Acute Effects of Split Pea-Enriched White Pan Bread on Postprandial Glycemic and Satiety Responses in Healthy Volunteers—A Randomized Crossover Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics and Study Design
2.3. Bread Products
2.4. Nutrient Analysis
2.5. Study Protocol
2.6. Glucose and Insulin Measurements
2.7. Satiety Assessment
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Blood Glucose and Plasma Insulin Responses
3.3. Satiety Response
3.4. Acceptability of Bread Products
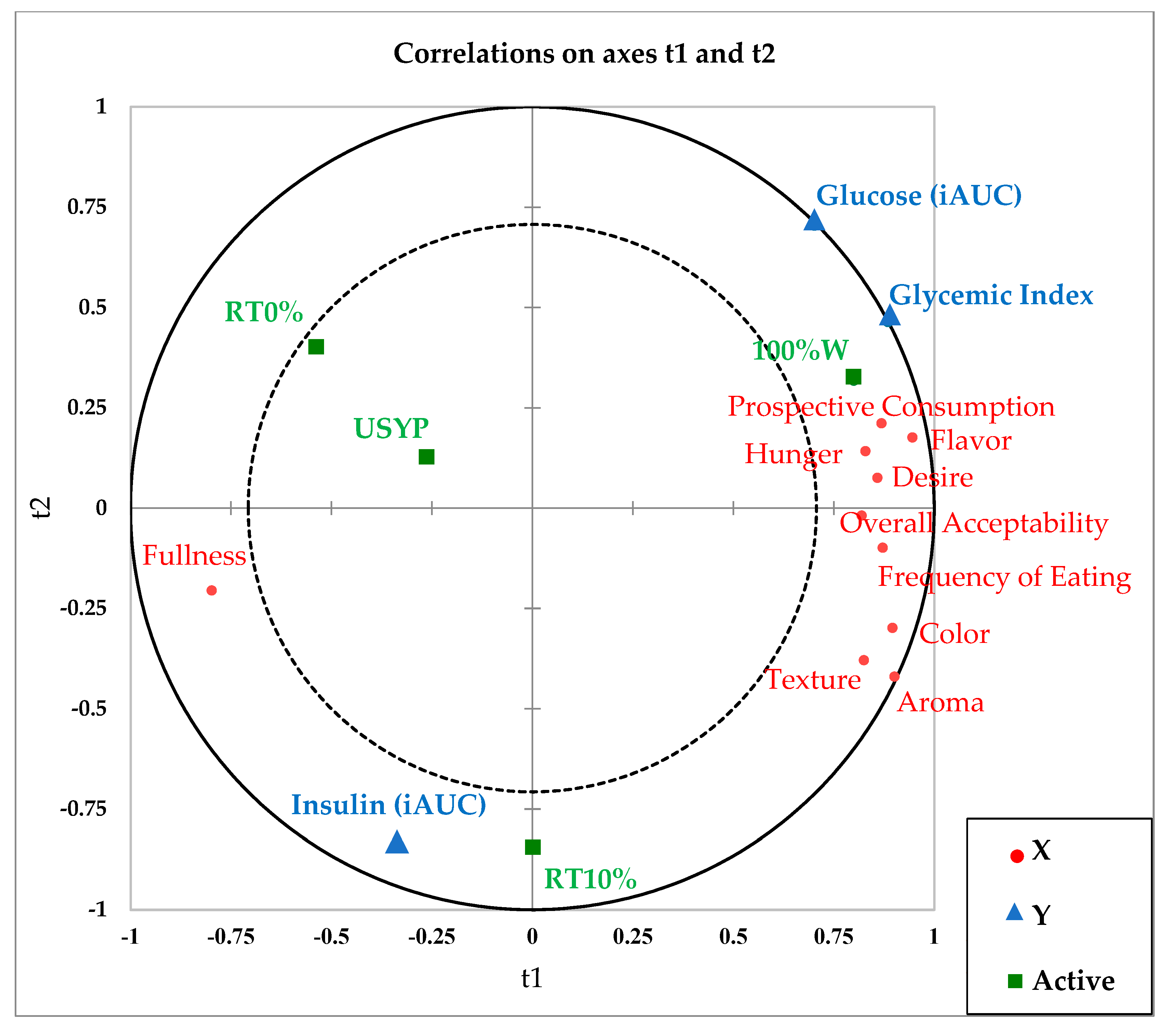
3.5. PLS-R
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Twells, L.K.; Janssen, I.; Kuk, J.L. Canadian Adult Obesity Clinical Practice Guidelines: Epidemiology of Adult Obesity. 2020. Available online: Https://Obesitycanada.ca/Guidelines/Epidemiology (accessed on 10 January 2022).
- World Health Organisation Obesity and Overweight (WHO). Available online: https://www.Who.Int/News-Room/Fact-Sheets/Detail/Obesity-and-Overweight (accessed on 13 September 2021).
- Public Health Agency of Canada Canadian Risk Factor Atlas (CRFA), 2020 Edition. 2020. Available online: Https://Health-Infobase.Canada.ca/Crfa/ (accessed on 10 January 2022).
- Barnes, A.S. The Epidemic of Obesity and Diabetes: Trends and Treatments. Tex. Heart Inst. J. 2011, 38, 3. [Google Scholar]
- Ghanem, S. Diabetes in Canada. 2020, p. 8. Available online: https://www.Diabetes.ca/DiabetesCanadaWebsite/Media/Advocacy-and-Policy/Backgrounder/2020_Backgrounder_Canada_English_FINAL.Pdf (accessed on 10 January 2022).
- Augustin, L.S.; Franceschi, S.; Jenkins, D.J.A.; Kendall, C.W.C.; La Vecchia, C. Glycemic Index in Chronic Disease: A Review. Eur. J. Clin. Nutr. 2002, 56, 1049–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Ibarrola-Jurado, N.; Rabassa-Soler, A.; Díaz-López, A.; Guasch-Ferré, M.; Hernández-Alonso, P.; Balanza, R.; Bulló, M. Effect of the Glycemic Index of the Diet on Weight Loss, Modulation of Satiety, Inflammation, and Other Metabolic Risk Factors: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2014, 100, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic Index of Foods: A Physiological Basis for Carbohydrate Exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic Index, Glycemic Load, and Chronic Disease Risk—A Meta-Analysis of Observational Studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef]
- Jayedi, A.; Soltani, S.; Jenkins, D.; Sievenpiper, J. Dietary Glycemic Index, Glycemic Load, and Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Crit. Rev. Food Sci. Nutr. 2020, 1–10. [Google Scholar]
- Ma, X.; Liu, J.; Song, Z. Glycemic Load, Glycemic Index and Risk of Cardiovascular Diseases: Meta-Analyses of Prospective Studies. Atherosclerosis 2012, 223, 491–496. [Google Scholar] [CrossRef]
- Du, H.; van der A, D.L.; van Bakel, M.M.E.; Slimani, N.; Forouhi, N.G.; Wareham, N.J.; Halkjær, J.; Tjønneland, A.; Jakobsen, M.U.; Overvad, K.; et al. Dietary Glycaemic Index, Glycaemic Load and Subsequent Changes of Weight and Waist Circumference in European Men and Women. Int. J. Obes. 2009, 33, 1280–1288. [Google Scholar] [CrossRef] [Green Version]
- Youn, S.; Woo, H.D.; Cho, Y.A.; Shin, A.; Chang, N.; Kim, J. Association between Dietary Carbohydrate, Glycemic Index, Glycemic Load, and the Prevalence of Obesity in Korean Men and Women. Nutr. Res. 2012, 32, 153–159. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Tobias, D.K.; Malik, V.S.; Pan, A.; Hruby, A.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic Index, Glycemic Load, and Risk of Type 2 Diabetes: Results from 3 Large US Cohorts and an Updated Meta-Analysis. Am. J. Clin. Nutr. 2014, 100, 218–232. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.-Y.; Zhang, L.; Zhang, Y.-H.; Qin, L.-Q. Dietary Glycaemic Index and Glycaemic Load in Relation to the Risk of Type 2 Diabetes: A Meta-Analysis of Prospective Cohort Studies. Br. J. Nutr. 2011, 106, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.L.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic Index, Glycemic Load, Carbohydrates, and Type 2 Diabetes: Systematic Review and Dose–Response Meta-Analysis of Prospective Studies. Diabetes Care 2013, 36, 4166–4171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, D.S.; Garvin, J.T.; Xu, H. Carbohydrate Quality, Glycemic Index, Glycemic Load and Cardiometabolic Risks in the US, Europe and Asia: A Dose–Response Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 853–871. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; McKeown-Eyssen, G.; Josse, R.G.; Silverberg, J.; Booth, G.L.; Vidgen, E.; Josse, A.R.; Nguyen, T.H.; Corrigan, S.; et al. Effect of a Low–Glycemic Index or a High–Cereal Fiber Diet on Type 2 Diabetes: A Randomized Trial. JAMA 2008, 300, 2742–2753. [Google Scholar] [CrossRef] [Green Version]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.L.; Gellar, L.; Nathanson, B.H.; Pbert, L.; Ma, Y.; Ockene, I.; Rosal, M.C. Decrease in Glycemic Index Associated with Improved Glycemic Control among Latinos with Type 2 Diabetes. J. Acad. Nutr. Diet. 2015, 115, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Diabetes Canada The Glycemic Index (GI). 2022. Available online: https://www.Diabetes.ca/En-CA/Resources/Tools---Resources/the-Glycemic-Index-(Gi) (accessed on 10 January 2022).
- Bekkering, E. Pulses in Canada. 2015. Available online: https://publications.gc.ca/collections/collection_2014/statcan/96-325-x/CS96-325-2011-7-eng.pdf (accessed on 10 January 2022).
- Fujiwara, N.; Hall, C.; Jenkins, A.L. Development of Low Glycemic Index (GI) Foods by Incorporating Pulse Ingredients into Cereal-Based Products: Use of In Vitro Screening and In Vivo Methodologies. Cereal. Chem. 2017, 94, 110–116. [Google Scholar] [CrossRef]
- McCrory, M.A.; Hamaker, B.R.; Lovejoy, J.C.; Eichelsdoerfer, P.E. Pulse Consumption, Satiety, and Weight Management. Adv. Nutr. 2010, 1, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Dahl, W.J. Health Benefits of Pulses. In Health Benefits of Pulses; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Singh, M.; Manickavasagan, A.; Shobana, S.; Mohan, V. Glycemic Index of Pulses and Pulse-Based Products: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1567–1588. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Kendall, C.W.C.; Esfahani, A.; Wong, J.M.W.; Carleton, A.J.; Jiang, H.Y.; Bazinet, R.P.; Vidgen, E.; Jenkins, D.J.A. Effect of Non-Oil-Seed Pulses on Glycaemic Control: A Systematic Review and Meta-Analysis of Randomised Controlled Experimental Trials in People with and without Diabetes. Diabetologia 2009, 52, 1479–1495. [Google Scholar] [CrossRef] [Green Version]
- Marinangeli, C.P.F. The Use of Postprandial Glycaemic Health Claims on Dry and Canned Whole Pulse Food Products for the Canadian Market. 2016, p. 7. Available online: https://www.Manitobapulse.ca/Wp-Content/Uploads/2015/10/Pulse-Canada-Final-Report-PPG-Whole-Pulses.Pdf (accessed on 10 September 2021).
- Health Canada Draft Guidance Document on Food Health Claims Related to the Reduction in Post-Prandial Glycaemic Response. 2013. Available online: https://www.Canada.ca/En/Health-Canada/Services/Food-Nutrition/Public-Involvement-Partnerships/Technical-Consultation-Draft-Guidance-Document-Food-Health-Claims-Related-Post-Prandial-Glycaemia.Html (accessed on 5 October 2021).
- Ramdath, D.; Renwick, S.; Duncan, A.M. The Role of Pulses in the Dietary Management of Diabetes. Can. J. Diabetes 2016, 40, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Kendall, C.W.C.; Souza, R.J.; Jayalath, V.H.; Cozma, A.I.; Ha, V.; Mirrahimi, A.; Chiavaroli, L.; Augustin, L.S.A.; Blanco Mejia, S.; et al. Dietary Pulses, Satiety and Food Intake: A Systematic Review and Meta-analysis of Acute Feeding Trials. Obesity 2014, 22, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Agriculture and Agri-Food Canada (AAFC): Outlook for Principal Field Crops, 2021-05-20. Available online: https://agriculture.canada.ca/en/canadas-agriculture-sectors/crops/reports-and-statistics-data-canadian-principal-field-crops/canada-outlook-principal-field-crops-2021-05-20 (accessed on 10 January 2022).
- Reid, I. Factors Influencing Pulse Consumption in Canada; Ipsos Reid: Calgary, AB, Canada, 2010. Available online: Https://www1.agric.gov.ab.ca/$department/deptdocs.nsf/ba3468a2a8681f69872569d60073fde1/da8c7aee8f2470c38725771c0078f0bb/$FILE/v3_factors_influencing_pulse_consumption_final_report_feb24_2010.pdf (accessed on 10 January 2022).
- Darabi, A.; Taleban, F.; Esmaili, M.; Valaii, N. Glycemic Index of Split Peas, Rice (Binam), Kidney Beans, Green Peas, “Lavash” Bread and Broad Bean Kernels in NIDDM Subjects. Acta Med. Iran 2000, 2, 79–83. [Google Scholar]
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor Components of Pulses and Their Potential Impact on Human Health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the Health Benefits of Peas (Pisum Sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef] [Green Version]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive Proteins and Peptides in Pulse Crops: Pea, Chickpea and Lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive Constituents in Pulses and Their Health Benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef] [Green Version]
- Tulbek, M.C.; Lam, R.S.H.; Wang, Y.C.; Asavajaru, P.; Lam, A. Pea. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 145–164. ISBN 978-0-12-802778-3. [Google Scholar]
- Schäfer, G.; Schenk, U.; Ritzel, U.; Ramadori, G.; Leonhardt, U. Comparison of the Effects of Dried Peas with Those of Potatoes in Mixed Meals on Postprandial Glucose and Insulin Concentrations in Patients with Type 2 Diabetes. Am. J. Clin. Nutr. 2003, 78, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.L.; Mollard, R.C.; Zafar, T.A.; Luhovyy, B.L.; Anderson, G.H. Food Intake and Satiety Following a Serving of Pulses in Young Men: Effect of Processing, Recipe, and Pulse Variety. J. Am. Coll. Nutr. 2009, 28, 543–552. [Google Scholar] [CrossRef]
- Yoshimoto, J.; Kato, Y.; Ban, M.; Kishi, M.; Horie, H.; Yamada, C.; Nishizaki, Y. Palatable Noodles as a Functional Staple Food Made Exclusively from Yellow Peas Suppressed Rapid Postprandial Glucose Increase. Nutrients 2020, 12, 1839. [Google Scholar] [CrossRef]
- Dodd, H.; Williams, S.; Brown, R.; Venn, B. Calculating Meal Glycemic Index by Using Measured and Published Food Values Compared with Directly Measured Meal Glycemic Index. Am. J. Clin. Nutr. 2011, 94, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Marinangeli, C.P.F.; Kassis, A.N.; Jones, P.J.H. Glycemic Responses and Sensory Characteristics of Whole Yellow Pea Flour Added to Novel Functional Foods. J. Food Sci. 2009, 74, S385–S389. [Google Scholar] [CrossRef] [PubMed]
- Marinangeli, C.P.F.; Jones, P.J.H. Whole and Fractionated Yellow Pea Flours Reduce Fasting Insulin and Insulin Resistance in Hypercholesterolaemic and Overweight Human Subjects. Br. J. Nutr. 2011, 105, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.E.; Mollard, R.C.; Luhovyy, B.L.; Anderson, G.H. The Effect of Yellow Pea Protein and Fibre on Short-Term Food Intake, Subjective Appetite and Glycaemic Response in Healthy Young Men. Br. J. Nutr. 2012, 108, S74–S80. [Google Scholar] [CrossRef] [Green Version]
- Mollard, R.C.; Luhovyy, B.L.; Smith, C.; Anderson, G.H. Acute Effects of Pea Protein and Hull Fibre Alone and Combined on Blood Glucose, Appetite, and Food Intake in Healthy Young Men – a Randomized Crossover Trial. Appl. Physiol. Nutr. Metab. 2014, 39, 1360–1365. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada Sector Trend Analysis—Baked Goods in the United States and Canada. 2018. Available online: Https://Sba.Ubc.ca/Blog/Industry-Overview-Bakeries-Canada (accessed on 10 January 2022).
- Hosseini, S.H.; Papanikolaou, Y.; Isalm, N.; Rashmi, P.; Shamloo, A.; Vatanparast, H. Consumption Patterns of Grain-Based Foods among Children and Adolescents in Canada: Evidence from Canadian Community Health Survey-Nutrition 2015. Nutrients 2019, 11, 623. [Google Scholar] [CrossRef] [Green Version]
- Borczak, B.; Sikora, M.; Sikora, E.; Dobosz, A.; Kapusta-Duch, J. Glycaemic Index of Wheat Bread: Glycaemic Index of Bread. Starch Stärke 2018, 70, 1700022. [Google Scholar] [CrossRef]
- Hall, R.S.; Thomas, S.J.; Johnson, S.K. Australian Sweet Lupin Flour Addition Reduces the Glycaemic Index of a White Bread Breakfast without Affecting Palatability in Healthy Human Volunteers. Asia Pac. J. Clin. Nutr. 2005, 14, 91. [Google Scholar]
- Johnson, S.K.; Thomas, S.J.; Hall, R.S. Palatability and Glucose, Insulin and Satiety Responses of Chickpea Flour and Extruded Chickpea Flour Bread Eaten as Part of a Breakfast. Eur. J. Clin. Nutr. 2005, 59, 169–176. [Google Scholar] [CrossRef]
- Zafar, T.A.; Al-Hassawi, F.; Al-Khulaifi, F.; Al-Rayyes, G.; Waslien, C.; Huffman, F.G. Organoleptic and Glycemic Properties of Chickpea-Wheat Composite Breads. J. Food Sci. Technol. 2015, 52, 2256–2263. [Google Scholar] [CrossRef] [Green Version]
- Bourré, L.; Young, G.; Hill, H.; Fraser, S.; Borsuk, Y.; Sarkar, A.; Sopiwnyk, E.; Jones, S.; Dyck, A.; Malcolmson, L. Effect of Dry and Steam Heating on the Functional and Bread Baking Properties of Yellow Pea and Navy Bean Flours. Cereal. Chem. 2019, 96, 1079–1092. [Google Scholar] [CrossRef]
- Jiang, Z.; Pulkkinen, M.; Wang, Y.; Lampi, A.-M.; Stoddard, F.L.; Salovaara, H.; Piironen, V.; Sontag-Strohm, T. Faba Bean Flavour and Technological Property Improvement by Thermal Pre-Treatments. LWT Food Sci. Technol. 2016, 68, 295–305. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Azarnia, S.; Simpson, B.K. Volatile Flavor Profile of Saskatchewan Grown Pulses as Affected by Different Thermal Processing Treatments. Int. J. Food Prop. 2016, 19, 2251–2271. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, T. Addition of Micronized Black Bean (Phaseolus Vulgaris) Flour Improves Sensory Qualities of Low Fat Beef Burgers. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2013. [Google Scholar]
- Paladugula, M.P.; Smith, B.; Morris, C.F.; Kiszonas, A. Incorporation of Yellow Pea Flour into White Pan Bread. Cereal. Chem. 2021, 98, 1020–1026. [Google Scholar] [CrossRef]
- Ribéreau, S.; Aryee, A.N.A.; Tanvier, S.; Han, J.; Boye, J.I. Composition, Digestibility, and Functional Properties of Yellow Pea as Affected by Processing. J. Food Process Preserv. 2018, 42, e13375. [Google Scholar] [CrossRef]
- Shariati-Ievari, S.; Ryland, D.; Edel, A.; Nicholson, T.; Suh, M.; Aliani, M. Sensory and Physicochemical Studies of Thermally Micronized Chickpea (Cicer Arietinum) and Green Lentil (Lens Culinaris) Flours as Binders in Low-Fat Beef Burgers. J. Food Sci. 2016, 81, S1230–S1242. [Google Scholar] [CrossRef]
- Fahmi, R.; Ryland, D.; Sopiwnyk, E.; Malcolmson, L.; Shariati-Ievari, S.; McElrea, A.; Barthet, V.; Blewett, H.; Aliani, M. Effect of Revtech Thermal Processing on Volatile Organic Compounds and Chemical Characteristics of Split Yellow Pea (Pisum Sativum L.) Flour. J. Food Sci. 2021, 86, 4330–4353. [Google Scholar] [CrossRef]
- Fahmi, R.; Ryland, D.; Sopiwnyk, E.; Aliani, M. Sensory and Physical Characteristics of Pan Bread Fortified with Thermally Treated Split Yellow Pea (Pisum Sativum L.) Flour. J. Food Sci. 2019, 84, 3735–3745. [Google Scholar] [CrossRef]
- Revtech Europe Revtech Process Systems. Retrieved 18 November 2020. Available online: https://revtech-process-systems.com/ (accessed on 18 November 2020).
- Health Canada, H. Draft Guidance Document - Satiety Health Claims on Food. 2012. Available online: https://www.Canada.ca/En/Health-Canada/Services/Food-Nutrition/Public-Involvement-Partnerships/Technical-Consultation-Health-Canada-Draft-Guidance-Document-Satiety-Health-Claims-Food/Consultation.Html (accessed on 5 October 2021).
- AOAC Official Methods of Analysis. In Method 930.15 Loss on Drying (Moisture) for Feeds (at 135 °C for 2 Hours)/Dry Matter on Oven Drying for Feeds (at 135 °C for 2 Hours), 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005.
- AOAC Official Methods of Analysis. In Method 990.03 Crude Protein in Animal Feed Combustion Analyser Method, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005.
- AOCS Official and Tentative Methods of the American Oil Chemists’ Society. Standard Procedure Am 5-04 Rapid Determination of Oil/Fat Utilizing High-Temperature Solvent Extraction; American Oil Chemists’ Society: Champaign, IL, USA, 2017. [Google Scholar]
- AOCS Official and Tentative Methods of the American Oil Chemists’ Society. Standard Procedure Ba 6a-05 Crude Fiber in Feed by Filter Bag Technique; American Oil Chemists’ Society: Champaign, IL, USA, 2017. [Google Scholar]
- AOAC Official Methods of Analysis. In Method 923.03 Ash of Flour, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005.
- AOAC Official Methods of Analysis. In Method 980.13 Fructose, Glucose, Lactose, Maltose, and Sucrose in Milk Chocolate; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2004.
- AACC International Approved Methods of Analysis 10th AACC Method 76-13. In Total Starch Assay Procedure; American Association of Cereal Chemists International: St. Paul, MN, USA, 2000.
- AACC International Approved Methods of Analysis 11th AACC Method 32–40.01. In Resistant Starch in Starch Samples and Plant Materials; American Association of Cereal Chemists International: St. Paul, MN, USA, 2002.
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M.S. Glycaemic Index Methodology. Nutr. Res. Rev. 2005, 18, 145. [Google Scholar] [CrossRef] [Green Version]
- Rabiee, A.; Magruder, J.T.; Grant, C.; Salas-Carrillo, R.; Gillette, A.; DuBois, J.; Shannon, R.P.; Andersen, D.K.; Elahi, D. Accuracy and Reliability of the Nova StatStrip® Glucose Meter for Real-Time Blood Glucose Determinations during Glucose Clamp Studies. J. Diabetes Sci. Technol. 2010, 4, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, N.; Blewett, H.; Storsley, J.; Thandapilly, S.J.; Zahradka, P.; Taylor, C. A Double-Blind Randomised Controlled Trial Testing the Effect of a Barley Product Containing Varying Amounts and Types of Fibre on the Postprandial Glucose Response of Healthy Volunteers. Br. J. Nutr. 2015, 113, 1373–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollard, R.C.; Wong, C.L.; Luhovyy, B.L.; Anderson, G.H. First and Second Meal Effects of Pulses on Blood Glucose, Appetite, and Food Intake at a Later Meal. Appl. Physiol. Nutr. Metab. 2011, 36, 634–642. [Google Scholar] [CrossRef]
- Mollard, R.C.; Zykus, A.; Luhovyy, B.L.; Nunez, M.F.; Wong, C.L.; Anderson, G.H. The Acute Effects of a Pulse-Containing Meal on Glycaemic Responses and Measures of Satiety and Satiation within and at a Later Meal. Br. J. Nutr. 2012, 108, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.C. Hammer and Roller Milling of Yellow Split Pea; North Dakota State University: Fargo, ND, USA, 2019. [Google Scholar]
- Martens, L.G. Pea Hull Fibre: Novel and Sustainable Fibre with Important Health and Functional Properties. EC Nutrition 2017, 10, 139–148. [Google Scholar]
- Lunde, M.S.H.; Hjellset, V.T.; Holmboe-Ottesen, G.; Høstmark, A.T. Variations in Postprandial Blood Glucose Responses and Satiety after Intake of Three Types of Bread. J. Nutr. Metab. 2011, 2011, 437587. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, A.; Johnson, S.K. In Vitro Carbohydrate Digestibility of Whole-Chickpea and Chickpea Bread Products. Int. J. Food Sci. Nutr. 2005, 56, 147–155. [Google Scholar] [CrossRef]
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The Glycemic Index: Methodology and Clinical Implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; MacLean, D.A. Plasma Glucagon and Insulin Responses Depend on the Rate of Appearance of Amino Acids after Ingestion of Different Protein Solutions in Humans. J. Nutr. 2002, 132, 2174–2182. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.J.; Saris, W.H.; Verhagen, H.; Wagenmakers, A.J. Plasma Insulin Responses after Ingestion of Different Amino Acid or Protein Mixtures with Carbohydrate. Am. J. Clin. Nutr. 2000, 72, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Byrne, D.V. Current Trends in Multidisciplinary Approaches to Understanding Consumer Preference and Acceptance of Food Products. Foods 2020, 9, 1380. [Google Scholar] [CrossRef] [PubMed]




| Bread Treatments | ||||
|---|---|---|---|---|
| 100%W | USYP | RT0% | RT10% | |
| Energy (Kcal) | 255.7 | 268.6 | 265.8 | 275.2 |
| Weight (g) | 100.2 | 103.9 | 103.0 | 107.3 |
| Energy density (kcal·g−1) | 2.6 | 2.6 | 2.6 | 2.6 |
| Available Carbohydrate (g) * | 50.0 | 50.0 | 50.0 | 50.0 |
| Protein (g) | 9.3 | 12.1 | 12.4 | 12.6 |
| Fiber (g) | 1.0 | 0.7 | 0.8 | 1.3 |
| Fat (g) | 2.2 | 2.6 | 2.6 | 2.6 |
| Ash (g) | 3.3 | 2.9 | 3.1 | 2.5 |
| Moisture (g) | 36.3 | 38.0 | 37.5 | 39.7 |
| Carbohydrate, by difference (g) | 49.0 | 48.4 | 47.5 | 50.0 |
| Baseline Characteristics | Female (n = 12) | Male (n = 12) | ||
|---|---|---|---|---|
| Mean (SD) 1 | Range | Mean (SD) 1 | Range | |
| Age (years) | 25 (3.8) | 19–31 | 24 (4.7) | 18–34 |
| BMI (kg/m2) | 22.4 (1.7) | 19.6–26.0 | 22.4 (1.1) | 21.0–23.9 |
| Body fat (%) | 27.7 (5.6) | 18.3–37.1 | 15.1 (6.1) | 3.1–24.1 |
| Waist circumference (cm) | 76.0 (5.6) | 69.0–89.5 | 80.1 (4.6) | 72.5–86.5 |
| Systolic blood pressure (mm Hg) | 102 (5.8) | 91–113 | 108 (8.4) | 92–120 |
| Diastolic blood pressure (mm Hg) | 70 (5.6) | 62–83 | 70 (8.0) | 57–84 |
| Ethnicity | Female (n = 12) | Male (n = 12) | ||
| American/Canadian | 8 | 7 | ||
| European | 1 | 2 | ||
| Asian | 1 | 2 | ||
| Latin | 1 | 0 | ||
| Africa | 1 | 0 | ||
| Other | 0 | 1 | ||
| Source of Variation (F-Value 3) | Mean Values 4 (Bread Treatments) | Mean Values 5 (Sex) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | Sex (S) | Order 6 (O) | ID 7 | T × S | T × O | 100%W | USYP | RT0% | RT10% | Male | Female | |
| Glucose iAUC 1 (mmol/L × min) | 0.64 NS | 15.89 *** | 11.82 *** | 5.65 *** | 1.02 NS | 0.47 NS | 173.04 (15.98) | 162.76 (13.60) | 160.79 (13.46) | 154.70 (16.11) | 143.83 b (11.16) | 181.82 a (8.81) |
| Insulin iAUC 1 (pmol/L × min) | 2.01 NS | 43.21 *** | 28.35 *** | 15.04 *** | 0.19 NS | 0.47 NS | 24,295.50 (2784.99) | 24,746.08 (2940.76) | 26,133.13 (2727.84) | 28,507.00 (3604.68) | 21,534.58 b (1972.17) | 30,306.27 a (2102.44) |
| Glycemic index 2 | 0.39 NS | 2.33 NS | 2.25 NS | 2.97 ** | 0.40 NS | 0.33 NS | - | 74.69 (7.78) | 69.90 (7.14) | 67.78 (6.11) | 65.78 (5.27) | 75.79 (6.05) |
| Appetite Sensation Variables 1 | Source of Variation (F-Value 2) | Mean Values 3 (Bread Treatments) | Mean Values 4 (Sex) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | Sex (S) | Order 5 (O) | ID 6 | T × S | T × O | 100%W | USYP | RT0% | RT10% | Male | Female | |
| Hunger iAUC (cm × min) | 4.02 * | 66.88 *** | 10.78 *** | 16.02 *** | 0.78 NS | 0.95 NS | 609.13 a (46.27) | 509.13 b (41.34) | 547.75 ab (51.89) | 553.19 ab (45.90) | 638.78 a (29.45) | 470.81 b (31.67) |
| Fullness iAUC (cm × min) | 3.13 * | 16.58 *** | 12.44 *** | 17.09 *** | 0.25 NS | 1.12 NS | 500.13 b (48.40) | 591.13 a (43.18) | 552.19 ab (47.84) | 553.94 ab (46.76) | 506.28 b (28.92) | 592.41 a (35.41) |
| Desire to eat iAUC (cm × min) | 4.58 * | 79.86 *** | 9.47 *** | 17.10 *** | 1.31 NS | 2.03 NS | 618.88 a (48.45) | 515.06 b (43.40) | 550.06 ab (51.18) | 564.13 ab (46.95) | 652.13 a (29.10) | 471.94 b (32.90) |
| Prospective food consumption iAUC (cm × min) | 5.93 * | 106.71 *** | 23.11 *** | 17.73 *** | 2.26 NS | 1.40 NS | 603.13 a (49.37) | 494.50 b (41.50) | 528.00 b (52.53) | 531.56 b (45.91) | 636.25 a (29.67) | 442.34 b (31.54) |
| Sensory Attributes | Mean Acceptability Values 3 | F-Value 4 | |||
|---|---|---|---|---|---|
| 100%W | USYP | RT0% | RT10% | ||
| Aroma 1 | 6.6 (0.2) | 6.3 (0.2) | 6.1 (0.2) | 6.5 (0.3) | 0.82 NS |
| Flavor 1 | 7.0 (0.3) | 6.6 (0.3) | 6.3 (0.3) | 6.5 (0.3) | 0.78 NS |
| Color 1 | 6.6 (0.3) | 6.3 (0.3) | 5.9 (0.3) | 6.4 (0.3) | 1.22 NS |
| Texture 1 | 7.3 a (0.3) | 6.8 ab (0.3) | 5.7 b (0.4) | 7.0 a (0.3) | 5.33 ** |
| Overall Acceptability 1 | 6.9 (0.3) | 6.7 (0.3) | 6.1 (0.3) | 6.5 (0.4) | 1.17 NS |
| Frequency of Eating 2 | 6.3 (0.3) | 6.0 (0.4) | 5.4 (0.4) | 5.9 (0.3) | 1.09 NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by Her Majesty the Queen in Right of Canada as represented by the Minister of Agriculture and Agri-Food and the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahmi, R.; Blewett, H.; Stebbing, J.-A.; Olson, N.; Ryland, D.; Aliani, M. Acute Effects of Split Pea-Enriched White Pan Bread on Postprandial Glycemic and Satiety Responses in Healthy Volunteers—A Randomized Crossover Trial. Foods 2022, 11, 1002. https://doi.org/10.3390/foods11071002
Fahmi R, Blewett H, Stebbing J-A, Olson N, Ryland D, Aliani M. Acute Effects of Split Pea-Enriched White Pan Bread on Postprandial Glycemic and Satiety Responses in Healthy Volunteers—A Randomized Crossover Trial. Foods. 2022; 11(7):1002. https://doi.org/10.3390/foods11071002
Chicago/Turabian StyleFahmi, Ronak, Heather Blewett, Jo-Ann Stebbing, Nancy Olson, Donna Ryland, and Michel Aliani. 2022. "Acute Effects of Split Pea-Enriched White Pan Bread on Postprandial Glycemic and Satiety Responses in Healthy Volunteers—A Randomized Crossover Trial" Foods 11, no. 7: 1002. https://doi.org/10.3390/foods11071002







