, I.; Błaszkiewicz, J.; Kulesza, R.; Matejuk, R.; Szkutnik, K. Influence of coffee brewing methods on the chromatographic and spectroscopic profiles, antioxidant and sensory properties. Sci. Rep. 2021, 11, 21377. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Cortese, M.; Sagratini, G.; Vittori, S. The influence of different types of preparation (espresso and brew) on coffee aroma and main bioactive constituents. Int. J. Food Sci. 2015, 66, 505–513. [Google Scholar] [CrossRef]
- Heo, J.; Choi, K.S.; Wang, S.; Adhikari, K.; Lee, J. Cold Brew Coffee: Consumer acceptability and characterization using the check-all-that-apply (CATA) method. Foods 2019, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, G.; Guerrini, L.; Masella, P.; Innocenti, M.; Bellumori, M.; Parenti, A. Characterization and comparison of cold brew and cold drip coffee extraction methods. J. Sci. Food Agric. 2019, 99, 391–399. [Google Scholar] [CrossRef]
- Guenther, H.; Hoenicke, K.; Biesterveld, S.; Gerhard-Rieben, E.; Lantz, I. Furan in coffee: Pilot studies on formation during roasting and losses during production steps and consumer handling. Food Addit. Contam. Part A 2010, 27, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical characteristics of hot and cold brew coffee chemistry: The effects of roast level and brewing temperature on compound extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What kind of coffee do you drink? An investigation on effects of eight different extraction methods. Food Res. Int. 2019, 116, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Hu, G.; Hong, D.; Guo, T.; Li, J.; Li, Z.; Qiu, M. Review on factors affecting coffee volatiles: From seed to cup. J. Sci. Food Agric. 2022, 102, 1341–1352. [Google Scholar] [CrossRef]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.M.; Ruiz, R.Y. Effect of grinding, extraction time and type of coffee on the physicochemical and flavour characteristics of cold brew coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef] [Green Version]
- Córdoba, N.; Moreno, F.L.; Osorio, C.; Velásquez, S.; Ruiz, Y. Chemical and sensory evaluation of cold brew coffees using different roasting profiles and brewing methods. Food Res. Int. 2021, 141, 110141. [Google Scholar] [CrossRef]
- Han, J.W.; Boo, H.; Chung, M.S. Effects of extraction conditions on acrylamide/furan content, antioxidant activity, and sensory properties of cold brew coffee. Food Sci. Biotechnol. 2020, 29, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The effect of brewing process parameters on antioxidant activity and caffeine content in infusions of roasted and unroasted arabica coffee beans originated from different countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef] [PubMed]
- Derossi, A.; Ricci, I.; Caporizzi, R.; Fiore, A.; Severini, C. How grinding level and brewing method (Espresso, American, Turkish) could affect the antioxidant activity and bioactive compounds in a coffee cup. J. Sci. Food Agric. 2018, 98, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Gavrilyuk, O.; Braaten, T.; Skeie, G.; Weiderpass, E.; Dumeaux, V.; Lund, E. High coffee consumption and different brewing methods in relation to postmenopausal endometrial cancer risk in the Norwegian women and cancer study: A population-based prospective study. BMC Womens Health 2014, 14, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothwell, J.A.; Loftfield, E.; Wedekind, R.; Freedman, N.; Kambanis, C.; Scalbert, A.; Sinha, R. A metabolomic study of the variability of the chemical composition of commonly consumed coffee brews. Metabolites 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Olechno, E.; Puścion-Jakubik, A.; Socha, K.; Zujko, M.E. Coffee infusions: Can they be a source of microelements with antioxidant properties? Antioxidants 2021, 10, 1709. [Google Scholar] [CrossRef]
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Kapczuk, P.; Kochman, J.; Rębacz-Maron, E.; Gutowska, I. Mineral composition and antioxidant potential of coffee beverages depending on the brewing method. Foods 2020, 9, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E.; Socha, K. influence of various factors on caffeine content in coffee brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef]
- Park, S.H.; Jo, A.; Lee, K.G. Effect of various roasting, extraction and drinking conditions on furan and 5-hydroxymethylfurfural levels in coffee. Food Chem. 2021, 358, 129806. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H. Analysis of the headspace volatiles of freshly brewed arabica coffee using solid-phase microextraction. J. Food Sci. 2007, 72, 388–396. [Google Scholar] [CrossRef] [PubMed]
- López-Galilea, I.; Fournier, N.; Cid, C.; Guichard, E. Changes in headspace volatile concentrations of coffee brews caused by the roasting process and the brewing procedure. J. Agric. Food Chem. 2006, 54, 8560–8566. [Google Scholar] [CrossRef]
- Angeloni, S.; Mustafa, A.M.; Abouelenein, D.; Alessandroni, L.; Acquaticci, L.; Nzekoue, F.K.; Petrelli, R.; Sagratini, G.; Vittori, S.; Torregiani, E.; et al. Characterization of the aroma profile and main key odorants of espresso coffee. Molecules 2021, 26, 3856. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-M.; Chu, M.; Park, H.; Park, J.; Lee, K.-G. Analysis of Volatile Compounds in Coffee Prepared by Various Brewing and Roasting Methods. Foods 2021, 10, 1347. [Google Scholar] [CrossRef]
- Lolli, V.; Acharjee, A.; Angelino, D.; Tassotti, M.; Del Rio, D.; Mena, P.; Caligiani, A. Chemical Characterization of Capsule-Brewed Espresso Coffee Aroma from the Most Widespread Italian Brands by HS-SPME/GC-MS. Molecules 2020, 25, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakidou, P.; Plati, F.; Matsakidou, A.; Varka, E.-M.; Blekas, G.; Paraskevopoulou, A. Single origin coffee aroma: From optimized flavor protocols and coffee customization to instrumental volatile characterization and chemometrics. Molecules 2021, 26, 4609. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Adhikari, K.; Choi, K.S.; Lee, J. Analysis of caffeine, chlorogenic acid, trigonelline, and volatile compounds in cold brew coffee using high-performance liquid chromatography and solid-phase microextraction-gas chromatography-mass spectrometry. Foods 2020, 9, 1746. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Murakami, K.; Hirano, Y.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H. Characterization of headspace aroma compounds of freshly brewed arabica coffees and studies on a characteristic aroma compound of Ethiopian coffee. J. Food Sci. 2008, 73, C335–C346. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, D.; Roach, J.A.; Gay, M.L.; Musser, S.M. Analysis of coffee for the presence of acrylamide by LC-MS/MS. J. Agric. Food Chem. 2004, 52, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Gruczyńska, E.; Kowalska, D.; Kozłowska, M.; Majewska, E.; Tarnowska, K. Furan in roasted, ground and brewed coffee. Rocz. Panstw. Zakl. Hig. 2018, 69, 111–118. [Google Scholar]
- Liu, R.; Zha, L.; Sobue, T.; Kitamura, T.; Ishihara, J.; Kotemori, A.; Ikeda, S.; Sawada, N.; Iwasaki, M.; Tsugane, S. Dietary acrylamide intake and risk of lung cancer: The Japan public health center based prospective study. Nutrients 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Başaran, B.; Aydın, F.; Kaban, G. The determination of acrylamide content in brewed coffee samples marketed in Turkey. Food Addit. Contam. Part A 2020, 37, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.; Rao, N.Z. The effect of time, roasting temperature, and grind size on caffeine and chlorogenic acid concentrations in cold brew coffee. Sci. Rep. 2017, 7, 17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeenfard, M.; Erny, G.L.; Alves, A. Variability of some diterpene esters in coffee beverages as influenced by brewing procedures. J. Food Sci. Technol. 2016, 53, 3916–3927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erny, G.L.; Moeenfard, M.; Alves, A. Liquid chromatography with diode array detection combined with spectral deconvolution for the analysis of some diterpene esters in Arabica coffee brew. J. Sep. Sci. 2015, 38, 612–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeenfard, M.; Alves, A. New trends in coffee diterpenes research from technological to health aspects. Food Res. Int. 2020, 134, 109207. [Google Scholar] [CrossRef]
- Islam, M.T.; Tabrez, S.; Jabir, N.R.; Ali, M.; Kamal, M.A.; da Silva Araujo, L.; De Oliveira Santos, J.V.; Da Mata, A.; De Aguiar, R.P.S.; de Carvalho Melo Cavalcante, A.A. An insight into the therapeutic potential of major coffee components. Curr. Drug Metab. 2018, 19, 544–556. [Google Scholar] [CrossRef]
- Claassen, L.; Rinderknecht, M.; Porth, T.; Röhnisch, J.; Seren, H.Y.; Scharinger, A.; Gottstein, V.; Noack, D.; Schwarz, S.; Winkler, G.; et al. Cold brew coffee-pilot studies on definition, extraction, consumer preference, chemical characterization and microbiological hazards. Foods 2021, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.d.S.G.; Scholz, M.B.d.S.; Kitzberger, C.S.G.; Benassi, M.d.T. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef]
- Salem, M.A.; Zayed, A.; Alseekh, S.; Fernie, A.R.; Giavalisco, P. The integration of MS-based metabolomics and multivariate data analysis allows for improved quality assessment of Zingiber officinale Roscoe. Phytochemistry 2021, 190, 112843. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.C.; Lima, L.; Heleno, S.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Food metabolites as tools for authentication, processing, and nutritive value assessment. Foods 2021, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Ting, H.; Jin-Lan, Z. Novel identification strategy for ground coffee adulteration based on UPLC-HRMS oligosaccharide profiling. Food Chem. 2016, 190, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.M.; Ali, E.; Gamal, M.; Farag, M.A. How do coffee substitutes compare to coffee? A comprehensive review of its quality characteristics, sensory characters, phytochemicals, health benefits and safety. Food Biosci. 2021, 43, 101290. [Google Scholar] [CrossRef]
- Flores-Valdez, M.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velázquez, T. Identification and quantification of adulterants in coffee (Coffea arabica L.) using FT-MIR spectroscopy coupled with chemometrics. Foods 2020, 9, 851. [Google Scholar] [CrossRef]
- Wang, X.; Lim, L.T.; Fu, Y. Review of analytical methods to detect adulteration in coffee. J. AOAC Int. 2020, 103, 295–305. [Google Scholar] [CrossRef]
- Cheah, W.L.; Fang, M. HPLC-based chemometric analysis for coffee adulteration. Foods 2020, 9, 880. [Google Scholar] [CrossRef]
- Thammarat, P.; Kulsing, C.; Wongravee, K.; Leepipatpiboon, N.; Nhujak, T. Identification of volatile compounds and selection of discriminant markers for elephant dung coffee using static headspace gas chromatography-mass spectrometry and chemometrics. Molecules 2018, 23, 1910. [Google Scholar] [CrossRef] [Green Version]
- Haile, M.; Bae, H.M.; Kang, W.H. Comparison of the antioxidant activities and volatile compounds of coffee beans obtained using digestive bio-processing (elephant dung coffee) and commonly known processing methods. Antioxidants 2020, 9, 408. [Google Scholar] [CrossRef]
- Sittipod, S.; Schwartz, E.; Paravisini, L.; Peterson, D.G. Identification of flavor modulating compounds that positively impact coffee quality. Food Chem. 2019, 301, 125250. [Google Scholar] [CrossRef]
- Sittipod, S.; Schwartz, E.; Paravisini, L.; Tello, E.; Peterson, D.G. Identification of Compounds that negatively impact coffee flavor quality using untargeted liquid chromatography/mass spectrometry analysis. J. Agric. Food Chem. 2020, 68, 10424–10431. [Google Scholar] [CrossRef]
- Gao, C.; Tello, E.; Peterson, D.G. Identification of coffee compounds that suppress bitterness of brew. Food Chem. 2021, 350, 129225. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, S.; Frank, O.; Hofmann, T. Quantitative studies on the influence of the bean roasting parameters and hot water percolation on the concentrations of bitter compounds in coffee brew. J. Agric. Food Chem. 2010, 58, 3720–3728. [Google Scholar] [CrossRef] [PubMed]
- Núñez, N.; Saurina, J.; Núñez, O. Authenticity assessment and fraud quantitation of coffee adulterated with chicory, barley, and flours by untargeted HPLC-UV-FLD fingerprinting and chemometrics. Foods 2021, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, I.W.; Martinez Farina, C.F.; Ragupathy, S.; Arunachalam, T.; Newmaster, S.; Berrué, F. Quantitative NMR methodology for the authentication of roasted coffee and prediction of blends. J. Agric. Food Chem. 2020, 68, 14643–14651. [Google Scholar] [CrossRef] [PubMed]
- Alfarhani, B. Analysis of acrylamide levels in various food types in the Iraqi markets using chromatography techniques. J. Mater. Environ. Sci. 2017, 8, 4902. [Google Scholar]
- Winkler-Moser, J.K.; Singh, M.; Rennick, K.A.; Bakota, E.L.; Jham, G.; Liu, S.X.; Vaughn, S.F. Detection of corn adulteration in Brazilian coffee (Coffea arabica) by tocopherol profiling and near-infrared (NIR) spectroscopy. J. Agric. Food Chem. 2015, 63, 10662–10668. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Couto, C.; Freitas-Silva, O.; Morais Oliveira, E.M.; Sousa, C.; Casal, S. Near-infrared spectroscopy applied to the detection of multiple adulterants inroasted and ground arabica coffee. Foods 2021, 11, 61. [Google Scholar] [CrossRef]
- Reis, N.; Botelho, B.G.; Franca, A.S.; Oliveira, L.S. Simultaneous detection of multiple adulterants in ground roasted coffee by ATR-FTIR spectroscopy and data fusion. Food Anal. Methods 2017, 10, 2700–2709. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).