Myofibrillar Protein Interacting with Trehalose Elevated the Quality of Frozen Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Extraction of Myofibrillar Proteins (MPs)
2.3. MP Interacting with Trehalose/COS
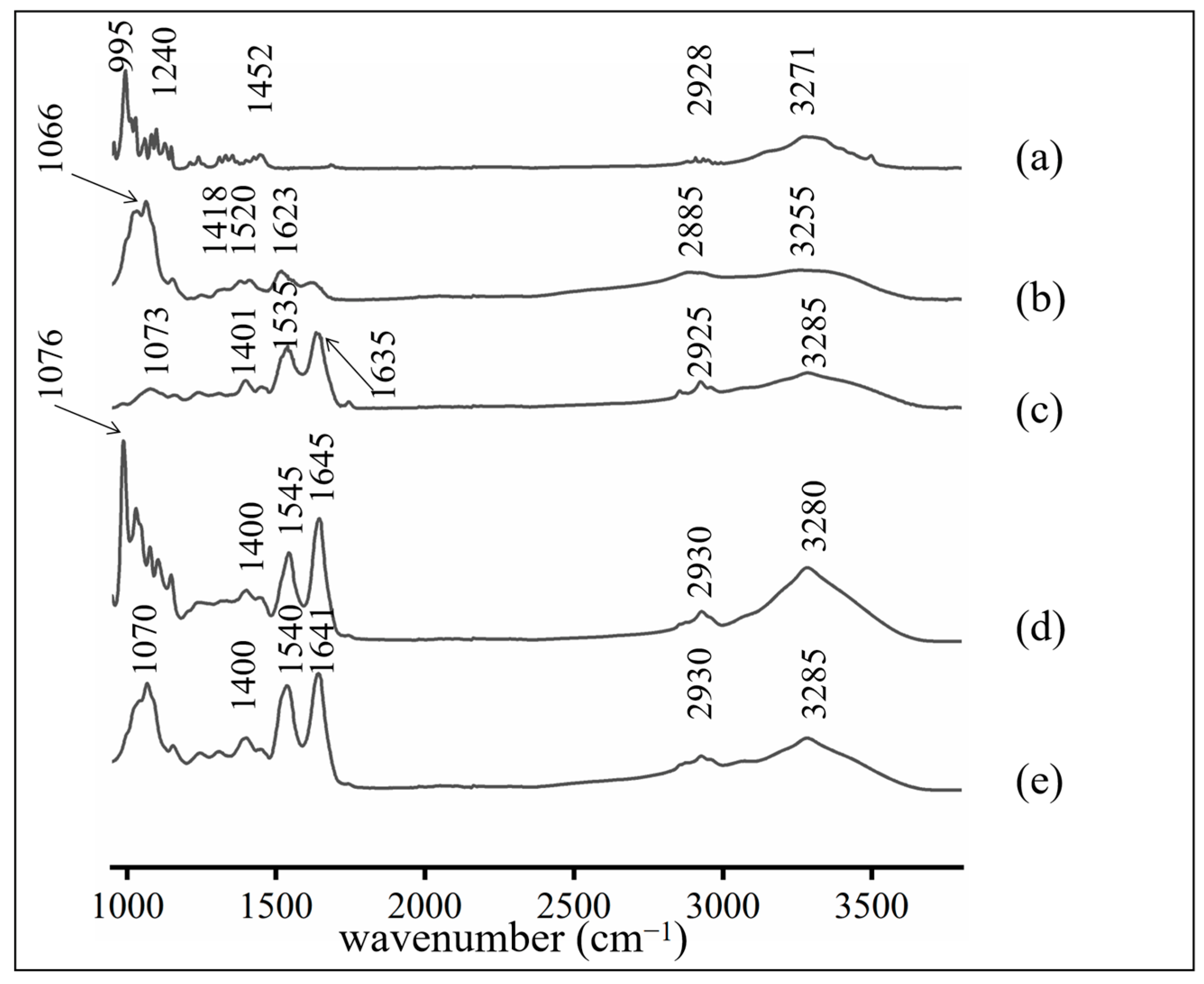
2.4. Fourier Transform Infrared (FTIR) Spectrum
2.5. Zeta Potential
2.6. Raman Spectroscopy
2.7. Treatment of Meat Samples
2.8. Thawing Loss
2.9. Ice Crystals in Meat Muscle
2.10. Surface Color
2.11. pH
2.12. Sensory Evaluation
2.13. Statistical Analysis
3. Results and Discussion
3.1. The Interaction of Myofibrillar Protein (MP) with Trehalose and COS
3.1.1. FTIR
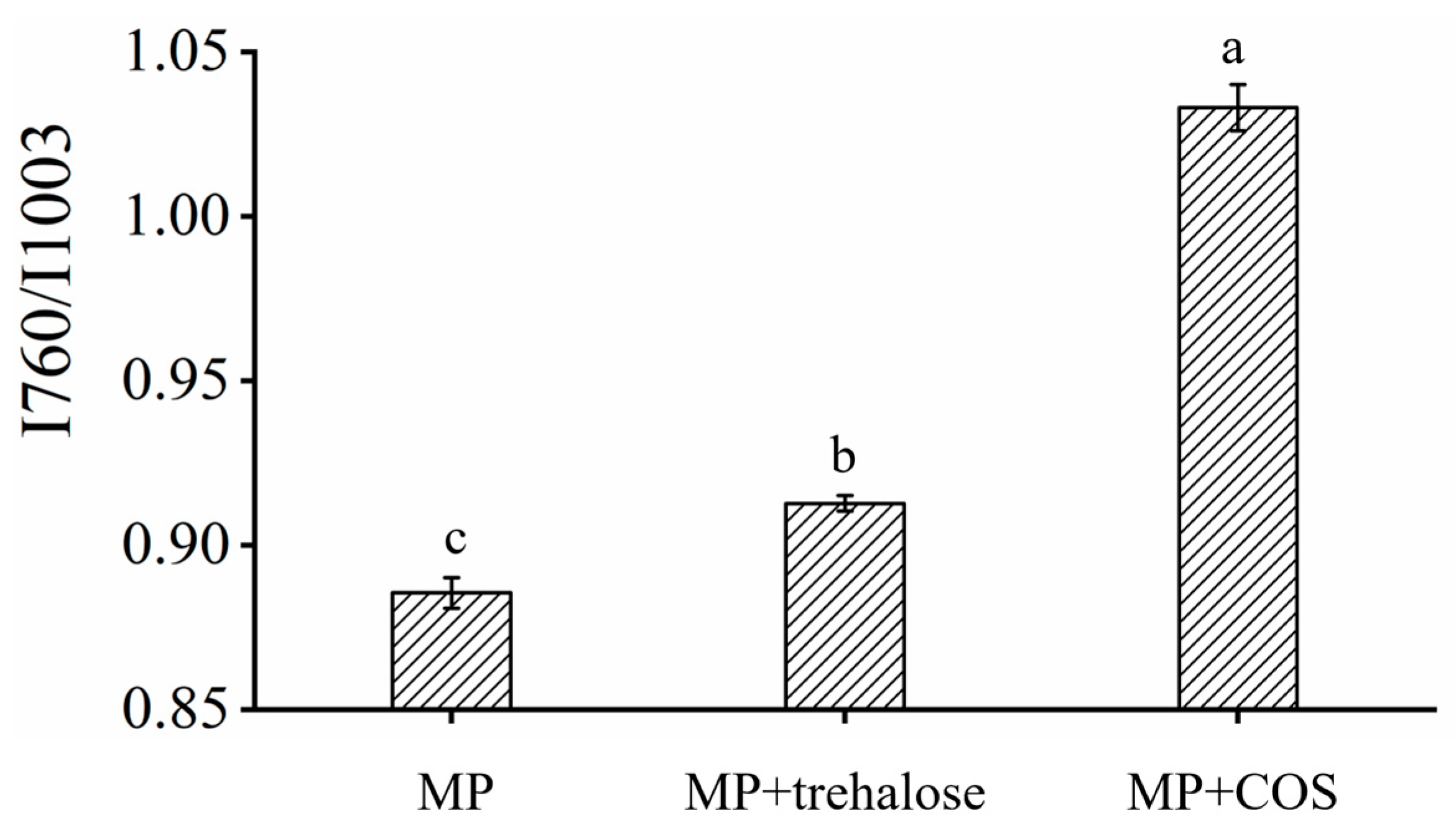
3.1.2. Zeta Potential
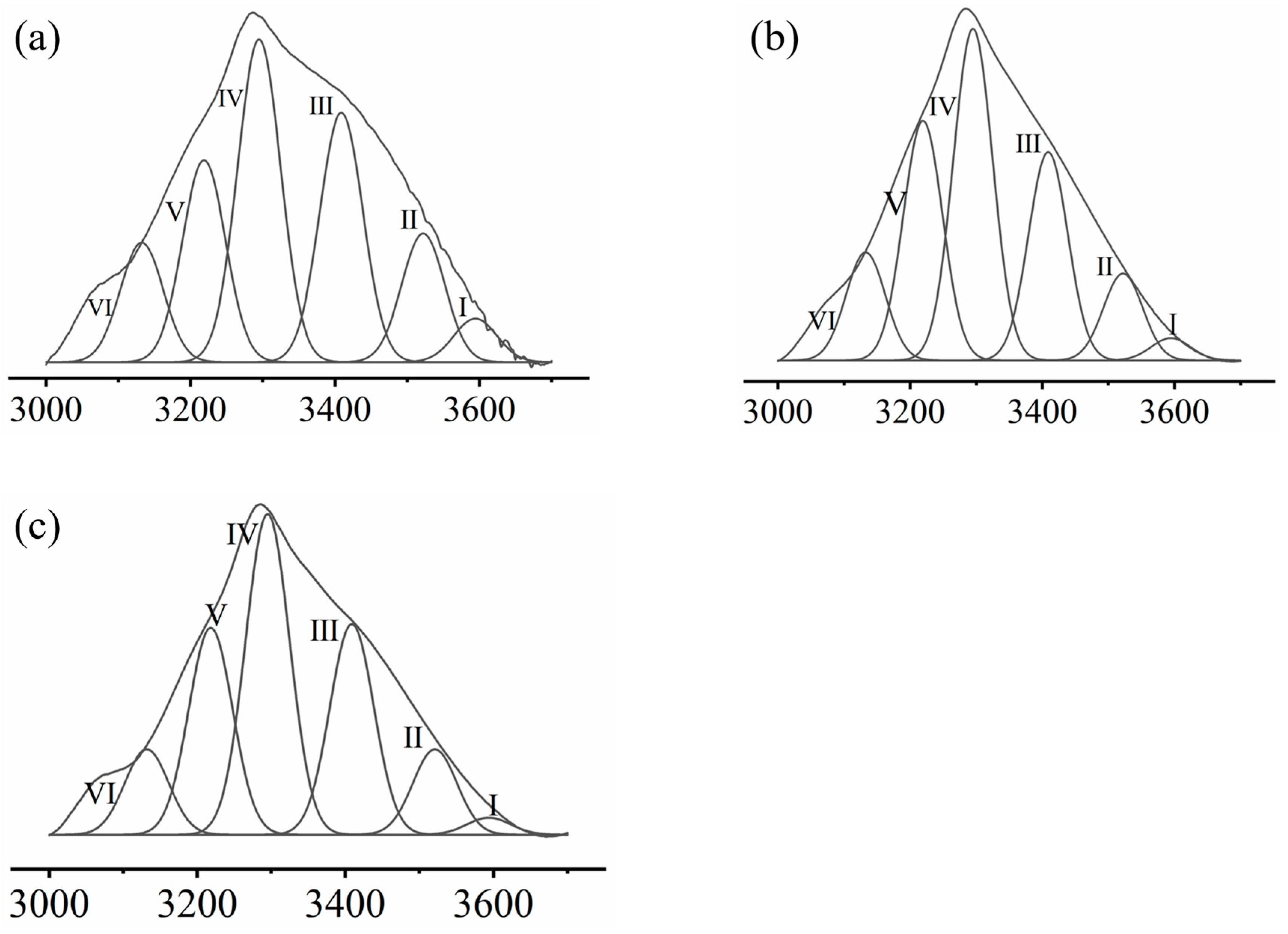
3.1.3. Raman Spectroscopy
3.2. Effect of Trehalose and COS on Meat Quality
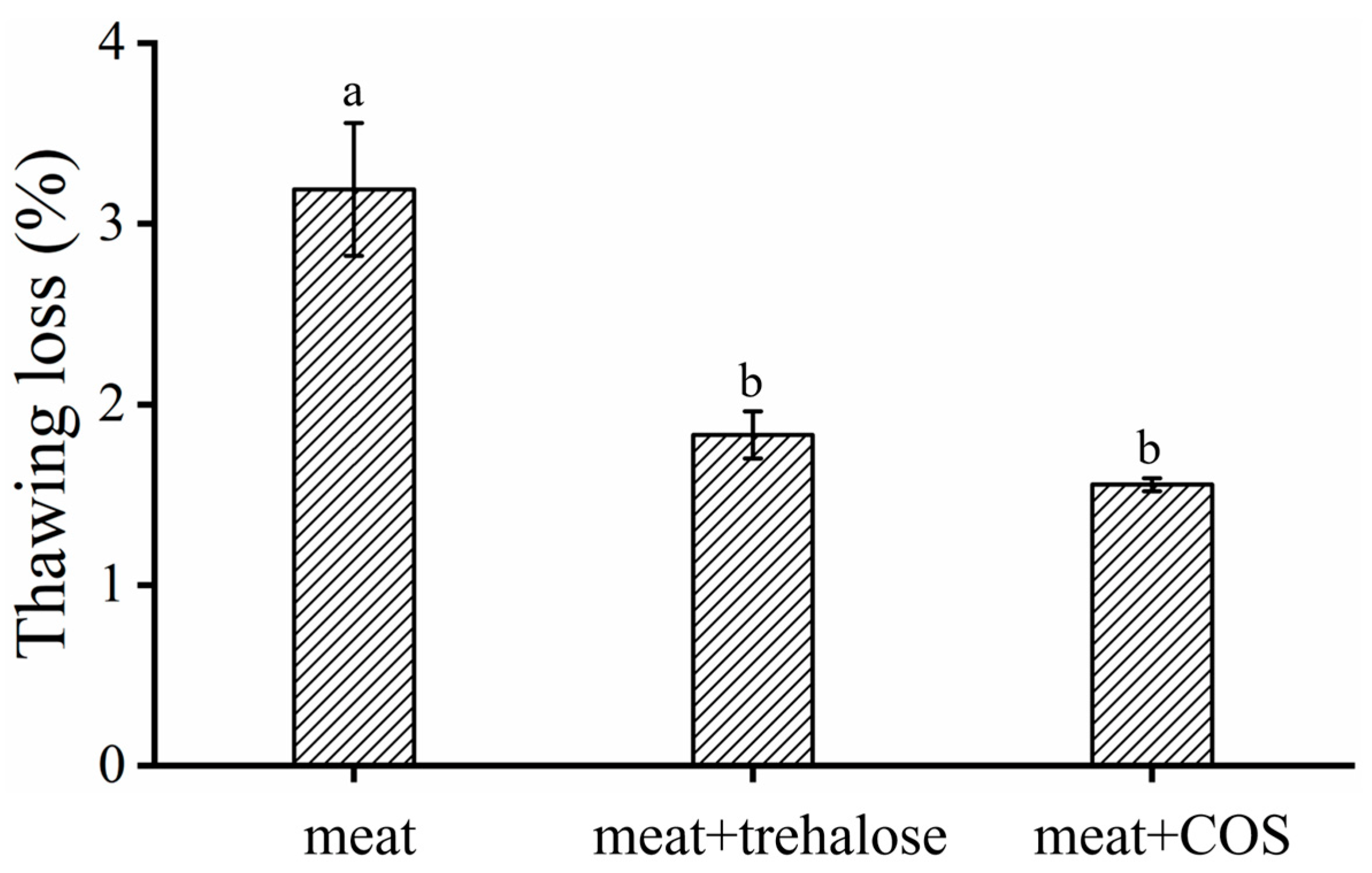
3.2.1. Thawing Loss
3.2.2. Ice Crystals in Meat Muscle
3.2.3. Surface Color
3.2.4. pH
3.2.5. Sensory Evaluation
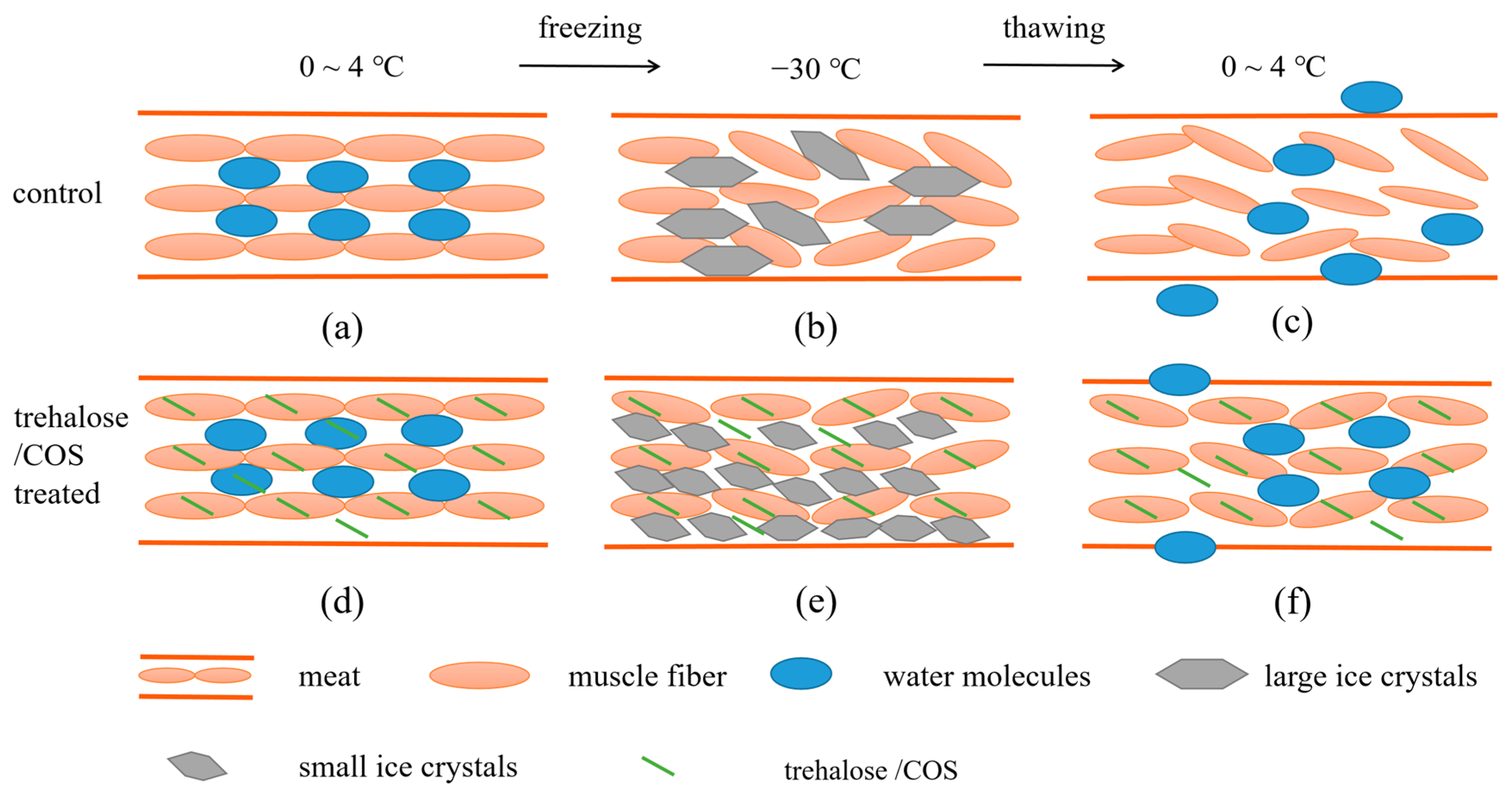
3.3. Mechanism of Trehalose and COS Reducing Frozen Meat Thawing Loss
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, J.; Tang, X.Y.; Xue, Y.; Lin, G.; Xiong, Y.L.L. Dietary linseed oil supplemented with organic selenium improved the fatty acid nutritional profile, muscular selenium deposition, water retention, and tenderness of fresh pork. Meat Sci. 2017, 131, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.H.H.; He, L.C.; Nawaz, A.; Jin, G.F.; Huang, X.; Ma, M.H.; Abdegadir, W.; Elgasim, E.A.; Khalifa, I. Effect of frozen and refrozen storage of beef and chicken meats on inoculated microorganisms and meat quality. Meat Sci. 2021, 175, 108453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Magro, A.; Puolanne, E.; Zotte, A.D.; Ertbjerg, P. Myofibrillar protein characteristics of fast or slow frozen pork during subsequent storage at −3 °C. Meat Sci. 2021, 176, 108468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Puolanne, E.; Ertbjerg, P. Mimicking myofibrillar protein denaturation in frozen-thawed meat: Effect of pH at high ionic strength. Food Chem. 2021, 338, 128017. [Google Scholar] [CrossRef]
- Shen, H.; Stephen, E.J.; Zhao, M.M.; Sun, W.Z. Effect of oxidation on the gel properties of porcine myofibrillar proteins and their binding abilities with selected flavour compounds. Food Chem. 2020, 329, 127032. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Yang, Y.L.; Tang, X.Z.; Chen, Y.J.; You, Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015, 188, 111–118. [Google Scholar] [CrossRef]
- Li, F.F.; Du, X.; Ren, Y.M.; Kong, B.H.; Wang, B.; Xia, X.F.; Bao, Y.H. Impact of ice structuring protein on myofibrillar protein aggregation behaviour and structural property of quick-frozen patty during frozen storage. Int. J. Biol. Macromol. 2021, 178, 136–142. [Google Scholar] [CrossRef]
- Walayat, N.; Rincón, M.Á.; Niaz, S.; Nawaz, A.; Niaz, N.; Zahid, F.M.; Ahmad, I.; Wang, P.K.; Zhang, Z.L. Egg white proteins and β-cyclodextrin: Effective cryoprotectant mixture against oxidative changes in the myofibrillar proteins of Culter alburnus. Int. J. Food Sci. Technol. 2021, 56, 4009–4016. [Google Scholar] [CrossRef]
- Jia, G.; Liu, H.; Nirasawa, S.; Liu, H. Effects of high-voltage electrostatic field treatment on the thawing rate and post-thawing quality of frozen rabbit meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 348–356. [Google Scholar] [CrossRef]
- Stefanello, R.F.; Machado, A.A.R.; Cavalheiro, C.P.; Santos, M.L.B.; Nabeshima, E.H.; Copetti, M.V.; Fries, L.L.M. Trehalose as a cryoprotectant in freeze-dried wheat sourdough production. LWT—Food Sci. Technol. 2018, 89, 510–517. [Google Scholar] [CrossRef]
- Pan, S.K.; Wu, S.J. Effect of chitooligosaccharides on the denaturation of weever myofibrillar protein during frozen storage. Int. J. Biol. Macromol. 2014, 65, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Pan, S.K.; Wang, H.B. Effect of trehalose on Lateolabrax japonicus myofibrillar protein during frozen storage. Food Chem. 2014, 160, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qi, X.E.; Mao, J.L.; Ying, X.G. Trehalose and alginate oligosaccharides affect the stability of myosin in whiteleg shrimp (Litopenaeus vannamei): The water-replacement mechanism confirmed by molecular dynamic simulation. LWT—Food Sci. Technol. 2020, 127, 109393. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, H.; Qi, H.; Zhang, X. Trehalose and alginate oligosaccharides increase the stability of muscle proteins in frozen shrimp (Litopenaeus vannamei). Food Funct. 2020, 11, 1270–1278. [Google Scholar] [CrossRef]
- Al-Naama, M.; Ewaze, J.O.; Green, B.J.; Scott, J.A. Trehalose accumulation in Baudoinia compniacensis following abiotic stress. Int. Biodegrad. 2009, 63, 765–768. [Google Scholar] [CrossRef]
- Andreas, W.; Reinhard, K.; Susanne, M. Three pathways for trehalose metabolism in Corynebacterium glutamicum ATCC13032 and their significance in response to osmotic stress. Mol. Microbiol. 2003, 49, 1119–1134. [Google Scholar] [CrossRef]
- Faieta, M.; Neri, L.; Di, M.A.; Di, M.C.D.; Pittia, P. High hydrostatic pressure treatment of Arthrospira (Spirulina) platensis extracts and the baroprotective effect of sugars on phycobiliproteins. Innov. Food Sci. Emerg. Technol. 2021, 70, 102693. [Google Scholar] [CrossRef]
- Tzvetkov, M.; Klopprogge, C.; Zelder, O.; Liebl, W. Genetic dissection of trehalose biosynthesis in Corynebacterium glutamicum: Inactivation of trehalose production leads to impaired growth and an altered cell wall lipid composition. Microbiology 2003, 149, 1659–1673. [Google Scholar] [CrossRef] [Green Version]
- Campo-Deaño, L.; Tovar, C.A.; Pombo, M.J.; Solas, M.T.; Borderías, A.J. Rheological study of giant squid surimi (Dosidicus gigas) made by two methods with different cryoprotectants added. J. Food Eng. 2009, 94, 26–33. [Google Scholar] [CrossRef]
- Fang, I.M.; Yang, C.M.; Yang, C.H. Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and inflammation in rats. Exp. Eye Res. 2015, 130, 38–50. [Google Scholar] [CrossRef]
- He, J.X.; Han, W.; Wang, J.; Qian, Y.C.; Saito, M.; Bai, W.B.; Song, J.Q.; Lv, G.H. Functions of Oligosaccharides in Improving Tomato Seeding Growth and Chilling Resistance. J. Plant Growth Regul. 2021, 41, 535–545. [Google Scholar] [CrossRef]
- Jabeen, N.; Ahmad, R. The activity of antioxidant enzymes in response to salt stress in safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) seedlings raised from seed treated with chitosan. J. Sci. Food Agric. 2013, 93, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Y.; Jia, Y.M.; Lu, X.R.; Li, H.J. Release and conformational changes in allergenic proteins from wheat gluten induced by high hydrostatic pressure. Food Chem. 2022, 368, 130805. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, K.; Xie, Y.; Nie, W.; Li, P.; Zhou, H.; Xu, B. Glutathione-mediated formation of disulfide bonds modulates the properties of myofibrillar protein gels at different temperatures. Food Chem. 2021, 364, 130356. [Google Scholar] [CrossRef] [PubMed]
- Velazqueza, G.; Méndez-Montealvo, M.G.; Welti-Chanes, J.; Ramírez, J.A.; Martínez-Maldonado, M.A. Effect of high pressure processing and heat treatment on the gelation properties of blue crab meat proteins. LWT 2021, 146, 111389. [Google Scholar] [CrossRef]
- Yang, L.J.; Guo, J.; Yu, Y.; An, Q.D.; Wang, L.Y.; Li, S.L.; Huang, X.L.; Mu, S.Y.; Qi, S.W. Hydrogen bonds of sodium alginate/Antarctic krill protein composite material. Carbohyd. Polym. 2016, 142, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Guo, J.J.; Wang, X.; Yang, K.; Wang, L.M.; Ma, J.; Zhou, Y.H.; Sun, W.Q. The direct current magnetic field improved the water retention of low-salt myofibrillar protein gel under low temperature condition. LWT—Food Sci. Technol. 2021, 151, 112034. [Google Scholar] [CrossRef]
- Monago-Maraña, O.; Wold, J.P.; Rødbotten, R.; Dankel, K.R.; Afseth, N.K. Raman, near-infrared and fluorescence spectroscopy for determination of collagen content in ground meat and poultry by-products. LWT 2021, 140, 110592. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, K.; Chen, B.; Wang, Y.; Nie, W.; Wu, S.; Wang, W.; Li, P.; Xu, B. Applying low voltage electrostatic field in the freezing process of beef steak reduced the loss of juiciness and textural properties. Innov. Food Sci. Emerg. Technol. 2021, 68, 102600. [Google Scholar] [CrossRef]
- Kaale, L.D.; Eikevik, T.M. A study of the ice crystal sizes of red muscle of pre-rigor Atlantic salmon (Salmo salar) fillets during superchilled storage. J. Food Eng. 2013, 119, 544–551. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, M.; Huang, Y.; Liu, X.; Zhong, L.; Ji, J.; Zhou, L.; Zeng, Q.; Ma, J.; Huang, L. The effect of purine content on sensory quality of pork. Meat Sci. 2021, 172, 108346. [Google Scholar] [CrossRef] [PubMed]
- Lages, Z.L.; Radünz, M.; Gonçalves, B.T.; Rosa, R.S.; Fouchy, M.V.; Conceiç, R.C.S.; Gularte, M.A.; Mendonça, C.R.B.; Gandra, E.A. Microbiological and sensory evaluation of meat sausage using thyme (Thymus vulgaris, L.) essential oil and powdered beet juice (Beta vulgaris L., Early Wonder cultivar). LWT 2021, 148, 111794. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ren, Y.X.; Zhang, K.S.; Xiong, Y.L.L.; Wang, Q.; Shang, K.; Dian, Z. Site-specific incorporation of sodium tripolyphosphate into myofibrillar protein from mantis shrimp (Oratosquilla oratoria) promotes protein crosslinking and gel network formation. Food Chem. 2020, 312, 126113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, H.C.; Tang, H.; Hao, G.J.; Zhang, Y.Y.; Deng, S.G. Insights into Cryoprotective Roles of Carrageenan Oligosaccharides in Peeled Whiteleg Shrimp (Litopenaeus vannamei) during Frozen Storage. J. Agric. Food Chem. 2017, 65, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Xiong, Y.L.L. Disruption of secondary structure by oxidative stress alters the cross-linking pattern of myosin by microbial transglutaminase. Meat Sci. 2015, 108, 97–105. [Google Scholar] [CrossRef]
- Zhou, F.B.; Zhao, M.M.; Su, G.W.; Cui, C.; Sun, W.Z. Gelation of salted myofibrillar protein under malondialdehyde-induced oxidative stress. Food Hydrocoll. 2014, 40, 153–162. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.M.; Yang, K.; Wu, D.; Ma, J.; Wang, S.J.; Zhang, Y.H.; Sun, W.Q. Radio frequency heating improves water retention of pork myofibrillar protein gel: An analysis from water distribution and structure. Food Chem. 2021, 350, 129265. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yan, J.K.; Ding, Y.H.; Rashid, M.T.; Ma, H.L. Effect of sweep frequency ultrasound and fixed frequency ultrasound thawing on gelling properties of myofibrillar protein from quick-frozen small yellow croaker and its possible mechanisms. LWT—Food Sci. Technol. 2021, 150, 111922. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, G.J.; Cao, H.J.; Tang, H.; Zhang, Y.Y.; Deng, S.G. The cryoprotectant effect of xylooligosaccharides on denaturation of peeled shrimp (Litopenaeus vannamei) protein during frozen storage. Food Hydrocoll. 2018, 77, 228–237. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, X.M.; Xiao, H.; Nowak-Wegrzyn, A.; Zhou, P. Insight into the allergenicity of shrimp tropomyosin glycated by functional oligosaccharides containing advanced glycation end products. Food Chem. 2020, 302, 125348. [Google Scholar] [CrossRef]
- Cao, Y.G.; Li, B.L.; Fan, X.; Wang, J.K.; Zhu, Z.B.; Huang, J.R.; Xiong, Y.L.L. Synergistic recovery and enhancement of gelling properties of oxidatively damaged myofibrillar protein by L-lysine and transglutaminase. Food Chem. 2021, 358, 129860. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Tao, F.; Cao, G.T.; Han, M.Y.; Xu, X.L.; Zhou, G.H.; Shen, Q. Stability improvement of reduced-fat reduced-salt meat batter through modulation of secondary and tertiary protein structures by means of high pressure processing. Meat Sci. 2021, 176, 108439. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.G.; Yao, Z.A.; Bai, X.F.; Du, Y.G.; Ma, X.J. Chitooligosaccharides inhibit nitric oxide mediated migration of endothelial cells in vitro and tumor angiogenesis in vivo. Carbohyd. Polym. 2010, 82, 927–932. [Google Scholar] [CrossRef]
- Peng, B.; Li, Y.Q.; Ding, S.Y.; Yang, J. Characterization of textural, rheological, thermal, microstructural, and water mobility in wheat flour dough and bread affected by trehalose. Food Chem. 2017, 233, 369–377. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zou, W.J.; Xia, M.Q.; Zeng, Q.; Cai, Z.X. Intelligent colorimetric film incorporated with anthocyanins-loaded ovalbumin-propylene glycol alginate nanocomplexes as a stable pH indicator of monitoring pork freshness. Food Chem. 2021, 368, 130825. [Google Scholar] [CrossRef]
- Faieta, M.; Neri, L.; Sacchetti, G.; Di Michele, A.; Pittia, P. Role of saccharides on thermal stability of phycocyanin in aqueous solutions. Food Res. Int. 2020, 132, 109093. [Google Scholar] [CrossRef]
- Carvalho, S.G.; Martins, D.S.A.; Silvestre, A.L.P.; Meneguin, A.B.; Ferreira, L.M.B.; Chorilli, M.; Gremião, M.P.D. New insights into physicochemical aspects involved in the formation of polyelectrolyte complexes based on chitosan and dextran sulfate. Carbohyd. Polym. 2021, 271, 118436. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Mao, X.Z. Chitopentaose protects HaCaT cells against H2O-induced oxidative damage through modulating MAPKs and Nrf2/ARE signaling pathways. J. Funct. Foods 2020, 72, 104086. [Google Scholar] [CrossRef]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Hamdi, M.; Nasri, R.; Azaza, Y.B.; Li, S.M.; Nasri, M. Conception of novel blue crab chitosan films crosslinked with different saccharides via the Maillard reaction with improved functional and biological properties. Carbohyd. Polym. 2020, 241, 116303. [Google Scholar] [CrossRef]
- Yeh, Y.; Omaye, S.T.; Ribeiro, F.A.; Calkins, C.R.; de Mello, A.S. Evaluation of palatability and muscle composition of novel value-added beef cuts. Meat Sci. 2018, 135, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, Z.; Aalhus, J.L.; Gibson, L.L.; Shand, P.J. Influence of blade tenderization, moisture enhancement and pancreatin enzyme treatment on the processing characteristics and tenderness of beef semitendinosus muscle. Meat Sci. 2009, 84, 512–517. [Google Scholar] [CrossRef] [PubMed]


| Attributes | Definition | Score Criterion (1~9 Scale) |
|---|---|---|
| Color | Appearance of color and brightness of meat surface. | 1 = Completely discolored, mold. 9 = Same red and bright as fresh meat. |
| Smell | The freshness and purity of smell from meat sample. | 1 = Strong unpleasant odor. 9 = Fresh and pleasant odor. |
| Texture | The extent of meat sample recovering after being gently pressed by the finger. | 1 = Not recover at all. 9 = Completely recover. |
| Overall acceptance | Rank of the samples regarding preference of overall impression. | 1 = Dislike extremely. 9 = Like extremely. |
| Hydrogen Bond Type | Abbreviations | Wave Number/cm−1 | Average Peak Area | Standard Deviations/% | Relative Strength/% | ||
|---|---|---|---|---|---|---|---|
| MP | Free hydroxyl | I | -OH | 3595 | 70.10 | 5.65 | 4.39 |
| Intramolecular hydrogen bond | III | OH...OH | 3409 | 351.85 | 5.19 | 22.04 | |
| V | Annular polymer | 3219 | 286.07 | 10.67 | 17.92 | ||
| Intermolecular hydrogen bond | II | OH...π | 3522 | 216.82 | 4.23 | 13.58 | |
| IV | OH...ether O | 3295 | 485.58 | 9.27 | 30.42 | ||
| VI | OH...N | 3132 | 185.66 | 4.23 | 11.63 | ||
| MP + trehalose | Free hydroxyl | I | -OH | 3595 | 29.12 | 6.18 | 1.77 |
| Intramolecular hydrogen bond | III | OH...OH | 3409 | 350.78 | 8.67 | 21.29 | |
| V | Annular polymer | 3219 | 397.56 | 5.70 | 24.13 | ||
| Intermolecular hydrogen bond | II | OH...π | 3522 | 148.34 | 8.67 | 9.00 | |
| IV | OH...ether O | 3295 | 587.25 | 10.48 | 35.64 | ||
| VI | OH...N | 3132 | 134.73 | 4.16 | 8.18 | ||
| MP + COS | Free hydroxyl | I | -OH | 3595 | 12.75 | 1.53 | 1.37 |
| Intramolecular hydrogen bond | III | OH...OH | 3409 | 218.42 | 6.29 | 23.51 | |
| V | Annular polymer | 3219 | 205.16 | 3.81 | 22.08 | ||
| Intermolecular hydrogen bond | II | OH...π | 3522 | 95.85 | 3.79 | 10.31 | |
| IV | OH...ether O | 3295 | 321.52 | 5.20 | 34.60 | ||
| VI | OH...N | 3132 | 75.53 | 3.06 | 8.13 | ||
| pH | Surface Color | |||
|---|---|---|---|---|
| L* | a* | b* | ||
| meat | 5.93 ± 0.03 a | 51.09 ± 0.09 a | 23.43 ± 0.14 a | 40.77 ± 0.29 a |
| meat + trehalose | 5.88 ± 0.03 b | 57.54 ± 0.08 b | 23.89 ± 0.34 a | 46.28 ± 0.36 b |
| meat + COS | 5.85 ± 0.04 b | 57.48 ± 0.02 b | 19.78 ± 0.05 b | 48.17 ± 0.08 c |
| Index | ||||
|---|---|---|---|---|
| Color | Smell | Texture | Overall Acceptance | |
| meat | 5.33 ± 0.94 b | 7.67 ± 0.94 ab | 7.00 ± 1.41 ab | 5.00 ± 0.82 bc |
| meat + trehalose | 5.33 ± 0.94 b | 8.50 ± 0.50 a | 8.75 ± 0.43 a | 7.00 ± 0.00 a |
| meat + COS | 6.25 ± 1.25 ab | 5.50 ± 0.50 c | 3.67 ± 0.47 c | 4.00 ± 0.82 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Li, P.; Han, F.; Zhou, H.; Zhou, K.; Wang, Y.; Cai, K.; Li, C.; Xu, B. Myofibrillar Protein Interacting with Trehalose Elevated the Quality of Frozen Meat. Foods 2022, 11, 1041. https://doi.org/10.3390/foods11071041
Xu S, Li P, Han F, Zhou H, Zhou K, Wang Y, Cai K, Li C, Xu B. Myofibrillar Protein Interacting with Trehalose Elevated the Quality of Frozen Meat. Foods. 2022; 11(7):1041. https://doi.org/10.3390/foods11071041
Chicago/Turabian StyleXu, Shijie, Ping Li, Fei Han, Hui Zhou, Kai Zhou, Ying Wang, Kezhou Cai, Cong Li, and Baocai Xu. 2022. "Myofibrillar Protein Interacting with Trehalose Elevated the Quality of Frozen Meat" Foods 11, no. 7: 1041. https://doi.org/10.3390/foods11071041
APA StyleXu, S., Li, P., Han, F., Zhou, H., Zhou, K., Wang, Y., Cai, K., Li, C., & Xu, B. (2022). Myofibrillar Protein Interacting with Trehalose Elevated the Quality of Frozen Meat. Foods, 11(7), 1041. https://doi.org/10.3390/foods11071041







