Drying Behavior of Bulgur and Its Effect on Phytochemical Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Bulgur Processing
2.3. Moisture Content
2.4. Phytochemical Analysis
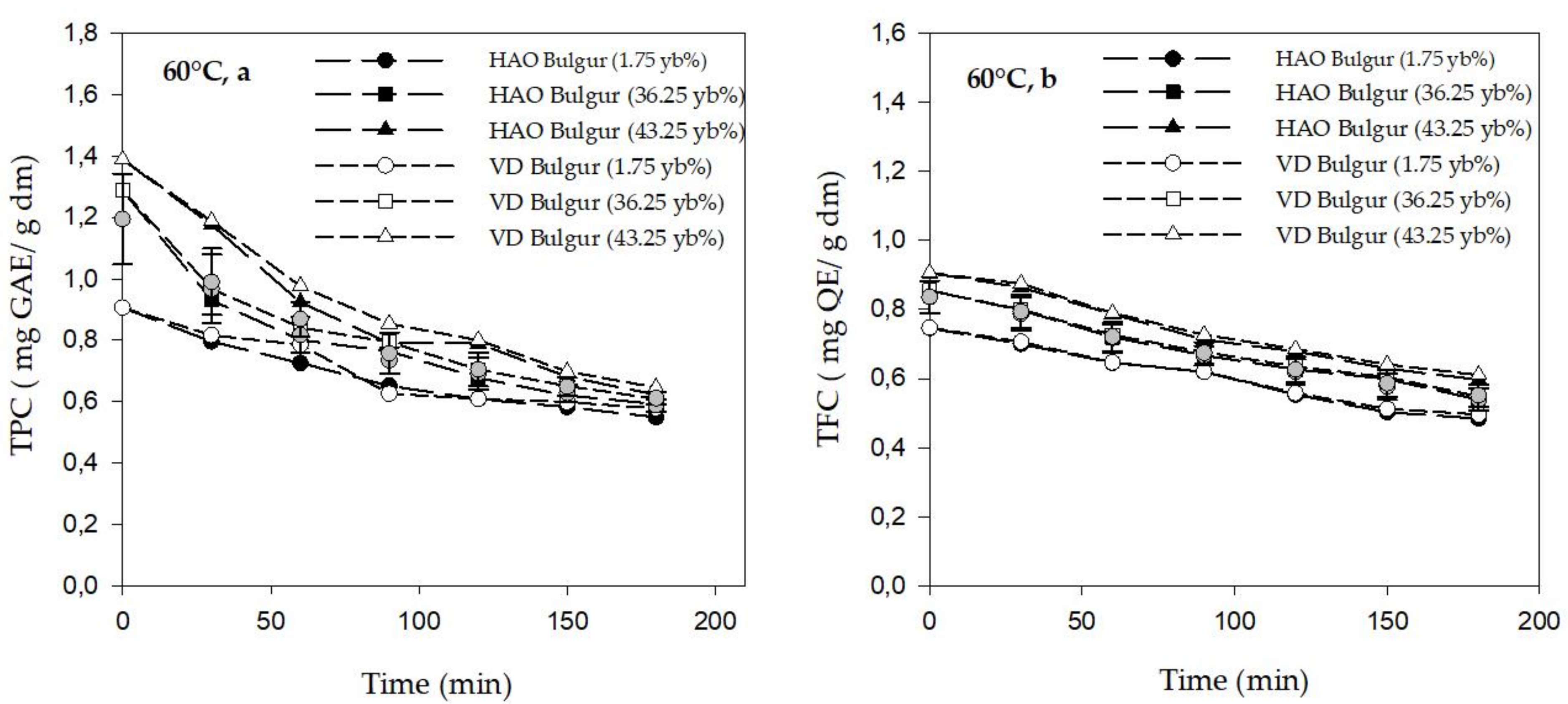
2.4.1. Total Polyphenol Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
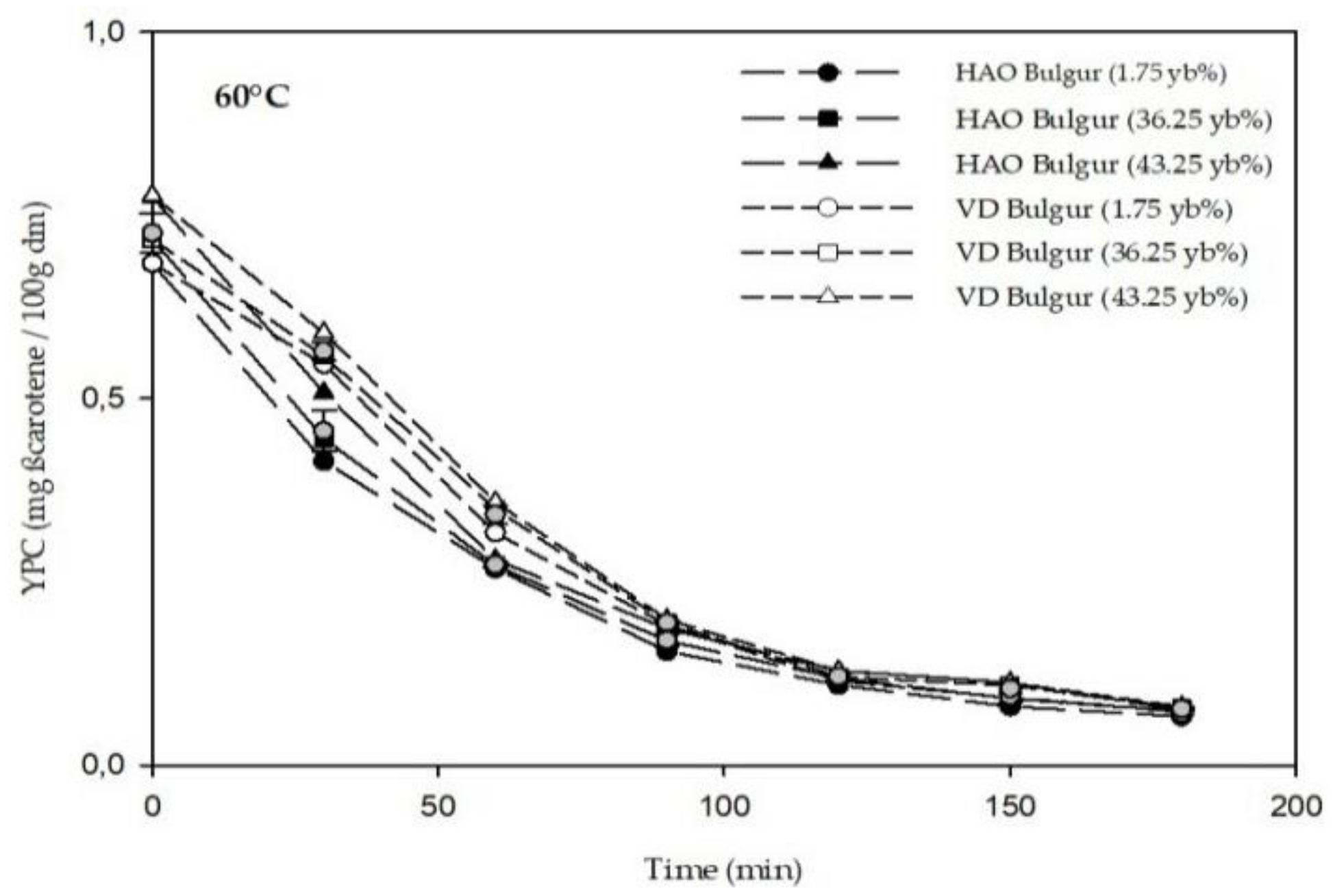
2.4.3. Yellow Pigment Content (YPC)
2.5. Modeling of the Drying Process
2.6. Effective Diffusion Coefficient
2.7. Statistical Analysis
3. Results
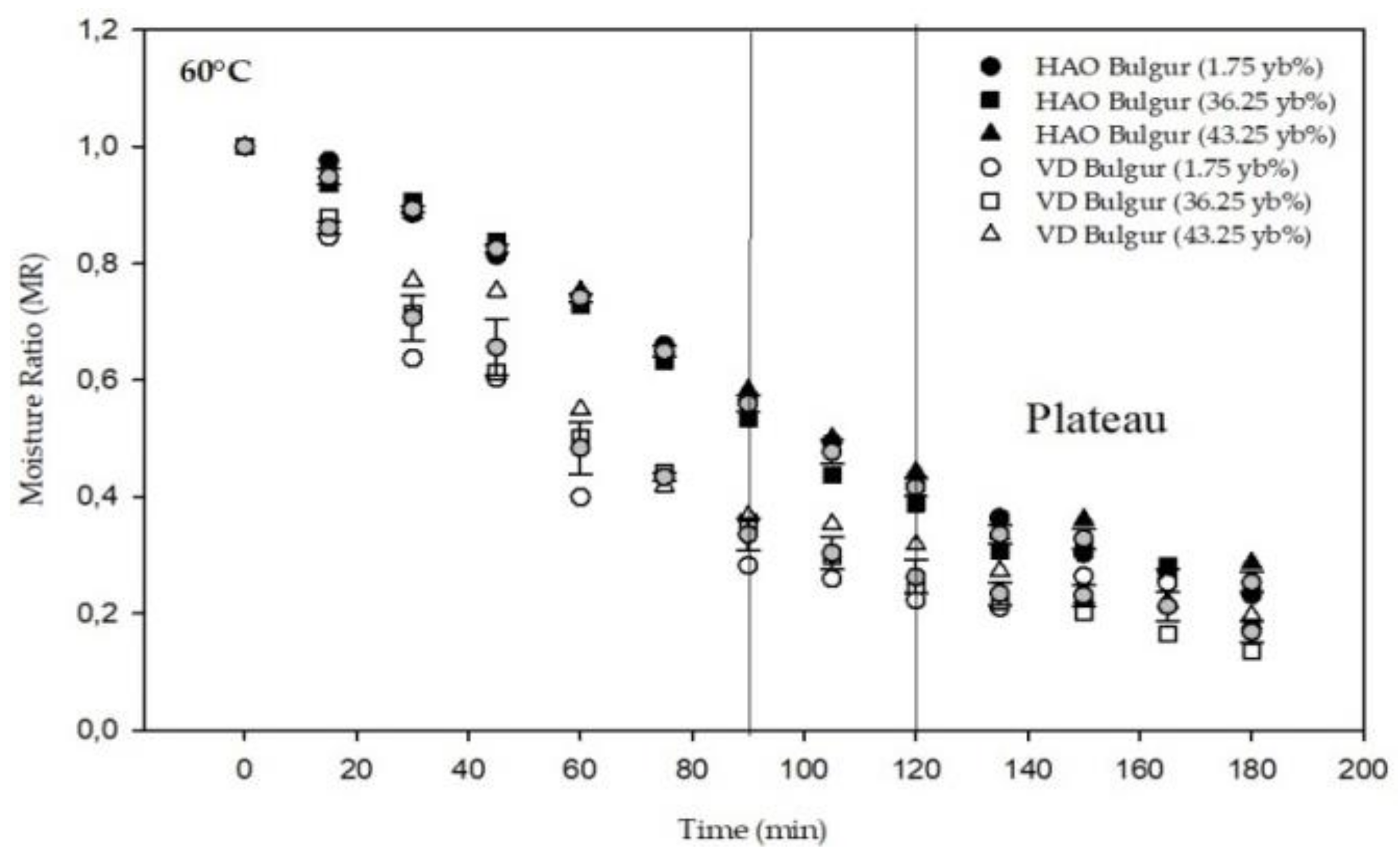
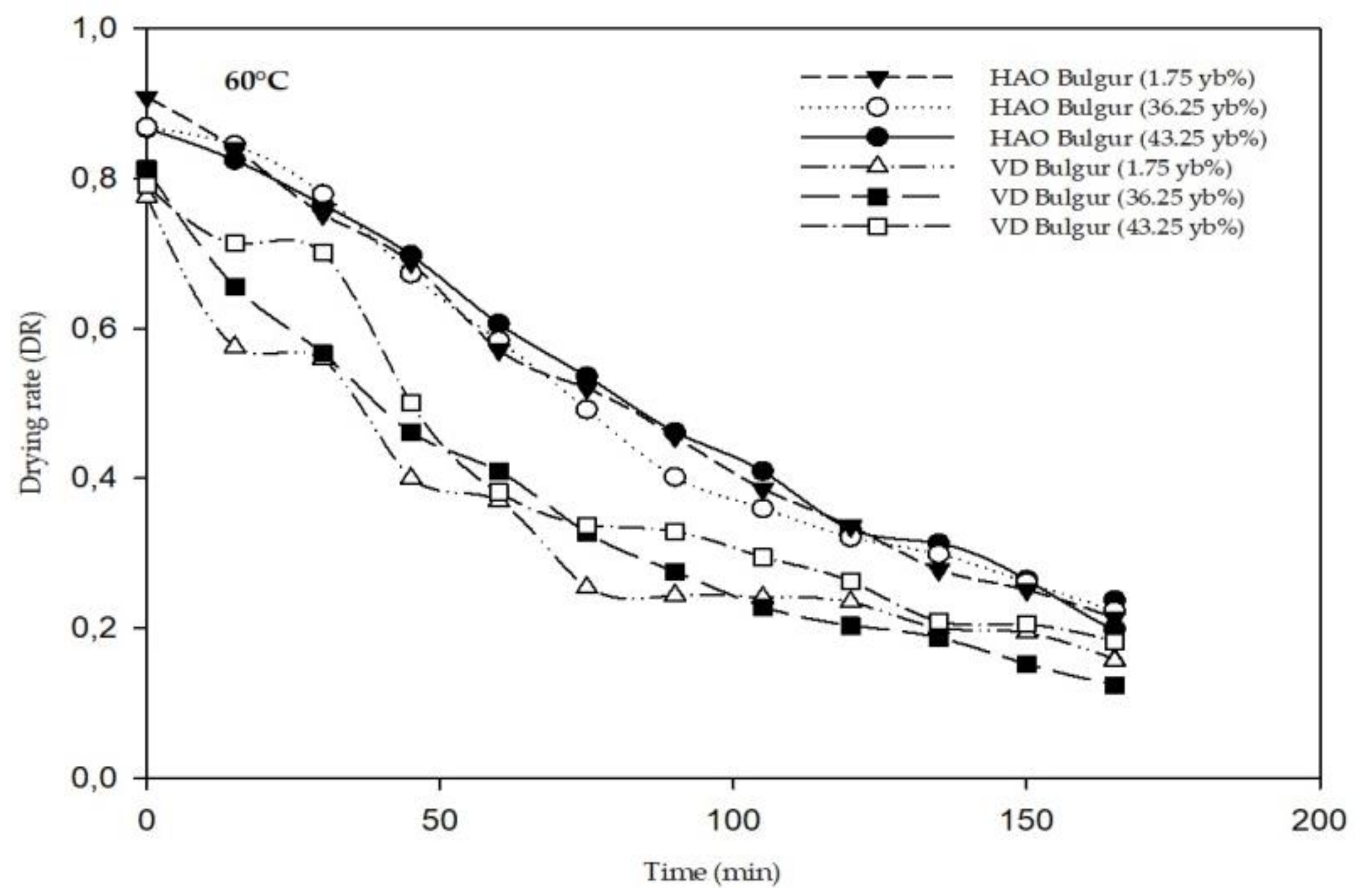
3.1. Drying Kinetics and Modeling
3.2. Phytochemicals Content of Bulgur
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miskelly, D. Optimisation of End-Product Quality for the Consumer. In Cereal Grains, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 653–688. [Google Scholar] [CrossRef]
- Caba, Z.T.; Boyacioglu, M.H.; Boyacioglu, D. Bioactive healthy components of bulgur. Inter. J. Food Sci. Nutr. 2012, 63, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bayram, M.; Öner, M.D. Bulgur milling using roller, double disc and vertical disc mills. J. Food Eng. 2007, 79, 181–187. [Google Scholar] [CrossRef]
- Dreher, M.L. Dietary fiber: Overview. In Handbook of Dietary Fiber; Sungsoo, S., Ed.; Marcel Dekker: New York, NY, USA, 2001; pp. 21–36. [Google Scholar]
- Jenkins, D.J.A.; Wolever, T.M.S.; Jenkins, A.L.; Giordano, C.; Giudici, S.; Thompson, L.U.; Kalmusky, J.; Josse, R.G.; Wong, G.S. Low glycemic response to traditionally processed wheat and rye products: Bulgur and pumper nickel bread. Am. J. Clin. Nutr. 1986, 43, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Yousif, S.I.; Bayram, M.; Kesen, S. Characterization of volatile compounds of Bulgur (AntepType) produced from durum wheat. J. Food Qual. 2018, 2018, 8564086. [Google Scholar] [CrossRef] [Green Version]
- Balci, F.; Bayram, M. Bulgur cooking process: Recovery of energy and waste water. J. Food Eng. 2020, 269, 109734. [Google Scholar] [CrossRef]
- Bayram, M. Bulgur around the world. Cereal Foods World 2000, 45, 80–82. [Google Scholar]
- Bayram, M.; Kaya, A.; Öner, M.D. Changes in properties of soaking water during production of soy-bulgur. J. Food Eng. 2004, 61, 221–230. [Google Scholar] [CrossRef]
- Yorgancilar, M.; Bilgiçli, N. Chemical and nutritional changes in bitter and sweet lupin seeds (Lupinus albus L.) during bulgur production. J. Food Sci. Technol. 2014, 51, 1384–1389. [Google Scholar] [CrossRef] [Green Version]
- Köksel, H.; Edney, M.J.; Özkaya, B. Barley bulgur: Effect of processing and cooking on chemical composition. J. Cereal Sci. 1999, 29, 185–190. [Google Scholar] [CrossRef]
- Bayram, M.; Öner, M.D.; Eren, S. Influence of soaking on the dimensions and colours of soybean for bulgur production. J. Food Eng. 2004, 61, 331–339. [Google Scholar] [CrossRef]
- Ertas, N.; Türker, S. Bulgur processes increase nutrition value: Possible role in in-vitro protein digestability, phytic acid, trypsin inhibitor activity and mineral bioavailability. J. Food Sci. Technol. 2014, 51, 1401–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özboy, Ö.; Köksel, H. An application of linear regression technique for predicting bulgur yield and quality of wheat cultivars. Nahr. Food 2002, 46, 21–24. [Google Scholar] [CrossRef]
- Ramirez-Wong, B.; Rodriguez-Félix, F.; Torres-Chavez, I.P.; Medina-Rodriguez, C.L.; Matus-Barba, E.A.; Ledesma-Osuna, A.I. Effects of Nitrogen and irrigation on gluten protein composition and their relationship to “Yellow Berry” disorder in wheat (Triticum aestivum). Pak. J. Bot. 2014, 46, 1797–1804. [Google Scholar]
- Anderson, W.K. Grain yields responses of barley and durum wheat to split nitrogen applications under rained conditions in a Mediterranean environment. Field Crop Res. 1985, 12, 191–202. [Google Scholar] [CrossRef]
- Herbert, F.R. Yellow berry in hard winter wheat. J. Agric. Res. 1919, 18, 155–168. [Google Scholar]
- López-Ahumada, G.A.; Ramírez-Wong, B.; Torres-Chávez, P.; Bello-Pérez, L.; Figueroa-Cardenas, J.; Garzón-Tiznado, J.; Gomez-Aldapa, C. Physicochemical characteristics of starch from bread wheat (Triticum aestivum) with “yellow berry”. Starch-Stärke 2010, 62, 517–523. [Google Scholar] [CrossRef]
- Ammiraju, J.S.S.; Dholakia, B.B.; Jawdekar, G.; Santra, D.K.; Gupta, V.S.; Roder, M.S.; Singh, H.; Lagu, M.D.; Dhaliwal, H.S.; Rao, V.S.; et al. Inheritance and identification of DNA markers associated with yellow berry tolerance in wheat (Triticum aestivum L.). Euphytica 2002, 123, 229–233. [Google Scholar] [CrossRef]
- Dexter, J.E.; Marchylo, B.A.; Macgregor, A.W.; Tkachuk, R. The structure and protein composition of vitreous, piebald and starchy durum-wheat kernels. J. Cereal Sci. 1989, 10, 19–32. [Google Scholar] [CrossRef]
- Behera, U.K.; Sharma, A.R.; Pandey, H.N. Sustaining of wheat-soybean cropping system through integrated nutrient management practices on the vertisols on central India. Plant Soil 2007, 297, 185–199. [Google Scholar] [CrossRef]
- Rodriguez-Félix, F.; Ramirez-Wong, B.; Torres-Chavez, P.I.; Álvarez-Avilés, A.; Moreno-Salazar, S.; Eugenia Renteria-Martinez, M.; Arturo Bello-Pérez, L. Yellow Berry, Protein and Agronomic Characteristics in bread wheat under different conditions of nitrogen and irrigation in north west Mexico. Pak. J. Bot. 2014, 46, 221–226. [Google Scholar]
- Sfayhi Terras, D.; Djebbali, K.; Jedidi, E. Influence of process parameters on bulgur quality. Qual. Assur. Saf. Crops Foods 2019, 11, 431–439. [Google Scholar] [CrossRef]
- Sfayhi Terras, D.; Hadjyahia, N.; Zarroug, Y. Effect of soaking time and temperature on water absorption capacity and dimensional changes of bulgur. Cereal Chem. 2021, 98, 851–857. [Google Scholar] [CrossRef]
- Stone, A.K.; Wang, S.; Tulbek, M.; Koksel, F.; Nickerson, M.T. Processing and quality aspects of bulgur from Triticum durum. Cereal Chem. 2020, 97, 1099–1110. [Google Scholar] [CrossRef]
- Chakraverty, A.; Kaushal, R.T. Determination of optimum drying conditions and development of drying equations for thin layer drying of parboiled wheat. Am. Mark. Assoc. 1982, 13, 33–36. [Google Scholar]
- Mohapatra, D.; Rao, P.S. A thin layer drying model of parboiled wheat. J. Food Eng. 2005, 66, 513–518. [Google Scholar] [CrossRef]
- Yildirim, A. Kinetics and thermodynamic properties of parboiled burgos wheat (Triticum durum) in Turkey during drying. Appl. Ecol. Environ. Res. 2018, 16, 495–510. [Google Scholar] [CrossRef]
- Becker, H.A. A study of diffusion in solids of arbitrary shape, with application to the drying of wheat kernel. J. Appl. Polym. Sci. 1959, 1, 212–226. [Google Scholar] [CrossRef]
- Watson, E.L.; Bhargava, V.K. Thin layer studies on wheat. Can. J. Agri. Eng. 1974, 16, 18–22. [Google Scholar]
- Bayram, M. Determination of the cooking degree for bulgur production using amylase/iodine, centre cutting and light scattering methods. Food Control 2006, 17, 331–335. [Google Scholar] [CrossRef]
- Bayram, M.; Öner, M.D.; Eren, S. Effect of cooking time and temperature on the dimensions and crease of the wheat kernel during bulgur production. J. Food Eng. 2004, 64, 43–51. [Google Scholar] [CrossRef]
- Rios, G.; Pinson-Gadais, L.; Abecassis, J.; Zakhia-Rozis, N.; LillienPellerin, V. Assessment of dehulling efficiency to reduce deoxynivalenol and Fusarium level in durum wheat grains. J. Cereal Sci. 2009, 49, 387–392. [Google Scholar] [CrossRef]
- Bilgiçli, N. Effects of cooking and drying processes on physical, chemical and sensory properties of legume based bulgur. J. Food Process. Preserv. 2008, 33, 590–604. [Google Scholar] [CrossRef]
- Savas, K.; Basman, A. Infrared drying: A promising technique for bulgur production. J. Cereal Sci. 2016, 68, 31–37. [Google Scholar] [CrossRef]
- Evlice, A.K.; Özkaya, H. Effects of wheat cultivar, cooking method, and bulgur type on nutritional quality characteristics of bulgur. J. Cereal Sci. 2020, 96, 103–124. [Google Scholar] [CrossRef]
- Hayta, M. Bulgur quality as affected by drying methods. J. Food Sci. 2001, 67, 2241–2243. [Google Scholar] [CrossRef]
- Kadakal, Ç.; Ekinci, R.; Yapar, A. The effect of cooking and drying on the water-soluble vitamins content of Bulgur. Food Sci. Technol. Inter. 2007, 13, 349–354. [Google Scholar] [CrossRef]
- Yilmaz, V.A.; Koca, A.F. Effect of different production techniques on bioactive compounds and antioxidant capacity of einkorn (Triticum monococcum L.) and durum (Triticum turgidum subsp. durum) bulgur. J. Sci. Food Agri. 2016, 97, 269–277. [Google Scholar] [CrossRef] [PubMed]
- American Association of Cereal Chemists. Approved Methods, 10th ed.; American Association of Cereal Chemists (AACC): Eagan, MN, USA, 2000. [Google Scholar]
- Sgrulletta, D.; DeStefanis, E. Simultaneous evaluation of quality parameters of durum wheat (Triticum durum) by near infrared spectroscopy. Ital. J. Food Sci. 1997, 9, 295–301. [Google Scholar]
- International Association for Cereal Science and Technology. Standard Methods of the ICC. In Determination of Ash in Cereals and Cereal Products; Schaifer: Detmold, Germany, 1990. [Google Scholar]
- Smirani, N.; Sfayhi, D. Bulgur modeling drying kinetic. In Proceedings of the 15 the International Cereal and Bread Congress, Istanbul, Turkey, 18–21 April 2016. [Google Scholar]
- Mau, J.L.; Chao, G.R.; Wu, K.T. Antioxidant properties of methanolic extracts from several ear mushrooms. J. Agri. Food Chem. 2001, 49, 5461–5467. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Determination of Yellow Pigment Content. In Durum Wheat Flour and Semolina; International Standardization Organization (ISO): Berlin, Germany, 1994; p. 11052.
- Bruce, D.M. Exposed-layer barley drying, three models fitted to new data up to 150 °C. J. Agri. Eng. Res. 1985, 32, 337–348. [Google Scholar] [CrossRef]
- Henderson, S.M.; Pabis, S. Grain drying theory II: Temperature effects on drying coefficients. J. Agri. Eng. Res. 1961, 6, 169–174. [Google Scholar]
- Toğrul, H. Suitable drying model for infrared drying of carrot. J. Food Eng. 2006, 77, 610–619. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z.A. New model for single-layer drying. Dry Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Rayaguru, K.; Routray, W. Mathematical modeling of thin layer drying kinetics of stone apple slices. Inter. Food Res. J. 2012, 19, 1503–1510. [Google Scholar]
- Kavak Akpinar, E. The Development of a Cyclone Type Dryer for Agricultural Products. Ph.D. Thesis, Firat University, Elazığ, Turkish, 2002. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, NY, USA, 1975. [Google Scholar]
- Kahyaoglu, L.N.; Sahin, S.; Sumnu, G. Physical properties of parboiled wheat and bulgur produced using spouted bed and microwave assisted spouted bed drying. J. Food Eng. 2010, 98, 159–169. [Google Scholar] [CrossRef]
- Singh, H.; Sodhi, N. Dehydration kinetics of onions. J. Food Sci. Technol. 2000, 37, 520–522. [Google Scholar]
- Parikh, D.M. Vacuum Drying: Basics and application. Chem. Eng. 2015, 122, 48–54. [Google Scholar]
- Lyon, T.L.; Keyser, A. Winter Wheat. In Bulletin of the Agricultural Experiment Station of Nebraska; Michigan Agricultural Experiment Station: East Lansing, MI, USA, 1905; Volume 17, pp. 1–28. [Google Scholar]
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J. Agric. Food Chem. 2009, 58, 1180–1188. [Google Scholar] [CrossRef]
- Cooke, D.; Michael, J.G. Loss of crystalline and molecular order during starch gelatinisation: Origin of the enthalpic transition. Carbohydr. Res. 1992, 227, 103–112. [Google Scholar] [CrossRef]
- Kumar, R.; Khatkar, B.S. Thermal, pasting and morphological properties of starch granules of wheat (Triticum aestivum L.) varieties. J. Food Sci. Technol. 2017, 54, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Wootton, M.; Bamunuarachchi, A. Application of Differential Scanning Calorimetry to Starch Gelatinization. II. Effect of Heating Rate and Moisture Level. Starch-Starke 1979, 31, 262–264. [Google Scholar] [CrossRef]
- Yu, L.; Cheng, Z. Antioxidant properties of wheat phenolic acids. In Wheat Antioxidants; Yu, L., Ed.; Wiley-Interscience: Hoboken, NJ, USA, 2008; pp. 54–72. [Google Scholar]
- Sosulski, F.; Krysztof, K.; Lawrence, H. Free, esterified, and insoluble-bound phenolic acids. 3. Composition of phenolic acids in cereal and potato flours. J. Agric Food Chem. 1982, 30, 337–340. [Google Scholar] [CrossRef]
- Ertaş, N. A comparison of industrial and homemade bulgur in turkey in terms of physical, chemical and nutritional properties. Chem. Ind. Chem. Eng. Q. 2017, 23, 341–348. [Google Scholar] [CrossRef]
- Yüksel, A.N.; Oner, M.D.; Bayram, M. Usage of undersize bulgur flour in production of short-cut pasta-like couscous. J. Cereal Sci. 2017, 77, 102–109. [Google Scholar] [CrossRef]
- Morel, I.; Lescoat, G.; Cogrel, P.; Sergent, O.; Pasdeloup, N.; Brissot, P.; Cillard, P.; Cillard, J. Antioxidant and Iron-Chelating activities of the flavonoids catechin, quercetin and diosmetin on Iron-Loaded rat hepatocyte. Biochem. Pharmacol. 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Ragaee, S.; Guzar, I.; Abdel-Aal, E.-S.M.; Seetharaman, K. Bioactive components and antioxidant capacity of Ontario hard and soft wheat varieties. Can. J. Plant Sci. 2011, 92, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Adom, K.; Liu, R. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Beta, T.; Nam, S.; Dexter, J.E.; Sapirstein, H.D. Phenolic content and antioxidant activity of pearled wheat and roller milled fractions. Cereal Chem. 2005, 82, 390–393. [Google Scholar] [CrossRef]
- Li, W.; Shan, F.; Sun, S.; Corke, H.; Beta, T. Free radicals cavenging properties and phenolic content of Chinese black-grained wheat. J. Agric. Food Chem. 2005, 53, 8533–8536. [Google Scholar] [CrossRef]
- Moore, J.; Hao, Z.; Zhou, K.; Luther, M.; Costa, J.; Yu, L. Carotenoid, tocopherol, phenolic acid, and antioxidant properties of Maryland-grown soft wheat. J. Agric. Food Chem. 2005, 53, 6649–6657. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.; Piironen, V.; Lampi, A.M.; Nystrom, L.; Li, L.; Rakszegi, M.; Fras, A.; Boros, D.; Gebruers, K.; Courtin, C.M. Phytochemical and fiber components in oat varieties in the health grain diversity screen. J. Agric. Food Chem. 2008, 56, 9777–9784. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, V.; Kranl, K.; Hollmann, J.; Lindhauer, M.G.; Böhm, V.; Bitsch, R. Spectrophotometric determination of yellow pigment content and evaluation of carotenoids by high-performance liquid chromatography in durum wheat grain. J. Agric. Food Chem. 2002, 50, 6663–6668. [Google Scholar] [CrossRef]
- Balcı, F.; Bayram, M. Modification of mechanical polishing operation using preheating systems to improve the bulgur color. J. Cereal Sci. 2017, 75, 108–115. [Google Scholar] [CrossRef]
- Burkhardt, S.; Böhm, V. Development of a new method for the complete extraction of carotenoids from cereals with special reference to durum wheat (Triticum durum Desf.). J. Agri. Food Chem. 2007, 55, 8295–8301. [Google Scholar] [CrossRef]
- Zhou, K.; Yin, J.J.; Yu, L. Phenolic acid, tocopherol and carotenoid compositions and antioxidant functions of hard red winter wheat bran. J. Agri. Food Chem. 2005, 53, 3916–3922. [Google Scholar] [CrossRef]
- Chen, Y.; Dunford, N.T.; Goad, C. Phytochemical composition of extracts from wheat grain fractions obtained by tangential abrasive dehulling. Food Sci. Technol. 2013, 54, 353–359. [Google Scholar] [CrossRef]
- Özboy, Ö.; Köksel, H. The effects of bulgur production on various chemical properties of wheats. J. Food 1998, 23, 449–457. [Google Scholar]
- Yilmaz, V.A. Effects of different cooking and drying methods on phenolicacids, carotenoids, and antioxidant activity of emmer (Triticum turgidums sp. dicoccum) bulgur. Cereal Chemistry 2019, 96, 1093–1102. [Google Scholar] [CrossRef]
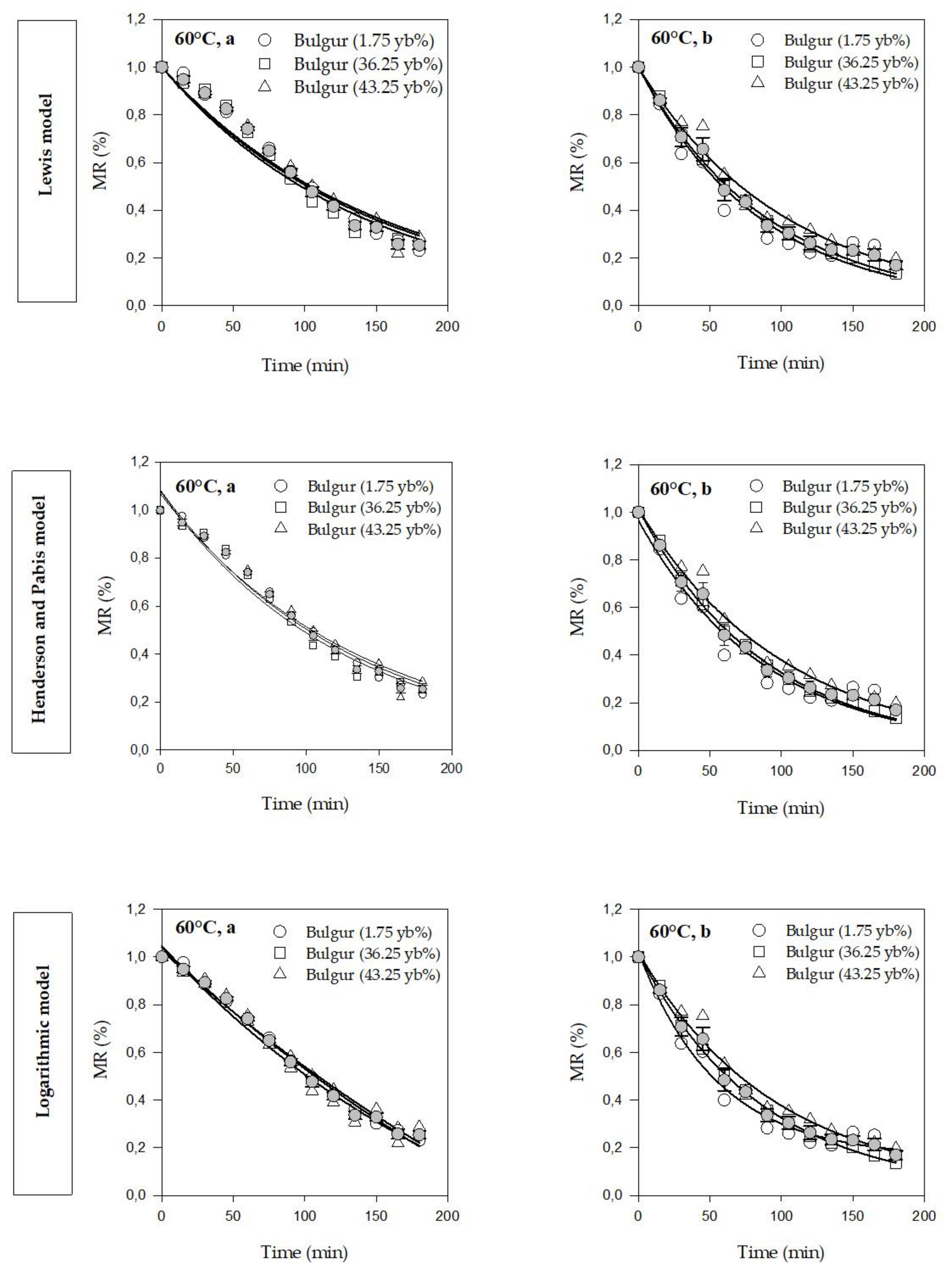
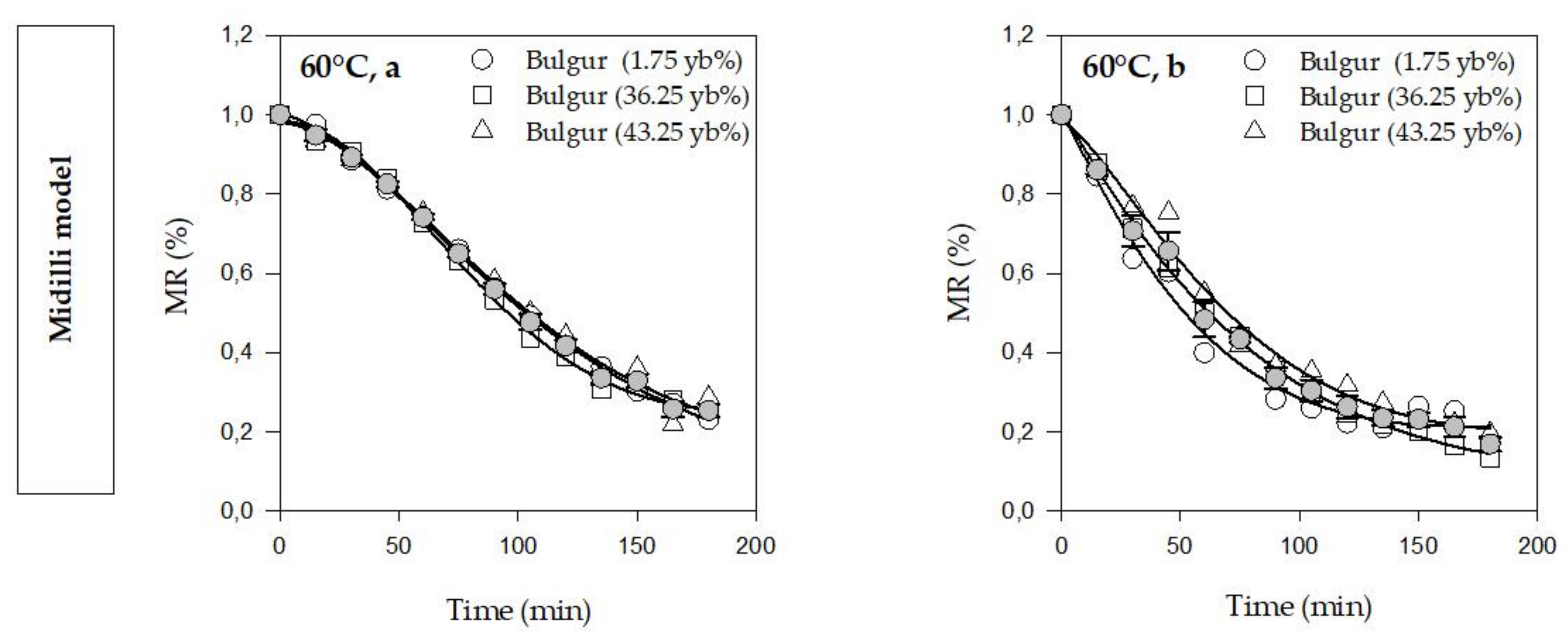
| Model Name | Model Equation | Reference |
|---|---|---|
| Lewis | [47] | |
| Henderson and Pabis | [48] | |
| Logarithmic | [49] | |
| Midilli | [50] |
| Slope | Bulgur 1.75 yb% | Bulgur 36.25 yb% | Bulgur 43.25 yb% | |
|---|---|---|---|---|
| Hot air oven | P1 | −5.51 × 10−3 ± 1.98 × 10−5 a | −5. 35 × 10−3 ± 0.68 × 10−5 a | −4.71 × 10−3 ± 5.94 × 10−5 a |
| Vacuum dryer | P2 | −7.48 × 10−3 ± 2.85×10−5 b | −6.97 × 10−3 ± 1.46 × 10−5 b | −6.56 × 10−3 ± 7.90 × 10−5 b |
| Hot Air Oven (P1) | Vacuum Dryer (P2) | |
|---|---|---|
| Bulgur 1.75 yb% | −5.51 × 10−3 ± 1.98 × 10−5 c | −7.48 × 10−3 ± 2.85 × 10−5 c |
| Bulgur 36.25 yb% | −5.35 × 10−3 ± 0.68 × 10−5 b | −6.97 × 10−3 ± 1.46 × 10−5 b |
| Bulgur 43.25 yb% | −4.71 × 10−3 ± 5.94 × 10−5 a | −6.56 × 10−3 ± 7.90 × 10−5 a |
| Samples | Model | Hot Air OvenDrying | Vacuum Drying | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | R2 | Std Err | RSS | Parameters | R2 | Std Err | RSS | ||
| Bulgur 1.75 yb% | Lewis | K 6.82 × 10−3 | 0.9570 | 0.0004 | 0.0381 | K 1.17 × 10−2 | 0.9486 | 0.0599 | 0.0431 |
| Henderson and Pabis | K 7.64 × 10−3 a 1.08 | 0. 9760 | 0.0440 | 0.0213 | K 1.13 × 10−2 a 9.95 × 10−1 | 0.9512 | 0.0610 | 0.0409 | |
| Logarithmic | K 2.23 × 10−3 a 2.43 b −1.41 × 10−1 | 0.9937 | 0. 0236 | 0. 0056 | K 1.80 × 10−2 a 8.60 × 10−1 b 1.59 × 10−1 | 0.9742 | 0.0465 | 0.0216 | |
| Midilli | K 6.93 × 10−4 a 1.00 b 3.30 × 10−4 n 1.51 | 0.9943 | 0.0324 | 0.0095 | K 9.51 × 10−3 a 1.00 b 9.72 × 10−4 n 1.12 | 0.9885 | 0.0462 | 0.0192 | |
| Bulgur 36.25 yb% | Lewis | K 7.12 × 10−3 | 0.9392 | 0.0661 | 0.0306 | K 1.12 × 10−2 | 0.9978 | 0.0132 | 0.0021 |
| Henderson and Pabis | K7.92 × 10−3 a 1.08 | 0.9570 | 0.0600 | 0.0216 | K 1.13 × 10−2 a 1.01 | 0.9981 | 0.0128 | 0.0018 | |
| Logarithmic | K 4.08 × 10−3 a 1.61 b−5.64 × 10−1 | 0.9830 | 0.0414 | 0.0086 | K 1.17 × 10−2 a 1.00 b 1.56 × 10−2 | 0.9982 | 0.0131 | 0.0017 | |
| Midilli | K 4.48 × 10−4 a 9.80 × 10−1 b 1.10 × 10−3 n 1.79 | 0.9961 | 0.0197 | 0.0035 | K 8.24 × 10−3 a 1.00 b 2.64 × 10−4 n 1.08 | 0.9994 | 0.0117 | 0.0012 | |
| Bulgur 43.25 yb% | Lewis | K 6.64 × 10−3 | 0.9604 | 0.0548 | 0.0420 | K 9.68 × 10−3 | 0.9778 | 0.0004 | 0.0198 |
| Henderson and Pabis | K 7.32 × 10−3 a 1.07 | 0.9719 | 0. 0478 | 0. 0298 | K 9.85 × 10−3 a 1.01 | 0.9782 | 0.0420 | 0.0194 | |
| Logarithmic | K 2.48 × 10−3 a 2.32 b −1.28 | 0. 9794 | 0.0427 | 0.0218 | K 1.06 × 10−2 a 9.84 × 10−1 b 3.60 × 10−2 | 0.9786 | 0.0436 | 0.0190 | |
| Midilli | K 2.55 × 10−4 a 9.79 × 10−1 b 7.75 × 10−4 n 1.60 | 0.9992 | 0.0087 | 0.0007 | K 3.07 × 10−3 a 9.84 × 10−1 b 8.04 × 10−4 n 1.31 | 0.9918 | 0.0403 | 0.0146 | |
| Bulgur 1.75 yb% | Bulgur 36.25 yb% | Bulgur 43.25 yb% | ||
|---|---|---|---|---|
| Deff (m2/s) | Hot air oven | 7.05 × 10−11 ± 3.17 × 10−22 a | 6.86 × 10−11 ± 2.05 × 10−21 a | 6.86 × 10−11 ± 4.52 × 10−21 a |
| Vacuum dryer | 7.82 × 10−11 ± 7.05 × 10−22 b | 7.73 × 10−11 ± 4.74 × 10−22 b | 7.73 × 10−11 ± 1.81 × 10−20 b | |
| Samples | Hot Air Oven | Vacuum Dryer | |
|---|---|---|---|
| Deff (m2/s) | Bulgur 1.75 yb% | 7.05 × 10−11 ± 3.17 × 10−22 b | 7.82 × 10−11 ± 7.05 × 10−22 b |
| Bulgur 36.25 yb% | 6.86 × 10−11 ± 2.05 × 10−21 a | 7.73 × 10−11 ± 4.74 × 10−22 a | |
| Bulgur 43.25 yb% | 6.86 × 10−11 ± 4.52 × 10−21 a | 7.73 × 10−11 ± 1.81 × 10−20 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorra, S.T.; Farah, D.; Nesrine, H.; Wafa, A.; Youkabed, Z. Drying Behavior of Bulgur and Its Effect on Phytochemical Content. Foods 2022, 11, 1062. https://doi.org/10.3390/foods11071062
Dorra ST, Farah D, Nesrine H, Wafa A, Youkabed Z. Drying Behavior of Bulgur and Its Effect on Phytochemical Content. Foods. 2022; 11(7):1062. https://doi.org/10.3390/foods11071062
Chicago/Turabian StyleDorra, Sfayhi Terras, Dridi Farah, Hadjyahia Nesrine, Allouch Wafa, and Zarroug Youkabed. 2022. "Drying Behavior of Bulgur and Its Effect on Phytochemical Content" Foods 11, no. 7: 1062. https://doi.org/10.3390/foods11071062
APA StyleDorra, S. T., Farah, D., Nesrine, H., Wafa, A., & Youkabed, Z. (2022). Drying Behavior of Bulgur and Its Effect on Phytochemical Content. Foods, 11(7), 1062. https://doi.org/10.3390/foods11071062






