Fabrication of Porous Spherical Beads from Corn Starch by Using a 3D Food Printing System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
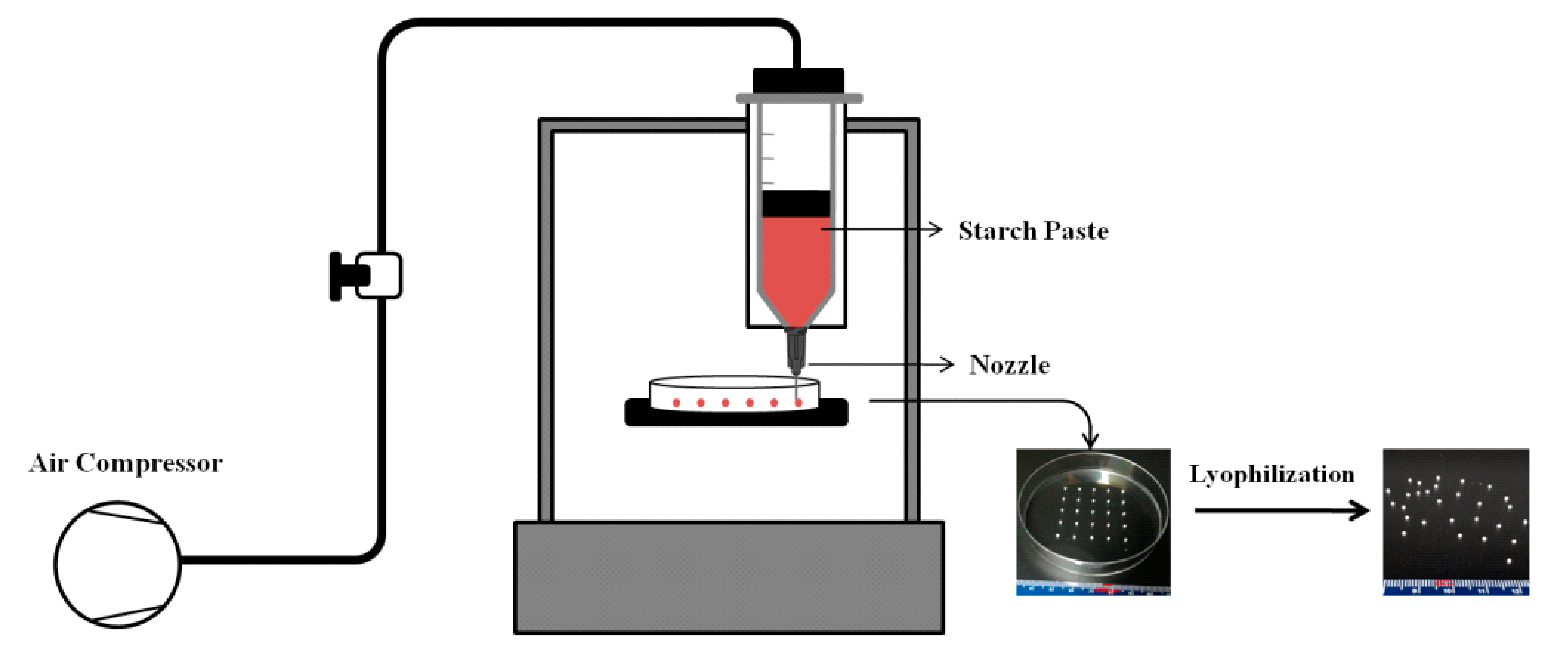
2.2. Preparation of Starch Beads by Extrusion-Based 3D Food Printing
2.3. Macroscopic and Microstructural Observations
2.4. Density and Porosity
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. X-ray Diffraction
2.7. Thermal Properties
2.8. Rheological Properties
2.9. Statistical Analysis
3. Results and Discussion
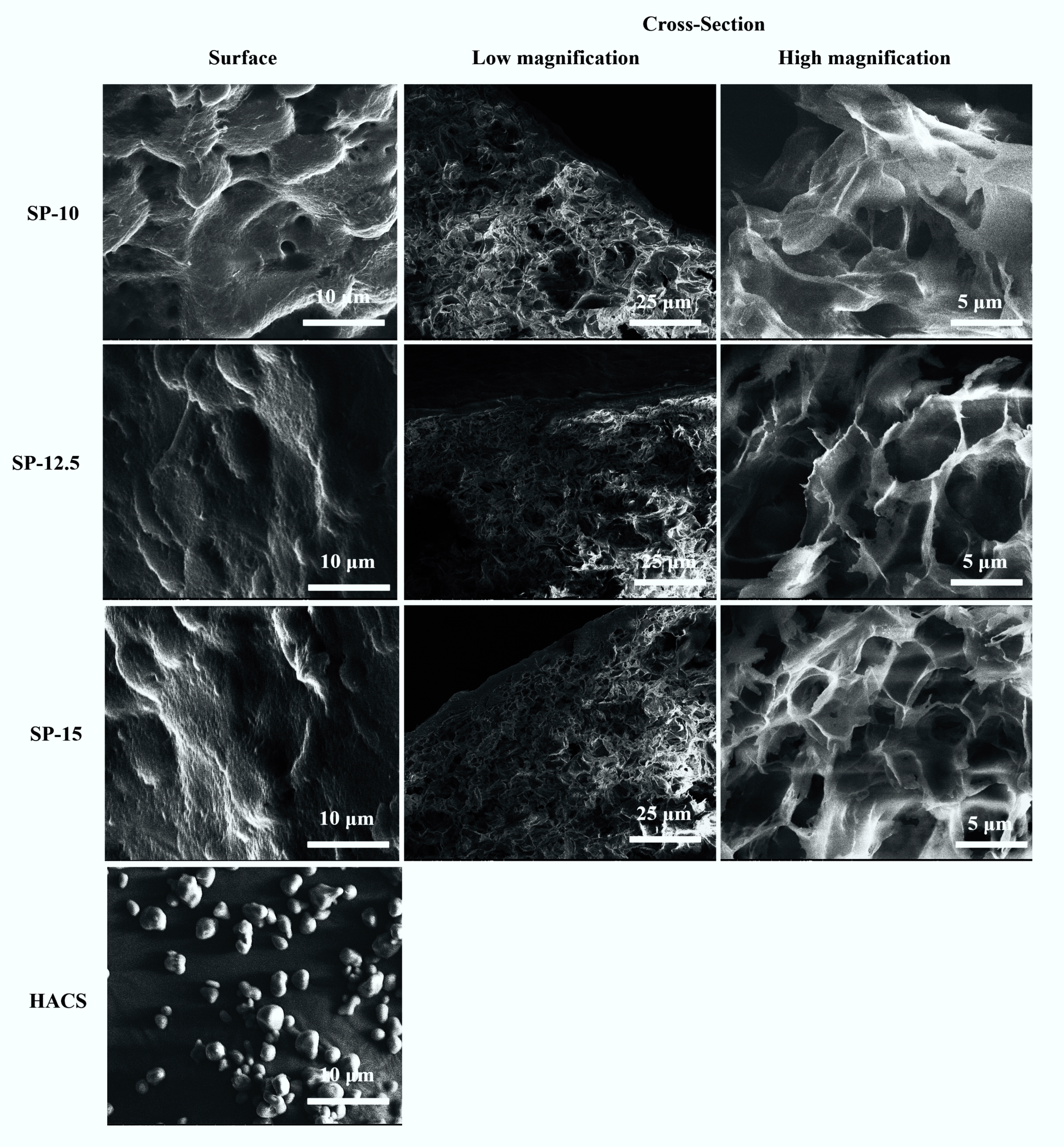
3.1. Macroscopic and Microstructural Observations
3.2. Density and Porosity
3.3. Fourier Transform Infrared Spectroscopy
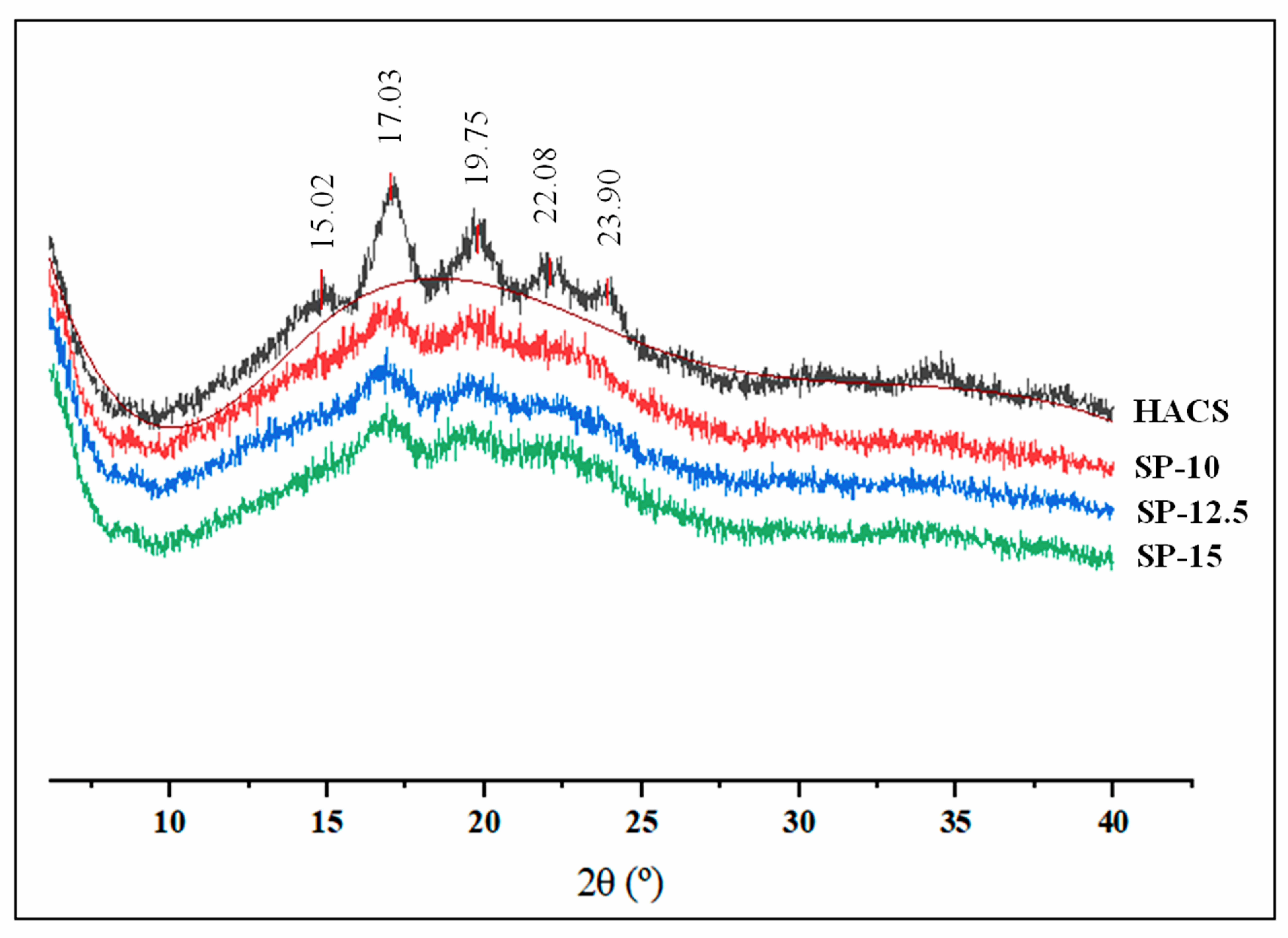
3.4. X-ray Diffraction
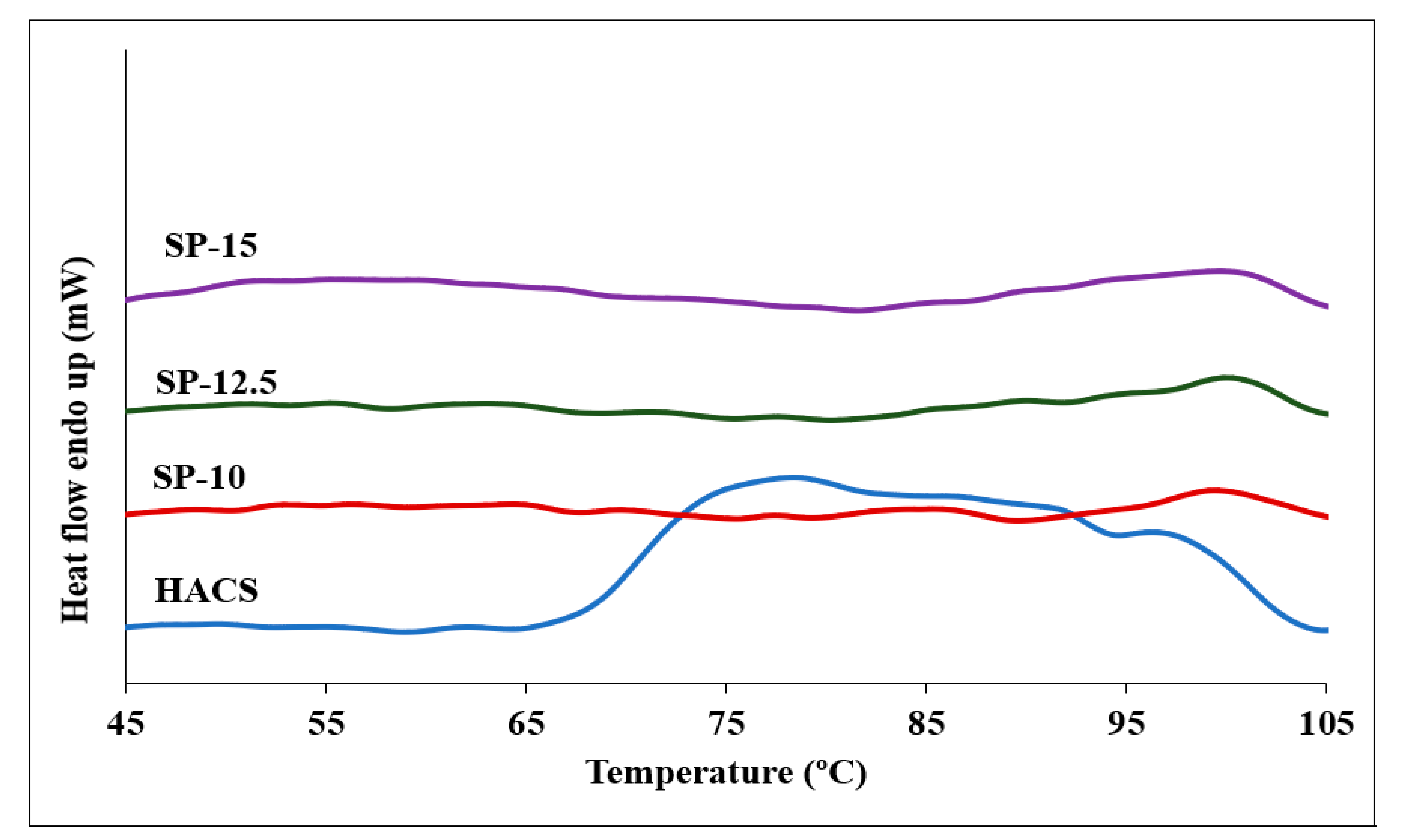
3.5. Thermal Properties
3.6. Rheological Properties
3.6.1. Viscosity
3.6.2. Temperature Sweeps
3.6.3. Stress Sweeps
3.6.4. Frequency Sweeps
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, Z.; Cui, H.; Zhang, H.; Wang, F.; Zhan, X.; Mayer, F.; Nestler, B.; Wegener, M.; Levkin, P.A. 3D printing of inherently nanoporous polymers via polymerization-induced phase separation. Nat. Commun. 2021, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Ubeyitogullari, A.; Ciftci, O.N. A novel and green nanoparticle formation approach to forming low-crystallinity curcumin nanoparticles to improve curcumin’s bioaccessibility. Sci. Rep. 2019, 9, 19112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovskaya, D.D.; Lebedev, A.E.; Menshutina, N.V. Aerogels as drug delivery systems: In Vitro and In Vivo evaluations. J. Supercrit. Fluids 2015, 106, 115–121. [Google Scholar] [CrossRef]
- Zhu, F. Starch based aerogels: Production, properties and applications. Trends Food Sci. Technol. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Zhuang, P.; Greenberg, Z.; He, M. Biologically Enhanced Starch Bio-Ink for Promoting 3D Cell Growth. Adv. Mater. Technol. 2021, 6, 2100551. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhao, L.; Huang, J.; Chen, Y.; Wei, C. Morphology, structure and gelatinization properties of heterogeneous starch granules from high-amylose maize. Carbohydr. Polym. 2014, 102, 606–614. [Google Scholar] [CrossRef]
- Zou, F.; Budtova, T. Tailoring the morphology and properties of starch aerogels and cryogels via starch source and process parameter. Carbohydr. Polym. 2021, 255, 117344. [Google Scholar] [CrossRef]
- Wang, M.; Ji, N.; Li, M.; Li, Y.; Dai, L.; Zhou, L.; Xiong, L.; Sun, Q. Fabrication and characterization of starch beads formed by a dispersion-inverse gelation process for loading polyphenols with improved antioxidation. Food Hydrocoll. 2020, 101, 105565. [Google Scholar] [CrossRef]
- García-González, C.A.; Uy, J.J.; Alnaief, M.; Smirnova, I. Preparation of tailor-made starch-based aerogel microspheres by the emulsion-gelation method. Carbohydr. Polym. 2012, 88, 1378–1386. [Google Scholar] [CrossRef]
- Lipton, J.I.; Cutler, M.; Nigl, F.; Cohen, D.; Lipson, H. Additive manufacturing for the food industry. Trends Food Sci. Technol. 2015, 43, 114–123. [Google Scholar] [CrossRef]
- Le-Bail, A.; Maniglia, B.C.; Le-Bail, P. Recent advances and future perspective in additive manufacturing of foods based on 3D printing. Curr. Opin. Food Sci. 2020, 35, 54–64. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Bhandari, B. Recent development in 3D food printing. Crit. Rev. Food Sci. Nutr. 2017, 57, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Dankar, I.; Haddarah, A.; Sepulcre, F.; Pujolà, M. Assessing Mechanical and Rheological Properties of Potato Puree: Effect of Different Ingredient Combinations and Cooking Methods on the Feasibility of 3D Printing. Foods 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J. A 3D Food Printing Process for the New Normal Era: A Review. Processes 2021, 9, 1495. [Google Scholar] [CrossRef]
- Feng, J.; Su, B.-L.; Xia, H.; Zhao, S.; Gao, C.; Wang, L.; Ogbeide, O.; Feng, J.; Hasan, T. Printed aerogels: Chemistry, processing, and applications. Chem. Soc. Rev. 2021, 5, 3842–3888. [Google Scholar] [CrossRef]
- Chen, H.; Xie, F.; Chen, L.; Zheng, B. Effect of rheological properties of potato, rice and corn starches on their hot-extrusion 3D printing behaviors. J. Food Eng. 2019, 244, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadeh, S.; Keramat, J.; Nasirpour, A.; Hamdami, N.; Behzad, T.; Aranda, L.; Vilasi, V.; Desobry, S. Structural and mechanical properties of clay nanocomposite foams based on cellulose for the food packaging industry. J. Appl. Polym. Sci. 2016, 133, 510–520. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Z.; Hu, B.; Wang, W.; Ye, H.; Sun, Y.; Wang, X.; Zeng, X. A new method for determining the relative crystallinity of chickpea starch by Fourier-transform infrared spectroscopy. Carbohydr. Polym. 2014, 108, 153–158. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Formation of nanoporous aerogels from wheat starch. Carbohydr. Polym. 2016, 147, 125–132. [Google Scholar] [CrossRef]
- Grace, J.R.A. Ebneyamini, Connecting particle sphericity and circularity. Particuology 2021, 54, 1–4. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. Starch: Chemistry and Technology; Elsevier Science & Technology: San Diego, CA, USA, 2009. [Google Scholar]
- Kenar, J.A.; Eller, F.J.; Felker, F.C.; Jackson, M.A.; Fanta, G.F. Starch aerogel beads obtained from inclusion complexes prepared from high amylose starch and sodium palmitate. Green Chem. 2014, 16, 1921–1930. [Google Scholar] [CrossRef]
- Putseys, J.A.; Lamberts, L.; Delcour, J.A. Amylose-inclusion complexes: Formation, identity and physico-chemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- De Marco, I.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical Gel Drying for the Production of Starch Aerogels for Delivery Systems. Chem. Engin. Trans. 2015, 43, 307–312. [Google Scholar]
- Schroeter, B.; Yonkova, V.P.; Goslinska, M.; Orth, M.; Pietsch, S.; Gurikov, P.; Smirnova, I.; Heinrich, S. Spray coating of cellulose aerogel particles in a miniaturized spouted bed. Cellulose 2021, 28, 7795–7812. [Google Scholar] [CrossRef]
- Putaux, J.-L.; Buléon, A.; Chanzy, H. Network Formation in Dilute Amylose and Amylopectin Studied by TEM. Macromolecules 2000, 33, 6416–6422. [Google Scholar] [CrossRef]
- Buchtová, N.; Budtova, T. Cellulose aero-, cryo- and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Groult, S.; Budtova, T. Tuning structure and properties of pectin aerogels. Eur. Polym. J. 2018, 108, 250–261. [Google Scholar] [CrossRef]
- Nakamatsu, J.; Torres, F.G.; Troncoso, O.P.; Yuan, M.-L.; Boccaccini, A.R. Processing and Characterization of Porous Structures from Chitosan and Starch for Tissue Engineering Scaffolds. Biomacromolecules 2006, 7, 3345–3355. [Google Scholar] [CrossRef]
- Svagan, A.J.; Samir, M.A.S.A.; Berglund, L.A. Biomimetic Foams of High Mechanical Performance Based on Nanostructured Cell Walls Reinforced by Native Cellulose Nanofibrils. Adv. Mater. 2008, 20, 1263–1269. [Google Scholar] [CrossRef]
- Ahmad, M.; Gani, A.; Hassan, I.; Huang, Q.; Shabbir, H. Production and characterization of starch nanoparticles by mild alkali hydrolysis and ultra-sonication process. Sci. Rep. 2020, 10, 3533. [Google Scholar] [CrossRef]
- Chun, A.; Lee, H.-J.; Hamaker, B.R.; Janaswamy, S. Effects of Ripening Temperature on Starch Structure and Gelatinization, Pasting, and Cooking Properties in Rice (Oryza sativa). J. Agric. Food Chem. 2015, 63, 3085–3093. [Google Scholar] [CrossRef] [PubMed]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Shi, M.; Gao, Q. Recrystallization and in vitro digestibility of wrinkled pea starch gel by temperature cycling. Food Hydrocoll. 2016, 61, 712–719. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, H.; Liu, P.; Wang, W.; Dong, H. Effects of acid hydrolysis on the physicochemical properties of pea starch and its film forming capacity. Food Hydrocoll. 2019, 87, 173–179. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, M.; Zhou, D.; Li, X.; Hu, X. The retrogradation characteristics of pullulanase debranched field pea starch: Effects of storage time and temperature. Int. J. Biol. Macromol. 2019, 134, 984–992. [Google Scholar] [CrossRef]
- Genkina, N.K.; Kozlov, S.S.; Martirosyan, V.V.; Kiseleva, V.I. Thermal behavior of maize starches with different amylose/amylopectin ratio studied by DSC analysis. Starch 2014, 66, 700–706. [Google Scholar] [CrossRef]
- Kiseleva, V.I.; Genkina, N.K.; Tester, R.; Wasserman, L.A.; Popov, A.A.; Yuryev, V.P. Annealing of normal, low and high amylose starches extracted from barley cultivars grown under different environmental conditions. Carbohydr. Polym. 2004, 56, 157–168. [Google Scholar] [CrossRef]
- Matveev, Y.I.; van Soest, J.J.G.; Nieman, C.; Wasserman, L.A.; Protserov, V.A.; Ezernitskaja, M.; Yuryev, V.P. The relationship between thermodynamic and structural properties of low and high amylose maize starches. Carbohydr. Polym. 2001, 44, 151–160. [Google Scholar] [CrossRef]
- Liu, H.; Yu, L.; Xie, F.; Chen, L. Gelatinization of cornstarch with different amylose/amylopectin content. Carbohydr. Polym. 2006, 65, 357–363. [Google Scholar] [CrossRef]
- Singh, N.; Inouchi, N.; Nishinari, K. Structural, thermal and viscoelastic characteristics of starches separated from normal, sugary and waxy maize. Food Hydrocoll. 2006, 20, 923–935. [Google Scholar] [CrossRef]
- Chaunier, L.; Guessasma, S.; Belhabib, S.; Della Valle, G.; Lourdin, D.; Leroy, E. Material extrusion of plant biopolymers: Opportunities & challenges for 3D printing. Addit. Manufac. 2018, 21, 220–233. [Google Scholar]
- Cui, Y.; Li, C.; Guo, Y.; Liu, X.; Zhu, F.; Liu, Z.; Liu, X.; Yang, F. Rheological & 3D printing properties of potato starch composite gels. J. Food Eng. 2022, 313, 110756. [Google Scholar]
- Le Tohic, C.; O’Sullivan, J.J.; Drapala, K.P.; Chartrin, V.; Chan, T.; Morrison, A.P.; Kerry, J.P.; Kelly, A.L. Effect of 3D printing on the structure and textural properties of processed cheese. J. Food Eng. 2018, 220, 56–64. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B.; Yang, C. Impact of rheological properties of mashed potatoes on 3D printing. J. Food Eng. 2018, 220, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Montoya, J.; Medina, J.; Molina, A.; Gutiérrez, J.; Rodríguez, B.; Marín, R. Impact of viscoelastic and structural properties from starch-mango and starch-arabinoxylans hydrocolloids in 3D food printing. Addit. Manuf. 2021, 39, 101891. [Google Scholar] [CrossRef]
- Chen, X.; Liu, P.; Shang, X.; Xie, F.; Jiang, H.; Wang, J. Investigation of rheological properties and conformation of cassava starch in zinc chloride solution: Rheological properties of starch solution. Die Stärke 2017, 69, 1600384. [Google Scholar] [CrossRef] [Green Version]
- Tajuddin, S.; Xie, F.; Nicholson, T.M.; Liu, P.; Halley, P.J. Rheological properties of thermoplastic starch studied by multipass rheometer. Carbohydr. Polym. 2011, 83, 914–919. [Google Scholar] [CrossRef]
- Ji, Z.; Yu, L.; Liu, H.; Bao, X.; Wang, Y.; Chen, L. Effect of pressure with shear stress on gelatinization of starches with different amylose/amylopectin ratios. Food Hydrocoll. 2017, 72, 331–337. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Feilden, E.; Blanca, E.G.-T.; Giuliani, F.; Saiz, E.; Vandeperre, L. Robocasting of structural ceramic parts with hydrogel inks. J. Eur. Ceram. Soc. 2016, 36, 2525–2533. [Google Scholar] [CrossRef]





| Sample | Starch Concentration (%, w/w) | Extrusion Temperature (°C) | Nozzle Size (ID, mm) |
|---|---|---|---|
| A1 | 10 | 95 | 23G (0.33) |
| A2 | 10 | 95 | 25G (0.25) |
| A3 | 10 | 95 | 30G (0.15) |
| A4 | 10 | 95 | 32G (0.10) |
| A5 | 10 | 95 | 34G (0.08) |
| B1 | 12.5 | 95 | 23G (0.33) |
| B2 | 12.5 | 95 | 25G (0.25) |
| B3 | 12.5 | 95 | 30G (0.15) |
| B4 | 12.5 | 95 | 32G (0.10) |
| B5 | 12.5 | 95 | 34G (0.08) |
| C1 | 15 | 95 | 23G (0.33) |
| C2 | 15 | 95 | 25G (0.25) |
| C3 | 15 | 95 | 30G (0.15) |
| C4 | 15 | 95 | 32G (0.10) |
| C5 | 15 | 95 | 34G (0.08) |
| Sample | Nozzle Diameter | ||||
|---|---|---|---|---|---|
| 23G | 25G | 30G | 32G | 34G | |
| (a) Mean bead size (µm) * | |||||
| SP-10 | 3501 ± 440 a,A | 3061 ± 502 b,A | 1814 ± 207 c,A | 1203 ± 319 d,A | 812 ± 91 e,A |
| SP-12.5 | 1869 ± 101 a,B | 1272 ± 103 b,B | 1151 ± 85 c,B | 870 ± 89 d,B | 712 ± 82 e,B |
| SP-15 | 1507 ± 75 a,B | 1158 ± 93 b,B | 1006 ± 76 c,C | 842 ± 103 d,B | 650 ± 55 e,B |
| (b) Sphericity * | |||||
| SP-10 | 0.74 ± 0.04 a,A | 0.72 ± 0.05 a,A | 0.70 ± 0.06 a,A | 0.71 ± 0.03 a,A | 0.70 ± 0.03 a,A |
| SP-12.5 | 0.88 ± 0.01 a,B | 0.80 ± 0.03 b,B | 0.78 ± 0.04 b,B | 0.79 ± 0.03 b,B | 0.77 ± 0.03 b,B |
| SP-15 | 0.98 ± 0.01 a,C | 0.86 ± 0.02 b,C | 0.87 ± 0.02 b,C | 0.90 ± 0.05 b,C | 0.87 ± 0.02 b,C |
| Sample | Rheological Parameters * | Power-Law Parameters * | |||||
|---|---|---|---|---|---|---|---|
| G′ (Pa, at 95 °C) | Tan δ (at 95 °C) | Yield Stress (Pa) | Flow Stress (Pa) | n | K | R2 | |
| SP-10 | 2216 ± 115 a | 0.215 ± 0.0169 c | 4.5 ± 0.5 a | 7.12 ± 0.81 a | 0.129 b | 22.3 a | 0.996 |
| SP-12.5 | 3851 ± 528 b | 0.088 ± 0.004 a | 11.3 ± 1.2 a | 22.53 ± 2.58 a | 0.143 c | 44.70 a | 0.996 |
| SP-15 | 8947 ± 292 c | 0.160 ± 0.004 b | 40.9 ± 8.8 b | 142.2 ± 16.3 b | 0.057 a | 162.57 b | 0.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadzadeh, S.; Ubeyitogullari, A. Fabrication of Porous Spherical Beads from Corn Starch by Using a 3D Food Printing System. Foods 2022, 11, 913. https://doi.org/10.3390/foods11070913
Ahmadzadeh S, Ubeyitogullari A. Fabrication of Porous Spherical Beads from Corn Starch by Using a 3D Food Printing System. Foods. 2022; 11(7):913. https://doi.org/10.3390/foods11070913
Chicago/Turabian StyleAhmadzadeh, Safoura, and Ali Ubeyitogullari. 2022. "Fabrication of Porous Spherical Beads from Corn Starch by Using a 3D Food Printing System" Foods 11, no. 7: 913. https://doi.org/10.3390/foods11070913






