Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects
Abstract
:1. Introduction
1.1. Botany and Molecular Phylogeny
1.2. Agroindustry and Phytochemistry
1.2.1. Market Insights
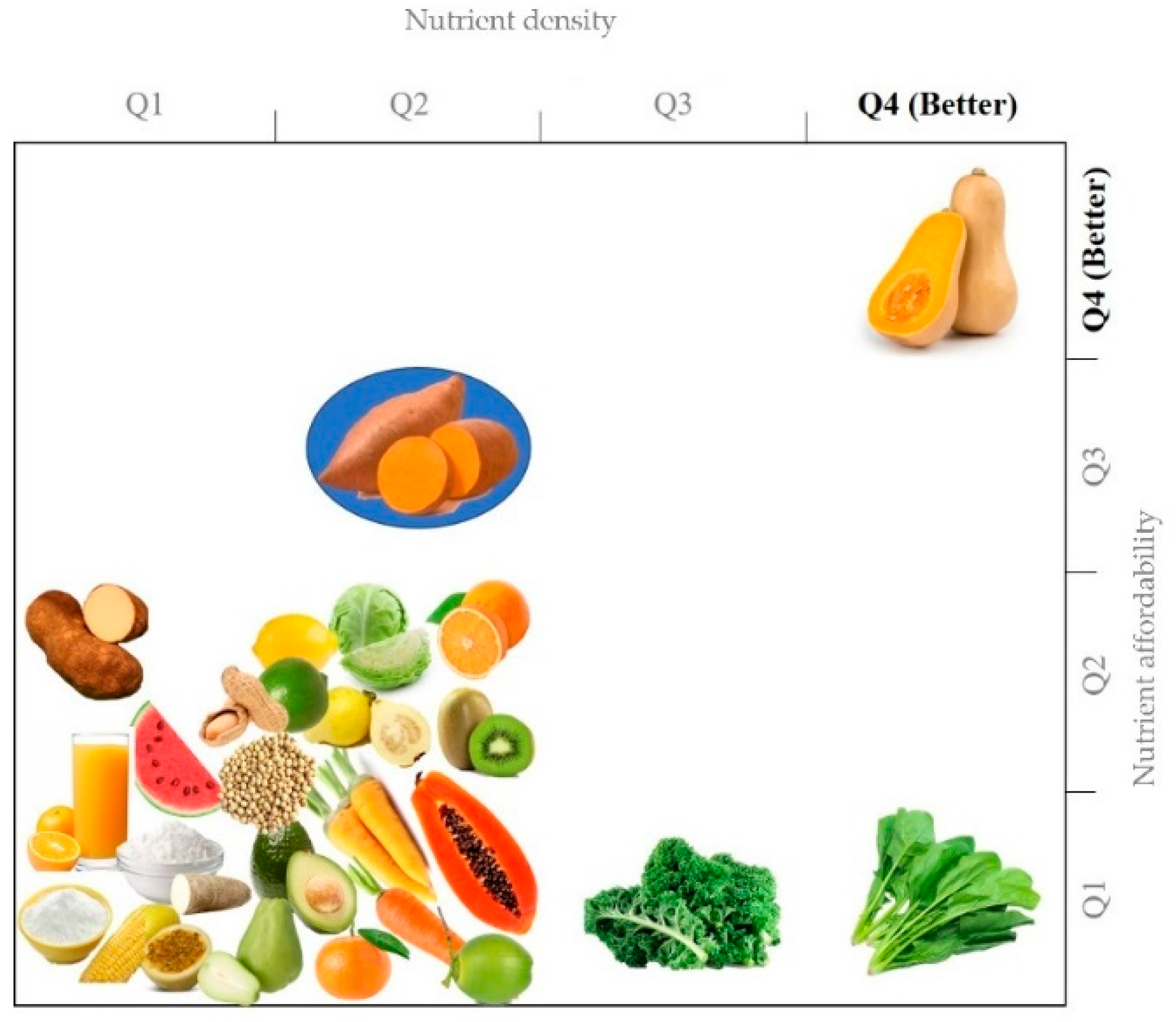
1.2.2. Nutritional Value
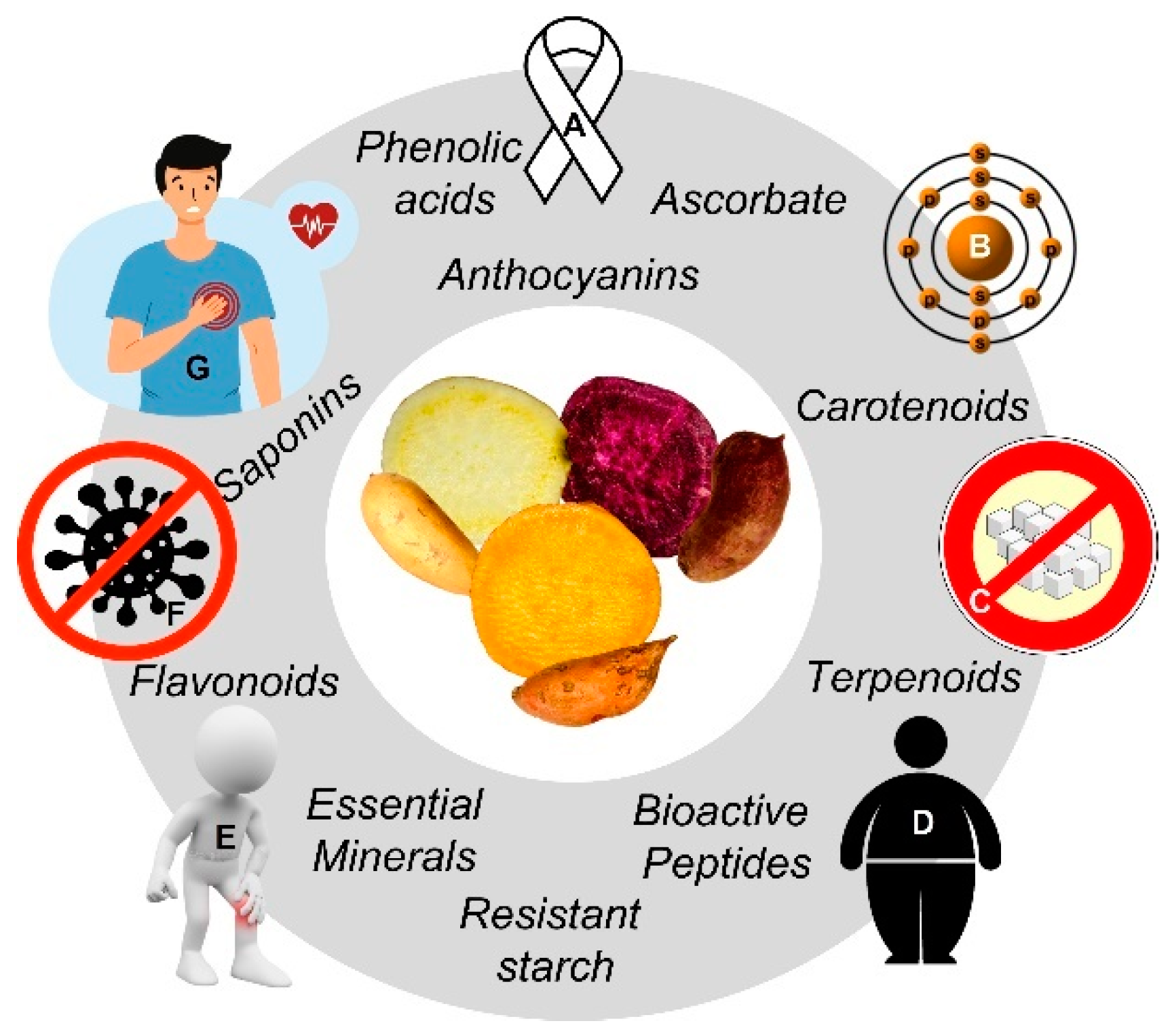
1.2.3. Functional Value
1.3. Health Effects and Metabolic Fate of SP’s Phytochemicals
1.3.1. SP and Cancer
1.3.2. SP and Cardiovascular Diseases (CVD)
2. Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guadarrama, L.M.; López, J.H. Patrones culinarios asociados al camote (Ipomoea batatas) y la yuca (Manihot esculenta) entre los mayas yucatecos, ch’oles y huastecos. Estud. Cult. Maya 2018, 52, 193–226. [Google Scholar] [CrossRef] [Green Version]
- Motsa, N.M.; Modi, A.T.; Mabhaudhi, T. Sweet potato (Ipomoea batatas L.) as a drought tolerant and food security crop. South Afr. J. Sci. 2015, 111, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Bonilla, L.; Cuevas, H.E.; Montero-Rojas, M.; Bird-Pico, F.; Luciano-Rosario, D.; Siritunga, D. Assessment of genetic diversity of sweet potato in Puerto Rico. PLoS ONE 2014, 9, e116184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartabiano-Leite, C.E.; Porcu, O.M.; de Casas, A.F. Sweet potato (Ipomoea batatas L. Lam) nutritional potential and social relevance: A review. History 2020, 11, 23–40. [Google Scholar] [CrossRef]
- Mwanga, R.O.; Andrade, M.I.; Carey, E.E.; Low, J.W.; Yencho, G.C.; Grüneberg, W.J. Sweetpotato (Ipomoea batatas L.). In Genetic Improvement of Tropical Crops; Campos, H., Caligari, P.D.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 181–218. [Google Scholar] [CrossRef]
- Xiao, S.; Xu, P.; Deng, Y.; Dai, X.; Zhao, L.; Heider, B.; Zhang, A.; Zhou, Z.; Cao, Q. Comparative analysis of chloroplast genomes of cultivars and wild species of sweetpotato (Ipomoea batatas [L.] Lam). BMC Genom. 2021, 22, 262. [Google Scholar] [CrossRef]
- Meira, M.; Da Silva, E.P.; David, J.M.; David, J.P. Review of the genus Ipomoea: Traditional uses, chemistry, and biological activities. Rev. Bras. Farmacogn. 2012, 22, 682–713. [Google Scholar] [CrossRef] [Green Version]
- de Albuquerque, T.M.R.; Sampaio, K.B.; de Souza, E.L. Sweet potato roots: Unrevealing an old food as a source of health promoting bioactive compounds–A review. Trends Food Sci. Technol. 2019, 85, 277–286. [Google Scholar] [CrossRef]
- de Albuquerque, T.M.R.; Borges, C.W.P.; Cavalcanti, M.T.; Lima, M.; Magnani, M.; de Souza, E.L. Potential prebiotic properties of flours from different varieties of sweet potato (Ipomoea batatas L.) roots cultivated in Northeastern Brazil. Food Biosci. 2020, 36, 100614. [Google Scholar] [CrossRef]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef]
- Khoury, C.K.; Heider, B.; Castañeda-Álvarez, N.P.; Achicanoy, H.A.; Sosa, C.C.; Miller, R.E.; Scotland, R.W.; Wood, J.R.I.; Rossel, G.; Eserman, L.A.; et al. Distributions, ex situ conservation priorities, and genetic resource potential of crop wild relatives of sweetpotato [Ipomoea batatas (L.) Lam., I. series Batatas]. Front. Plant Sci. 2015, 6, 251. [Google Scholar] [CrossRef]
- Mohanraj, R.; Sivasankar, S. Sweet Potato (Ipomoea batatas [L.] Lam)—A valuable medicinal food: A review. J. Med. Food 2014, 17, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.H.; Singh, J. Sweet potato: Chemistry, processing, and nutrition—An introduction. In Sweet Potato; Mu, T.H., Singh, J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Truong, V.D.; Avula, R.Y.; Pecota KYencho, G. Handbook of Vegetables and Vegetable Processing. In Handbook of Vegetables and Vegetable Processing; Siddiq, M., Uebersax, M.A., Eds.; Wiley-Blackwell Publishing Co.: Ames, IA, USA, 2018. Available online: https://www.ars.usda.gov/ARSUserFiles/60701000/Sweetpotato%20Publications/s158.pdf (accessed on 5 March 2022).
- Cartier, A.; Woods, J.; Sismour, E.; Allen, J.; Ford, E.; Githinji, L.; Xu, Y. Physiochemical, nutritional and antioxidant properties of fourteen Virginia-grown sweet potato varieties. J. Food Meas. Charact. 2017, 11, 1333–1341. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, S.J.; Lee, C.S.; Ren, C.; Kim, S.S.; Shin, M. Functional properties of different Korean sweet potato varieties. Food Sci. Biotechnol. 2011, 20, 1501–1507. [Google Scholar] [CrossRef]
- Anchundia, M.Á.; Pérez, E.; Torres, F. Composición química, perfil de aminoácidos y contenido de vitaminas de harinas de batata tratadas térmicamente. Rev. Chil. Nutr. 2019, 46, 137–143. [Google Scholar] [CrossRef]
- Kourouma, V.; Mu, T.H.; Zhang, M.; Sun, H.N. Effects of cooking process on carotenoids and antioxidant activity of orange-fleshed sweet potato. LWT 2019, 104, 134–141. [Google Scholar] [CrossRef]
- Failla, M.L.; Thakkar, S.K.; Kim, J.Y. In vitro bioaccessibility of β-carotene in orange fleshed sweet potato (Ipomoea batatas, Lam.). J. Agric. Food Chem. 2009, 57, 10922–10927. [Google Scholar] [CrossRef]
- Rios-Romero, E.A.; Ochoa-Martínez, L.A.; Bello-Pérez, L.A.; Morales-Castro, J.; Quintero-Ramos, A.; Gallegos-Infante, J.A. Effect of ultrasound and steam treatments on bioaccessibility of β-carotene and physicochemical parameters in orange-fleshed sweet potato juice. Heliyon 2021, 7, e06632. [Google Scholar] [CrossRef]
- Mbogo, D.; Muzhingi, T.; Janaswamy, S. Starch digestibility and β-carotene bioaccessibility in the orange-fleshed sweet potato puree-wheat bread. J. Food Sci. 2021, 86, 901–906. [Google Scholar] [CrossRef]
- Yang, Z.W.; Tang, C.E.; Zhang, J.L.; Zhou, Q.; Zhang, Z.C. Stability and antioxidant activity of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) subjected to simulated in vitro gastrointestinal digestion. Int. J. Food Sci. Technol. 2019, 54, 2604–2614. [Google Scholar] [CrossRef]
- Huamán, Z. Botánica Sistemática y Morfología De La Planta De Batata o Camote. International Potato Center. 1992. Available online: https://docplayer.es/24188174-Botanica-sistematica-y-morfologia-de-la-planta-de-batata-o-camote.html (accessed on 5 March 2022).
- Amoanimaa-Dede, H.; Su, C.; Yeboah, A.; Chen, C.; Yang, S.; Zhu, H.; Chen, M. Flesh color diversity of sweet potato: An overview of the composition, functions, biosynthesis, and gene regulation of the major pigments. Phyton 2020, 89, 805–833. [Google Scholar] [CrossRef]
- Laurie, S.M.; Calitz, F.J.; Adebola, P.O.; Lezar, A. Characterization and evaluation of South African sweet potato (Ipomoea batatas (L.) LAM) land races. South Afr. J. Bot. 2013, 85, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Moeinzadeh, M.-H.; Kuhl, H.; Helmuth, J.; Xiao, P.; Haas, S.; Liu, G.; Zheng, J.; Sun, Z.; Fan, W.; et al. Haplotype-resolved sweet potato genome traces back its hexaploidization history. Nat. Plants 2017, 3, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Rodríguez, P.; Carruthers, T.; Wood, J.R.; Williams, B.R.; Weitemier, K.; Kronmiller, B.; Goodwin, Z.; Sumadijaya, A.; Anglin, N.; Filer, D.; et al. A taxonomic monograph of Ipomoea integrated across phylogenetic scales. Nat. Plants 2019, 5, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Gemenet, D.C.; da Silva Pereira, G.; De Boeck, B.; Wood, J.C.; Mollinari, M.; Olukolu, B.A.; Buell, C.R. Quantitative trait loci and differential gene expression analyses reveal the genetic basis for negatively associated β-carotene and starch content in hexaploid sweetpotato [Ipomoea batatas (L.) Lam.]. Theor. Appl. Genet. 2020, 133, 23–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roullier, C.; Benoit, L.; McKey, D.B.; Lebot, V. Historical collections reveal patterns of diffusion of sweet potato in Oceania obscured by modern plant movements and recombination. Proc. Natl. Acad. Sci. USA 2013, 110, 2205–2210. [Google Scholar] [CrossRef] [Green Version]
- Loebenstein, G. Origin, Distribution and Economic Importance. In The Sweetpotato; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Samiyarsih, S.; Azizah, E.; Herawati, W. Anatomical profile and genetic variability of sweet potato (Ipomoea batatas) cultivars in Banyumas, Central Java, based on RAPD markers. Biodiversitas J. Biol. Divers. 2020, 21, 1755–1766. [Google Scholar] [CrossRef]
- Otoboni, M.E.F.; De Oliveira, D.J.L.S.F.; Vargas, P.F.; Pavan, B.E.; Andrade, M.I. Genetic parameters and gain from selection in sweet potato genotypes with high beta-carotene content. Crop Breed. Appl. Biotechnol. 2020, 20, e31632038. [Google Scholar] [CrossRef]
- Rahajeng, W.; Rahayuningsih, S.A. Agronomic performance, variance component, and diversity of sixty-two sweet potato accessions. Biodiversitas 2017, 18, 95–100. [Google Scholar] [CrossRef]
- Low, J.W.; Ortiz, R.; Vandamme, E.; Andrade, M.; Biazin, B.; Grüneberg, W.J. Nutrient-dense orange-fleshed sweetpotato: Advances in drought-tolerance breeding and understanding of management practices for sustainable next-generation cropping systems in sub-Saharan Africa. Front. Sustain. Food Syst. 2020, 4, 50. [Google Scholar] [CrossRef]
- Tanaka, M.; Ishiguro, K.; Oki, T.; Okuno, S. Functional components in sweetpotato and their genetic improvement. Breed. Sci. 2017, 67, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Wang, W.; Kang, L.; Kim, S.-E.; Lee, C.-J.; Park, S.-C.; Park, W.S.; Ahn, M.-J.; Kwak, S.-S. Metabolic engineering of low-molecular-weight antioxidants in sweetpotato. Plant Biotechnol. Rep. 2020, 14, 193–205. [Google Scholar] [CrossRef]
- Andre, C.M.; Burgos, G.; Ziebel, J.; Guignard, C.; Hausman, J.-F.; Felde, T.Z. In vitro iron bioaccessibility and uptake from orange-fleshed sweet potato (Ipomoea batatas (L.) Lam.) clones grown in Peru. J. Food Compos. Anal. 2018, 68, 79–86. [Google Scholar] [CrossRef]
- Shumbusha, D.; Shimelis, H.; Laing, M.; Rukundo, P. Gene action and heritability of yield components of dual-purpose sweetpotato clones. Euphytica 2019, 215, 122. [Google Scholar] [CrossRef]
- Transparency Market Research. Sweet Potatoes Market—Global Industry Analysis, Sice, Share, Growth, Trends and Forecast 2016–2024. 2020. Available online: https://www.transparencymarketresearch.com/sweet-potatoes-market.html (accessed on 5 March 2022).
- The Insight Partners. Sweet potato Market to 2027—Global Analysis and Forecasts. 2019. Available online: https://www.theinsightpartners.com/reports/sweet-potato-market (accessed on 5 March 2022).
- Chandrasekara, A.; Josheph Kumar, T. Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. Int. J. Food Sci. 2016, 2016, 3631647. [Google Scholar] [CrossRef] [Green Version]
- Afzal, N.; Afionis, S.; Stringer, L.; Favretto, N.; Sakai, M.; Sakai, P. Benefits and trade-offs of smallholder sweet potato cultivation as a pathway toward achieving the sustainable development goals. Sustainability 2021, 13, 552. [Google Scholar] [CrossRef]
- Rukundo, P.; Shimelis, H.; Laing, M.; Mashilo, J. Genotype-by-environment interaction for dual-purpose traits in sweetpotato. J. Crop Improv. 2020, 34, 800–823. [Google Scholar] [CrossRef]
- Laxminarayana, K.; Mishra, S.; Soumya, S. Good agricultural practices in tropical root and tuber crops (Chapter 4). In Tropical Roots and Tubers: Production, Processing and Technology; Sharma, H.K., Njintang, N.Y., Singhal, R.S., Kaushal, P., Eds.; John Wiley Sons, Ltd.: West Sussex, UK, 2016; pp. 183–224. [Google Scholar] [CrossRef]
- Kihurani, A.W.; Kaushal, P. Storage techniques and commercialization (Chapter 6). In Tropical Roots and Tubers: Production, Processing and Technology; Sharma, H.K., Njintang, N.Y., Singhal, R.S., Kaushal, P., Eds.; John Wiley Sons, Ltd.: West Sussex, UK, 2016; pp. 281–300. [Google Scholar] [CrossRef]
- Tavva, S.; Nedunchezhiyan, M. Global status of sweet potato cultivation. Fruit Veg. Cereal Sci. Biotechnol. 2012, 6, 143–147. Available online: http://www.globalsciencebooks.info/Online/GSBOnline/images/2012/FVCSB_6(SI1)/FVCSB_6(SI1)143-147o.pdf (accessed on 8 March 2022).
- FAO. FAOSTAT Database. Available online: www.fao.org/faostat/en/#data/QC (accessed on 5 March 2022).
- Mello, A.F.S.; Silva, G.O.D.; Nunes, M.U.C.; Celestino Filho, P.; Silva, W.B.; Moita, A.W.; de Carvalho, J.L.V.; Nuti, M.R. Performance of sweet potato genotypes in Brazilian regions. Sci. Agric. 2022, 79, e20210082. [Google Scholar] [CrossRef]
- Scott, G.J.; Maldonado, L. Sweetpotato for the New Millennium: Trends in Production and Utilization in Developing Countries; Program Report 1997–1998; Centro Internacional de la Papa (CIP): Lima, Peru, 1999; pp. 329–335. Available online: http://www.sweetpotatoknowledge.org/wp-content/uploads/2016/04/Sweetpotato-for-the-New-Millennium-Trends-in-Production-and-Utilization-in-Developing-Countries.pdf (accessed on 8 March 2022).
- Choquechambi, L.A.; Callisaya, I.R.; Ramos, A.; Bosque, H.; Mújica, A.; Jacobsen, S.E.; Sørensen, M.; Leidi, E.O. Assessing the nutritional value of root and tuber crops from Bolivia and Peru. Foods 2019, 8, 526. [Google Scholar] [CrossRef] [Green Version]
- Livia-Tacza, C.; Sánchez, G. Soil arthropods associated with sweetpotato crop (Ipomoea batata L.) in La Molina, Lima, Peru. Peruv. J. Agron. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Torquato-Tavares, A.; Nascimento, I.R.D.; Pascual-Reyes, I.D.; Santana, W.R.D.; Silveira, M.A.D. Potencial de cruzas simples de camote (Ipomoea batatas (L.) Lam.) para mejorar la producción de etanol. Revista Chapingo. Ser. Hortic. 2017, 23, 59–74. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.-J.; Kim, S.-E.; Ji, C.Y.; Kim, S.-T.; Kim, J.-S.; Kim, S.; Kwak, S.-S. Current status on global sweetpotato cultivation and its prior tasks of mass production. J. Plant Biotechnol. 2018, 45, 190–195. [Google Scholar] [CrossRef]
- Prakash, P.; Kishore, A.; Roy, D.; Behura, D.; Immanuel, S. Biofortification for reducing hidden hunger: A value chain analysis of sweet potato. Agric. Econ. Res. Rev. 2017, 30, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Nanbol, K.; Namo, O. The Contribution of Root and Tuber Crops to Food Security: A Review. J. Agric. Sci. Technol. B 2019, 9. [Google Scholar] [CrossRef]
- Food Navigator—USA.com. Sweet Potatoes Destined for Further Market Penetration, Says Innova Market Insights. Available online: https://www.foodnavigator-usa.com/Article/2019/05/07/Sweet-potatoes-destined-for-further-market-penetration-says-Innova-Market-Insights# (accessed on 5 March 2022).
- PMR. Sweet Potato Fries Market. 2020. Available online: www.persistencemarketresearch.com/market-research/sweet-potato-fries-market.asp (accessed on 8 March 2022).
- Laryea, D.; Koomson, D.; Oduro, I.; Carey, E. Evaluation of 10 genotypes of sweetpotato for fries. Food Sci. Nutr. 2019, 7, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Leksrisompong, P.P.; Whitson, M.E.; Truong, V.D.; Drake, M.A. Sensory attributes and consumer acceptance of sweet potato cultivars with varying flesh colors. J. Sens. Stud. 2012, 27, 59–69. [Google Scholar] [CrossRef]
- Drewnowski, A. The nutrient rich foods index helps to identify healthy, affordable foods. Am. J. Clin. Nutr. 2010, 91, 1095S–1101S. [Google Scholar] [CrossRef]
- USDA. Sweet Potato, Raw, Unprepared. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168482/nutrients (accessed on 8 March 2022).
- Neela, S.; Fanta, S.W. Review on nutritional composition of orange-fleshed sweet potato and its role in management of vitamin A deficiency. Food Sci. Nutr. 2019, 7, 1920–1945. [Google Scholar] [CrossRef] [Green Version]
- Brabet, C.; Reynoso, D.; Dufour, D.; Mestres, C.; Arredondo, J.; Scott, G. Starch Content and Properties of 106 Sweetpotato Clones from the World Germplasm Collection Held at CIP, Peru; CIP Program Report 1997–1998; CIP: Lima, Peru, 2013; pp. 279–286. Available online: http://www.sweetpotatoknowledge.org/wp-content/uploads/2015/10/Starch-Content-and-Properties-of-106-Sweetpotato-Clones-From-The-World-Germplasm-Collection-Held-at-CIP-Peru.pdf (accessed on 8 March 2022).
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.; Liu, J.; Zhang, X.; Wu, X.; Tang, S.; Sun, R.; Qian, C.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef]
- Lu, H.; Gui, Y.; Guo, T.; Wang, Q.; Liu, X. Effect of the particle size of cellulose from sweet potato residues on lipid metabolism and cecal conditions in ovariectomized rats. Food Funct. 2015, 6, 1185–1193. [Google Scholar] [CrossRef]
- Song, H.Y.; Lee, S.Y.; Choi, S.J.; Kim, K.M.; Kim, J.S.; Han, G.J.; Moon, T.W. Digestibility and physicochemical properties of granular sweet potato starch as affected by annealing. Food Sci. Biotechnol. 2014, 23, 23–31. [Google Scholar] [CrossRef]
- Lv, Q.Q.; Li, G.Y.; Xie, Q.T.; Zhang, B.; Li, X.M.; Pan, Y.; Chen, H.Q. Evaluation studies on the combined effect of hydrothermal treatment and octenyl succinylation on the physic-chemical, structural and digestibility characteristics of sweet potato starch. Food Chem. 2018, 256, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Morphological, structural and digestibility properties of RS4 enriched octenyl succinylated sweet potato, banana and lentil starches. Food Hydrocoll. 2018, 82, 219–229. [Google Scholar] [CrossRef]
- Kwon, C.; Kim, H.R.; Moon, T.W.; Lee, S.H.; Lee, C.J. Structural and physicochemical characteristics of granular malic acid-treated sweet potato starch containing heat-stable resistant starch. J. Chem. 2019, 2019, 2903252. [Google Scholar] [CrossRef]
- Yu, S.X.; Mu, T.H.; Zhang, M.; Ma, M.M.; Zhao, Z.K. Effects of retrogradation and further acetylation on the digestibility and physicochemical properties of purple sweet potato flour and starch. Starch-Stärke 2015, 67, 892–902. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Physicochemical properties and bioactive compounds of different varieties of sweet potato flour treated with high hydrostatic pressure. Food Chem. 2019, 299, 125129. [Google Scholar] [CrossRef]
- Trung, P.T.B.; Ngoc, L.B.B.; Hoa, P.N.; Tien, N.N.T.; Van Hung, P. Impact of heat-moisture and annealing treatments on physicochemical properties and digestibility of starches from different colored sweet potato varieties. Int. J. Biol. Macromol. 2017, 105, 1071–1078. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Q.; Li, B.; Lin, L.; Tundis, R.; Loizzo, M.R.; Zheng, B.; Xiao, J. Characterization and prebiotic effect of the resistant starch from purple sweet potato. Molecules 2016, 21, 932. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Li, X.; Janaswamy, S.; Chi, C.; Chen, L.; Wu, Y.; Liang, Y. Insights on the structure and digestibility of sweet potato starch: Effect of postharvest storage of sweet potato roots. Int. J. Biol. Macromol. 2020, 145, 694–700. [Google Scholar] [CrossRef]
- Babu, A.S.; Parimalavalli, R. Effect of pullulanase debranching and storage temperatures on structural characteristics and digestibility of sweet potato starch. J. Saudi Soc. Agric. Sci. 2018, 17, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Mu, T.H.; Tan, S.S.; Xue, Y.L. The amino acid composition, solubility and emulsifying properties of sweet potato protein. Food Chem. 2009, 112, 1002–1005. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, T.H.; Sun, M.J. Sweet potato protein hydrolysates: Antioxidant activity and protective effects on oxidative DNA damage. Int. J. Food Sci. Technol. 2012, 47, 2304–2310. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Sampaio, S.L.; Di Gioia, F.; Tzortzakis, N.; Rouphael, Y.; Kyriacou, M.C.; Ferreira, I. Grown to be blue—Antioxidant properties and health effects of colored vegetables. Part I: Root vegetables. Antioxidants 2019, 8, 617. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.U.; Mu, T.H.; Sun, H.N. Sweet potato and potato residual flours as potential nutritional and healthy food material. J. Integr. Agric. 2017, 16, 2632–2645. [Google Scholar] [CrossRef]
- Teow, C.C.; Truong, V.D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Padda, M.; Picha, D. Quantification of phenolic acids and antioxidant activity in sweetpotato genotypes. Sci. Hortic. 2008, 119, 17–20. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lee, S.Y.; Yang, J.W.; Lee, J.-S.; Oh, S.-D.; Oh, S.; Lee, S.M.; Lim, M.-H.; Park, S.K.; Jang, J.-S.; et al. Comparative analysis of phytochemicals and polar metabolites from colored sweet potato (Ipomoea batatas L.) tubers. Food Sci. Biotechnol. 2016, 25, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cai, W.; Xu, B. Profiles of phenolics, carotenoids and antioxidative capacities of thermal processed white, yellow, orange, and purple sweet potatoes grown in Guilin, China. Food Sci. Hum. Wellness 2015, 4, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Kammona, S.; Othman, R.; Jaswir, I.; Jamal, P. Characterisation of carotenoid content in diverse local sweet potato (Ipomoea batatas) flesh tubers. Int. J. Pharm. Pharm. Sci. 2015, 2, 347–351. [Google Scholar]
- Makori, S.I.; Mu, T.H.; Sun, H.N. Total polyphenol content, antioxidant activity, and individual phenolic composition of different edible parts of 4 sweet potato cultivars. Nat. Prod. Commun. 2020, 15, 1934578X20936931. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Saponins in Ipomoea batatas tubers: Isolation, characterization, quantification and antioxidant properties. Food Chem. 2009, 113, 411–419. [Google Scholar] [CrossRef]
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Chen, C.C.; Lin, K.H.; Chao, P.Y.; Lin, H.H.; Huang, M.Y. Bioactive compounds, antioxidants, and health benefits of sweet potato leaves. Molecules 2021, 26, 1820. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.M.; Faber, M.; Claasen, N. Incorporating orange-fleshed sweet potato into the food system as a strategy for improved nutrition: The context of South Africa. Food Res. Int. 2018, 104, 77–85. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Dikilitas, M. Role of antioxidant phytochemicals in prevention, formation, and treatment of cancer in Reactive Oxygen Species (ROS) in Living Cells. In Reactive Oxygen Species (ROS) in Living Cells; InTech Open: Rijeka, Croatia, 2018; pp. 21–45. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.J.; Sheu, M.J.; Chen, H.J.; Chang, Y.S.; Lin, Y.H. Growth inhibition and induction of apoptosis in NB4 promyelocytic leukemia cells by trypsin inhibitor from sweet potato storage roots. J. Agric. Food Chem. 2007, 55, 2548–2553. [Google Scholar] [CrossRef]
- Karna, P.; Gundala, S.R.; Gupta, M.V.; Shamsi, S.A.; Pace, R.D.; Yates, C.; Narayan, S.; Aneja, R. Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis 2011, 32, 1872–1880. [Google Scholar] [CrossRef] [Green Version]
- Taira, J.; Uehara, M.; Tsuchida, E.; Ohmine, W. Inhibition of the β-catenin/Tcf signaling by caffeoylquinic acids in sweet potato leaf through down regulation of the Tcf-4 transcription. J. Agric. Food Chem. 2014, 62, 167–172. [Google Scholar] [CrossRef]
- Mantilla, C.; Suárez Mellado, I.; Duque Jaramillo, A.; Navas, M.C. Mecanismos de señalización por β-catenina y su papel en la carcinogénesis. CES Med. 2015, 29, 109–127. Available online: http://www.scielo.org.co/pdf/cesm/v29n1/v29n1a10.pdf (accessed on 8 March 2022).
- Xu, H.; Li, Y.; Han, B.; Li, Z.; Wang, B.; Jiang, P.; Zhang, J.; Ma, W.; Zhou, D.; Li, X.; et al. Anti-breast-cancer activity exerted by β-sitosterol-D-glucoside from sweet potato via upregulation of microRNA-10a and via the PI3K–Akt signaling pathway. J. Agric. Food Chem. 2018, 66, 9704–9718. [Google Scholar] [CrossRef]
- Patel, K.; Kumar, V.; Verma, A.; Rahman, M.; Patel, D.K. β-sitosterol: Bioactive compounds in foods, their role in health promotion and disease prevention “a concise report of its phytopharmaceutical importance”. Curr. Tradit. Med. 2017, 3, 168–177. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The role of angiogenesis in cancer treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-M.; Li, S.-C.; Au, H.-K.; Shih, C.-K.; Hsu, C.-Y.; Liu, J.-F. Constituents in purple sweet potato leaves inhibit in vitro angiogenesis with opposite effects ex vivo. Nutrition 2011, 27, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, Y.; Wang, M.; Xu, H.; Han, B.; Jiang, P.; Ma, H.; Li, Y.; Tian, C.; Zhou, D.; et al. Anti-breast cancer activity of SPG-56 from sweet potato in MCF-7 bearing mice in situ through promoting apoptosis and inhibiting metastasis. Sci. Rep. 2019, 9, 146. [Google Scholar] [CrossRef]
- Lim, S. Anthocyanin-Enriched Purple Sweet Potato for Colon Cancer Prevention; Kansas State University, ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2012; Available online: https://www.proquest.com/openview/aac0a1d3911587677e77c25f72859b42/1?pq-origsite=gscholar&cbl=18750 (accessed on 8 March 2022).
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N. Comparative study on the chemical structure and in vitro antiproliferative activity of anthocyanins in purple root tubers and leaves of sweet potato (Ipomoea batatas). J. Agric. Food Chem. 2019, 67, 2467–2475. [Google Scholar] [CrossRef]
- Lee, J.H.; Woo, K.S.; Lee, H.-U.; Nam, S.S.; Lee, B.W.; Lee, Y.-Y.; Lee, B.; Kim, H.-J. Intracellular reactive oxygen species (ros) removal and cytoprotection effects of sweet potatoes of various flesh colors and their polyphenols, including anthocyanin. Prev. Nutr. Food Sci. 2019, 24, 293–298. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, T.H. Contribution of different molecular weight fractions to anticancer effect of sweet potato protein hydrolysates by six proteases on HT-29 colon cancer cells. Int. J. Food Sci. Technol. 2018, 53, 525–532. [Google Scholar] [CrossRef]
- Han, Y.T.; Chen, X.H.; Xie, J.; Zhan, S.M.; Wang, C.B.; Wang, L.X. Purple sweet potato pigments scavenge ROS, reduce p53 and modulate Bcl-2/Bax to inhibit irradiation-induced apoptosis in murine thymocytes. Cell. Physiol. Biochem. 2011, 28, 865–872. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. A review of therapeutic potentials of sweet potato: Pharmacological activities and influence of the cultivar. Trop. J. Pharm. Res. 2016, 15, 2751–2761. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Zhou, J.; Liu, W.; Tao, W.; He, J.; Jin, W.; Guo, H.; Yang, N.; Li, Y. The anti-inflammatory potential of protein-bound anthocyanin compounds from purple sweet potato in LPS-induced RAW264. 7 macrophages. Food Res. Int. 2020, 137, 109647. [Google Scholar] [CrossRef]
- Sugata, M.; Lin, C.Y.; Shih, Y.C. Anti-inflammatory and anticancer activities of taiwanese purple-fleshed sweet potatoes (Ipomoea batatas L. Lam) extracts. BioMed Res. Int. 2015, 2015, 768093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Xu, J.; Kim, J.; Chen, T.-Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef] [Green Version]
- Sesso, H.D.; Liu, S.; Gaziano, J.M.; Buring, J.E. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J. Nutr. 2003, 133, 2336–2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.M.; Li, S.C.; Lin, Y.L.; Hsu, C.Y.; Shieh, M.J.; Liu, J.F. Consumption of purple sweet potato leaves modulates human immune response: T-lymphocyte functions, lytic activity of natural killer cell and antibody production. World J. Gastroenterol. 2005, 11, 5777–5781. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Q.; Long, L.; Li, J.; Yang, R.; Gao, D. Antidiabetic activity of flavone from Ipomoea batatas leaf in non-insulin dependent diabetic rats. Int. J. Food Sci. Technol. 2007, 42, 80–85. [Google Scholar] [CrossRef]
- Johnson, M.; Pace, R.D. Sweet potato leaves: Properties and synergistic interactions that promote health and prevent disease. Nutr. Rev. 2010, 68, 604–615. [Google Scholar] [CrossRef]
- Torres-Urrutia, C.; Guzmán, L.; Schmeda-Hirschmann, G.; Moore-Carrasco, R.; Alarcón, M.; Astudillo, L.; Gutierrez, M.; Carrasco, G.; Yuri, J.A.; Aranda, E.; et al. Antiplatelet, anticoagulant, and fibrinolytic activity in vitro of extracts from selected fruits and vegetables. Blood Coagul. Fibrinolysis 2011, 22, 197–205. [Google Scholar] [CrossRef]
- Chen, C.-M.; Lin, Y.-L.; Hsu, C.-Y.; Shieh, M.-J.; Liu, J.-F. Consumption of purple sweet potato leaves decreases lipid peroxidation and DNA damage in humans. Asia Pac. J. Clin. Nutr. 2008, 17, 408–414. Available online: https://apjcn.nhri.org.tw/server/APJCN/17/3/408.pdf (accessed on 5 March 2022). [PubMed]
- Shih, C.K.; Chen, C.M.; Hsiao, T.J.; Liu, C.W.; Li, S.C. White sweet potato as meal replacement for overweight white-collar workers: A randomized controlled trial. Nutrients 2019, 11, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.; Pace, R.D.; Dawkins, N.L. The antioxidant potential of sweet potato greens in preventing cardiovascular disease risk in hamster. FASEB J. 2007, 21, A1091. [Google Scholar] [CrossRef]
- Runnie, I.; Salleh, M.N.; Mohamed, S.; Head, R.J.; Abeywardena, M.Y. Vasorelaxation induced by common edible tropical plant extracts in isolated rat aorta and mesenteric vascular bed. J. Ethnopharmacol. 2004, 92, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; Mu, T.H.; Zhang, M. Preparation and identification of angiotensin I-converting enzyme inhibitory peptides from sweet potato protein by enzymatic hydrolysis under high hydrostatic pressure. Int. J. Food Sci. Technol. 2020, 55, 482–489. [Google Scholar] [CrossRef]
- Chintha, P.; Sarkar, D.; Pecota, K.; Dogramaci, M.; Shetty, K. Improving Phenolic Bioactive-Linked Functional Qualities of Sweet Potatoes Using Beneficial Lactic Acid Bacteria-Based Biotransformation Strategy. Horticulturae 2021, 7, 367. [Google Scholar] [CrossRef]
- Jawi, I.M.; Mahendra, A.N.; Subawa, A.A.N.; Yasa, I.S.; Gunawan, W.G. Comparison of antihypertensive and antioxidative effect of Mahogany (Swietenia Mahagoni (L.) Jacq.) seed extract and purple sweet potato (Ipomoea batatas) tuber extract on rodent model of hypertension. Biomed. Pharmacol. J. 2017, 10, 577–582. [Google Scholar] [CrossRef]
- Herawati, E.R.N.; Santosa, U.; Sentana, S.; Ariani, D. Protective effects of anthocyanin extract from purple sweet potato (Ipomoea batatas L.) on blood MDA levels, liver and renal activity, and blood pressure of hyperglycemic rats. Prev. Nutr. Food Sci. 2020, 25, 375–379. [Google Scholar] [CrossRef]
- Suastika, L.; Oktaviono, Y.; Soemantri, D.; Sandra, F. Purple sweet potato extract and vitamin C increase the proliferation of endothelial progenitor cells from stable coronary artery disease patients. Bali Med. J. 2021, 10, 243–248. [Google Scholar] [CrossRef]
- Choi, J.H.; Hwang, Y.P.; Park, B.H.; Choi, C.Y.; Chung, Y.C.; Jeong, H.G. Anthocyanins isolated from the purple-fleshed sweet potato attenuate the proliferation of hepatic stellate cells by blocking the PDGF receptor. Environ. Toxicol. Pharmacol. 2011, 31, 212–219. [Google Scholar] [CrossRef]
- Satriyasa, B.K. Purple sweet potato ethanolic extract reduces aortic VCAM expression in rabbit with high-cholesterol diet. Bali Med. J. 2017, 6, S26–S28. [Google Scholar] [CrossRef]
- Rahmawati, E.; Sasangka Prasetyawan, C.M.; Srihardyastutie, A.; Adnyana, M.O. Potential of Purple Sweet Potato (Ipomoea batatas L.) To Increase BDNF Level and VEGF Expression in The Cerebellum of Ischemic Stroke Rats. J. Pure Appl. Chem. Res. 2018, 7, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Rahmawati, I.S.; Soetjipto, S.; Adi, A.C.; Aulanni’am, A.A. The effectiveness of ethanol extract of purple sweet potato var. Ayamurasaki as a natural antihypertensive mitigator in deoxycorticosterone acetate-salt hypertensive rats. Drug Invent. Today 2019, 11, 3179–3193. [Google Scholar]
- Zheng, G.-H.; Shan, Q.; Mu, J.-J.; Wang, Y.-J.; Zhang, Z.-F.; Fan, S.-H.; Hu, B.; Li, M.-Q.; Xie, J.; Chen, P.; et al. Purple sweet potato color attenuates kidney damage by blocking VEGFR2/ROS/NLRP3 signaling in high-fat diet-treated mice. Oxidative Med. Cell. Longev. 2019, 2019, 5189819. [Google Scholar] [CrossRef] [PubMed]
- Shafe, M.; Eze, E.; Ubhenin, A.; Tende, J. Effects of Aqueous Tuber Extract of Ipomea batatas on Cardiac Enzymes, Lipid Profile and Organ Weights in Wistar Rats. J. Basic Appl. Res. Biomed. 2016, 2, 414–417. [Google Scholar]
- Chang, H.H.; Lan, Y.C.; Chung, S.D.; Chien, C.T. Sweet potato leaf feeding decreases cholesterol, oxidative stress and thrombosis formation in syrian hamsters with a high-cholesterol diet. Life 2021, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Osuntokun, O.T.; Yusuf-Babatunde, M.A.; Fasila, O.O. Components and Bioactivity of Ipomoea batatas (L.)(Sweet Potato) Ethanolic Leaf Extract. Asian J. Adv. Res. Rep. 2020, 10, 10–26. [Google Scholar] [CrossRef]
- Li, J.; Shi, Z.; Mi, Y. Purple sweet potato color attenuates high fat-induced neuroinflammation in mouse brain by inhibiting MAPK and NF-κB activation. Mol. Med. Rep. 2018, 17, 4823–4831. [Google Scholar] [CrossRef]

| (a) | Global | China | Nigeria | ||
| 1999 | |||||
| Area (Ha) | 9,749,117 | 5,945,975 | 817,000 | ||
| Production (tons) | 147,214,978 | 126,143,701 | 2,354,000 | ||
| Yield (Hg/Ha) | 89,239 ± 63,996 | 212,150 | 43,673 | ||
| 2009 | |||||
| Area (Ha) | 7,848,391 | 3,253,056 | 1,100,000 | ||
| Production (tons) | 96,424,362 | 70,040,593 | 3,300,000 | ||
| Yield (Hg/Ha) | 100,364 ± 73,753 | 215,307 | 30,000 | ||
| 2019 | |||||
| Area (Ha) | 7,769,851 | 2,373,737 | 1,717,659 | ||
| Production (tons) | 91,820,732 | 51,992,156 | 4,145,488 | ||
| Yield (Hg/Ha) | 110,770 ± 82,415 | 219,031 | 24,135 | ||
| (b) | 1999 | 2009 | 2019 | ΔYield/y | R2 |
| Senegal | 87,469 | 400,000 | 385,997 | 15,396 | 0.69 |
| Australia | 275,000 | 250,797 | 363,976 | 4805 | 0.59 |
| Egypt | 240,545 | 285,425 | 320,537 | 4197 | 0.99 |
| Cook Islands | 263,478 | 266,667 | 291,667 | 1502 | 0.86 |
| Component | White | Yellow | Orange | Purple |
|---|---|---|---|---|
| Total carbohydrates | 85.3–87.3 | 81.3–85.7 | 83.1–87.0 | 84.5–85.0 |
| Digestible starch | 54.6–64.1 | 51.2–61.1 | 42.3–60.0 | 53.4–54.8 |
| Sucrose | 5.0–12.9 | 7.7–11.6 | 4.7–16.5 | 5.8–8.1 |
| Protein | 4.1–5.8 | 5.1–5.9 | 4.3–6.2 | 5.4–5.8 |
| Resistant starch | 2.5–3.7 | 1.6–4.3 | 0.6–3.8 | 1.8–2.7 |
| Ash | 2.3–3.4 | 2.6–2.8 | 3.3–4.5 | 1.5–2.9 |
| Crude fiber | 1.6–2.6 | 1.3–1.4 | 1.9–3.3 | 1.1–1.5 |
| Fructose | 0.5–4.5 | 0.8–4.3 | 0.9–6.6 | 1.9–2.4 |
| Glucose | 0.6–4.8 | 0.9–1.3 | 1.0–6.5 | 1.8–2.3 |
| Fat | 1.3–1.7 | 1.8–2.1 | 1.3–2.2 | 1.3–1.8 |
| Parameter | White | Yellow | Orange | Purple |
|---|---|---|---|---|
| Total phenols (mg GAE) | 1.4–2.5 | 3.3–3.5 | 2.9–4.6 | 11.5–12.3 |
| Flavonoids (mg QE) | 5.8–12.2 | 27.3–29.6 | 14.6–29.6 | 76.2–84.4 |
| DPPH (mg TE) | 3.2–17.6 | 9.5–13.5 | 7.0–11.8 | 17.2–17.9 |
| Anthocyanins (mg Cy3GE) | - | - | - | 1.4–1.6 |
| Carotenoids (mg) | 4.5 | 16.0 | 180.0 | 2.9 |
| Variety | Phytochemical | Mechanism | Action |
|---|---|---|---|
| Initiation | |||
| Tainong 57 | Trypsin inhibitor | DNA damage reparation | ↑ P53 leukemic cells |
| -- | Polyphenols | ↓ ROS | ↓ Oxidative damage induced by H2O2 in HepG2 cells. |
| Mixuan No. 1 | Protein hydrolysate | ↓ ROS | ↑ antioxidant activity, ↓ oxidative damage to DNA |
| Ayamurasaki | Anthocyanins | ↓ROS | ↓ Oxidative damage induced by radiation in thymocytes |
| Tainong 57 | Trypsin inhibitor | Cell cycle arrest | Phase G1 arrest |
| TU-155 | Polyphenols | Cell cycle arrest | ↓ciclin D1, A y E, ↑ Cip1/p21 |
| Promotion | |||
| NING No. 1 | Polysaccharides | Anti-inflammatory | ↓ IL-1β, IL-6 y TNF-α |
| TNG 73 | Anthocyanins | Anti-inflammatory | ↓ activation of NF-κβ in RAW 264.7 cells induced by LPS |
| -- | Caffeic acid and derivates | Inhibition in cell proliferation | β-catenin and Tcf-4 pathway suppression |
| Progression | |||
| Bhu Krishna | Anthocyanins | Cell death induction | Apoptosis—↑ caspases |
| Diverse | Anthocyanins | Cell death induction | ↑ caspase 3 in colonic cells |
| -- | Polyphenols | Angiogenesis inhibition | ↓ VEGF165 in a dose-dependent manner |
| -- | BSG | Invasion inhibition | PI3K-Akt signaling pathway suppression |
| Zhongshu-1 | SPG-56 Glycoprotein | Invasion inhibition | Regulation in the expression of proteins (MMP2, MMP9, VEGF, ocludin, and claudin) related with metastasis. |
| TNG 73 | Anthocyanins | Invasion inhibition | Cell migration suppression (MCF-7 cells) |
| Phytochemical | Mechanism | Action |
|---|---|---|
| Heart | ||
| Anthocyanins | ↓ Malondialdehyde | Antioxidant ↓ Lipid peroxidation |
| Flavonoids/ anthocyanin | Vasodilation induction/ ↓ endothelin—1 | Antihypertensive |
| Tannins/saponins/ Flavonoids/terpenoids | ↓ Creatine kinase ↓ Lactate dehydrogenase | Prevention in ischemic damage |
| Vascular | ||
| Aqueous extracts | ↑ Telomerase activity preventing cell senescence | Prevention of coronary artery disease |
| Anthocyanins | Inhibition of PDGF receptor-β | Regulation of platelet aggregation |
| Chlorogenic acid | ACE Inhibition | Antihypertensive |
| Anthocyanins/ethanolic extract | ↓ VCAM | Prevention of atherosclerosis |
| SP leaves | Elongate arterial occlusion time | Prevention of thrombotic events |
| Purple SP extract | ↓ cyclooxygenase-2, ↓ inducible nitric oxide synthase ↓ tumor necrosis factor-α | ↓Inflammation |
| Brain and Kidney | ||
| Anthocyanins | ↑ BDNF | Neuroprotection after ischemic stroke |
| Flavonoids/ acetylated anthocyanins | Blocking VEGFR2/ROS/NLRP3 signaling | ↓ Kidney damage |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Puentes, A.A.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects. Foods 2022, 11, 1058. https://doi.org/10.3390/foods11071058
Escobar-Puentes AA, Palomo I, Rodríguez L, Fuentes E, Villegas-Ochoa MA, González-Aguilar GA, Olivas-Aguirre FJ, Wall-Medrano A. Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects. Foods. 2022; 11(7):1058. https://doi.org/10.3390/foods11071058
Chicago/Turabian StyleEscobar-Puentes, Alberto A., Iván Palomo, Lyanne Rodríguez, Eduardo Fuentes, Mónica A. Villegas-Ochoa, Gustavo A. González-Aguilar, Francisco J. Olivas-Aguirre, and Abraham Wall-Medrano. 2022. "Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects" Foods 11, no. 7: 1058. https://doi.org/10.3390/foods11071058
APA StyleEscobar-Puentes, A. A., Palomo, I., Rodríguez, L., Fuentes, E., Villegas-Ochoa, M. A., González-Aguilar, G. A., Olivas-Aguirre, F. J., & Wall-Medrano, A. (2022). Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects. Foods, 11(7), 1058. https://doi.org/10.3390/foods11071058










