Encapsulation of a Desmodium intortum Protein Isolate Pickering Emulsion of β-Carotene: Stability, Bioaccesibility and Cytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Emulsion-Encapsulated β-Carotene
2.3. Extraction and Quantification of β-Carotene
2.4. Encapsulation Efficiency of β-Carotene
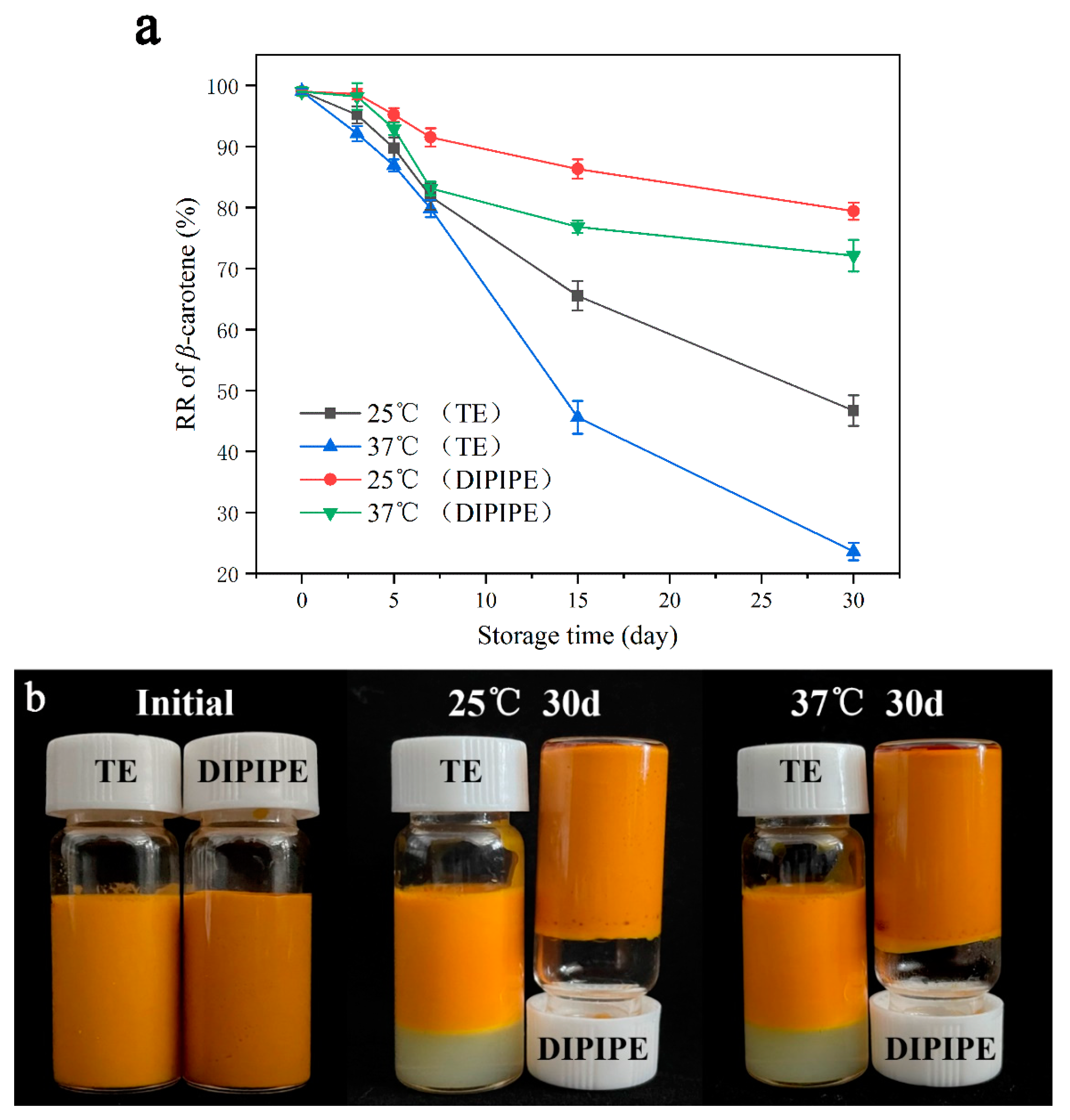
2.5. Retention Rate of β-Carotene
2.6. Construction of an In Vitro Simulated Gastrointestinal Digestion Model
2.6.1. Digestion Sample Solution
2.6.2. Simulated Gastric Fluid
2.6.3. Simulated Intestinal Fluid
2.7. Microscopic Morphology of Digested Products
2.8. Bioaccessibility of β-Carotene
2.9. Cytotoxicity Testing
3. Results and Discussion
3.1. Encapsulation Efficiency and Retention Rate of β-Carotene
3.2. Simulated In Vitro Digestion
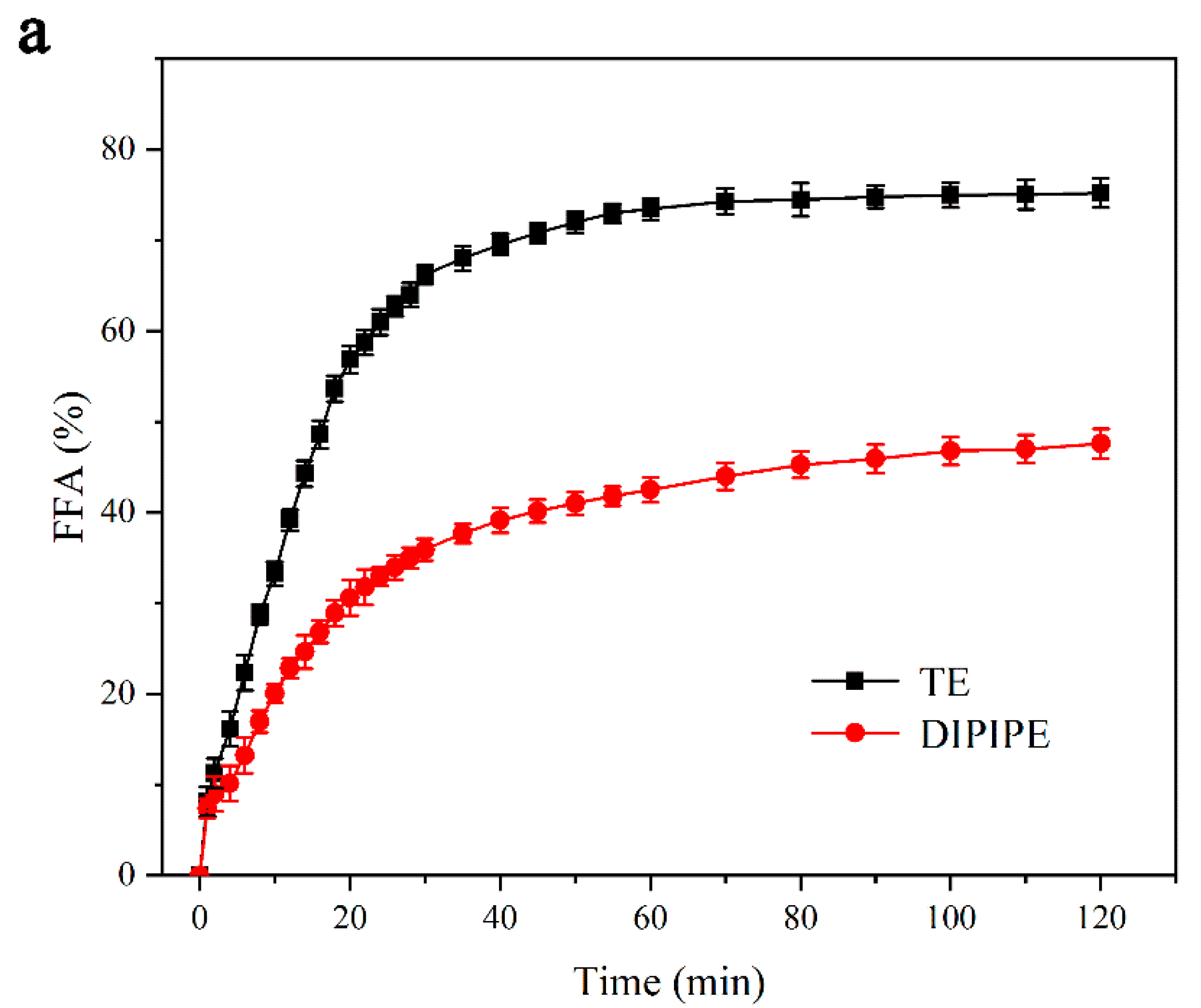
3.2.1. Simulation of the Release of FFA in Intestinal Digestion
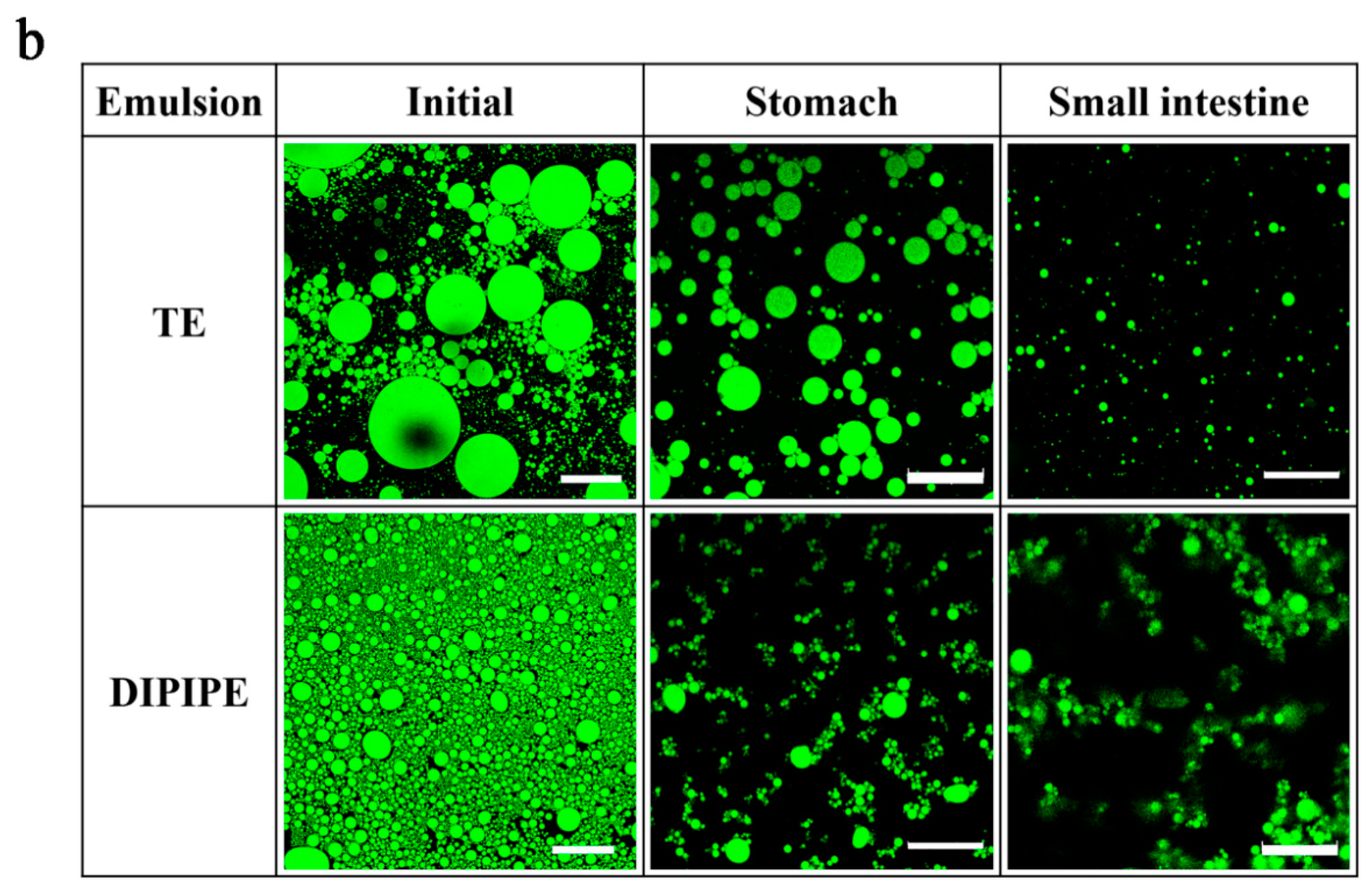
3.2.2. Effect of In Vitro Digestion on the Microstructure of the Emulsion
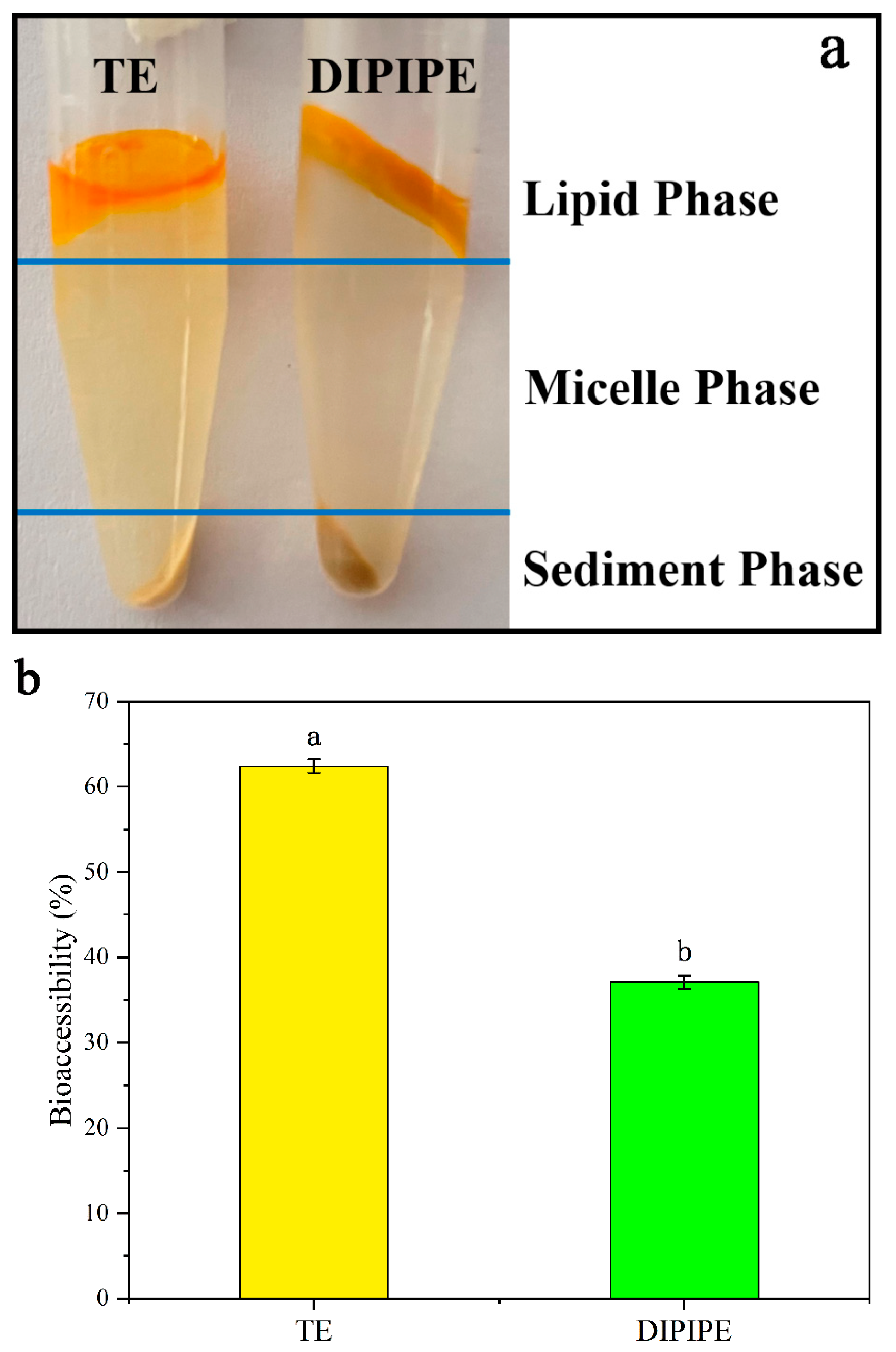
3.2.3. Bioaccessibility of β-Carotene
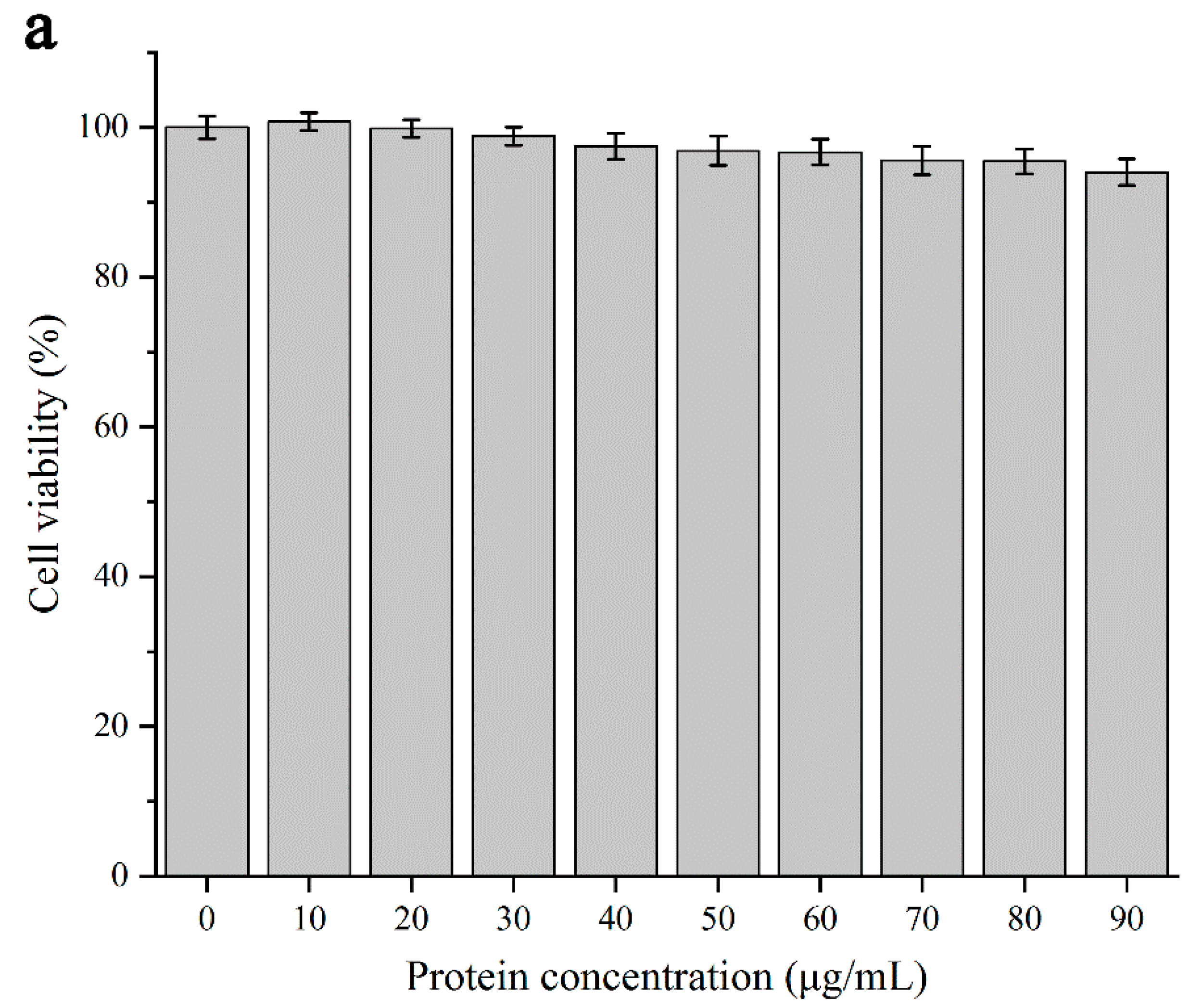
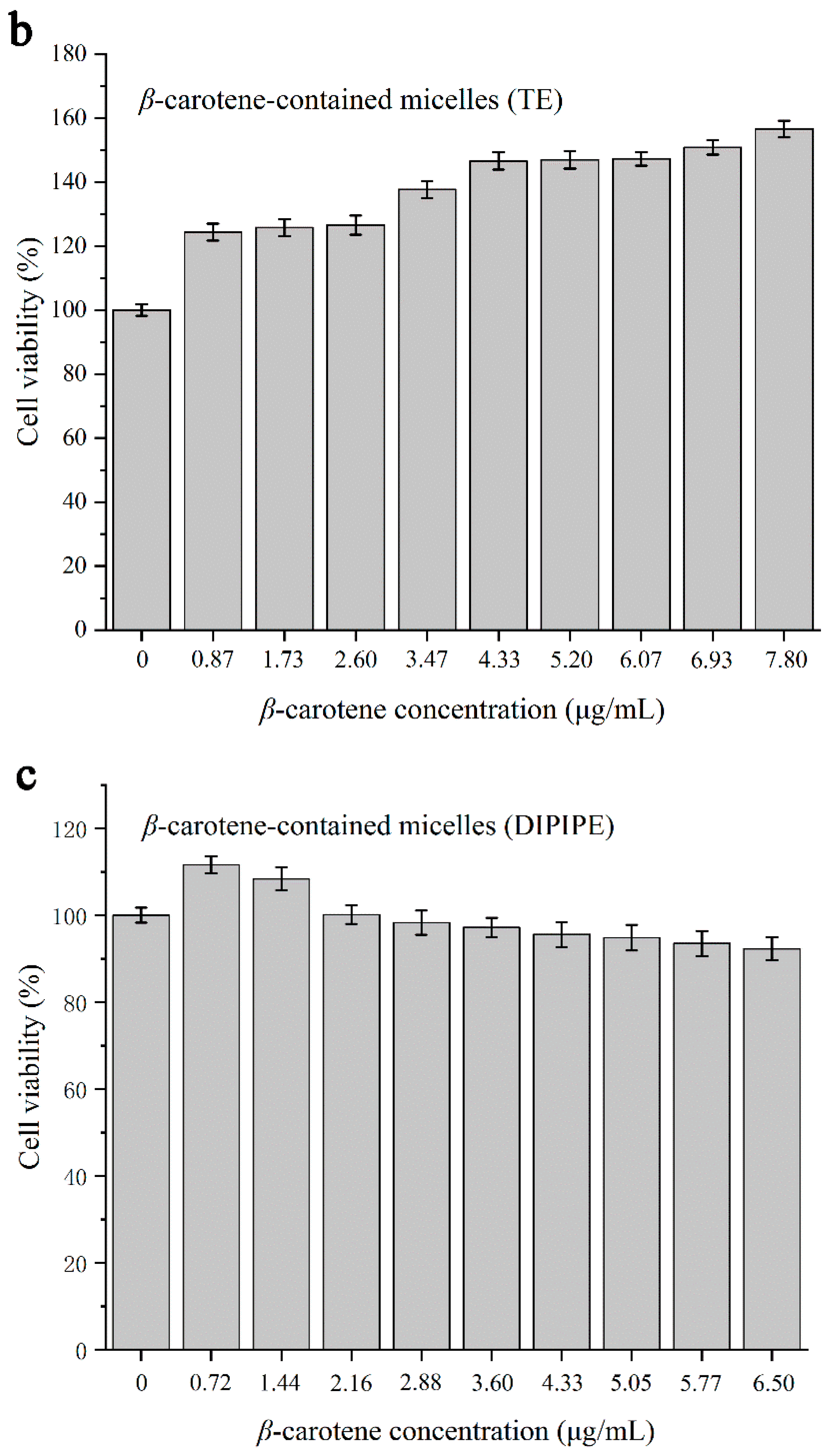
3.2.4. Cytotoxicity Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-grade pickering emulsions for encapsulation and delivery of bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Shi, A.; Feng, X.; Wang, Q.; Adhikari, B. Pickering and high internal phase pickering emulsions stabilized by protein-based particles: A review of synthesis, application and prospective. Food Hydrocoll. 2020, 109, 106117. [Google Scholar] [CrossRef]
- Yan, C.; Wu, X.; Wang, Y.; Peng, S.; Chen, J.; Zou, L.; McClements, D.J.; Liu, W. Utilization of polysaccharide-based high internal phase emulsion for nutraceutical encapsulation: Enhancement of carotenoid loading capacity and stability. J. Funct. Foods 2021, 84, 104601. [Google Scholar] [CrossRef]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical characteristics, applications and research trends of edible pickering emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of beta-carotene in wheat gluten nanoparticle-xanthan gum-stabilized pickering emulsions: Enhancement of carotenoid stability and bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Lv, P.; Wang, D.; Dai, L.; Wu, X.; Gao, Y.; Yuan, F. Pickering emulsion gels stabilized by high hydrostatic pressure-induced whey protein isolate gel particles: Characterization and encapsulation of curcumin. Food Res. Int. 2020, 132, 109032. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.-H. Soy glycinin as food-grade pickering stabilizers: Part. III. Fabrication of gel-like emulsions and their potential as sustained-release delivery systems for beta-carotene. Food Hydrocoll. 2016, 60, 631–640. [Google Scholar] [CrossRef]
- Marefati, A.; Bertrand, M.; Sjoo, M.; Dejmek, P.; Rayner, M. Storage and digestion stability of encapsulated curcumin in emulsions based on starch granule pickering stabilization. Food Hydrocoll. 2017, 63, 309–320. [Google Scholar] [CrossRef]
- Parker, R.S. Carotenoids in human blood and tissues. J. Nutr. 1989, 119, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin a on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Failla, M.L.; Chitchumroonchokchai, C.; Ishida, B.K. In vitro micellarization and intestinal cell uptake of cis isomers of lycopene exceed those of all-trans lycopene. J. Nutr. 2008, 138, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Luisa Bonet, M.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Concepcion, M.; Avalos, J.; Luisa Bonet, M.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Carmen Limon, M.; Melendez-Martinez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid. Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; McClements, D.J.; Decker, E.A. Design of foods with bioactive lipids for improved health. Annu. Rev. Food. Sci. Technol 2013, 4, 35–36. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Potential biological fate of ingested nanoemulsions: Influence of particle characteristics. Food Funct. 2012, 3, 202–220. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. The potential of zein nanoparticles to protect entrapped beta-carotene in the presence of milk under simulated gastrointestinal (gi) conditions. LWT 2016, 72, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Zhao, X.; Liu, Y.; Liang, X.; Yang, Y. Fabricating multilayer emulsions by using osa starch and chitosan suitable for spray drying: Application in the encapsulation of beta-carotene. Food Hydrocoll. 2019, 93, 102–110. [Google Scholar] [CrossRef]
- Tang, X.M.; Yao, G.L. Protein isolated from Desmodium intortum (Mill.) Urb.: Characterization and its utilization in Pickering emulsions. Food Res. Int. 2022; under review. [Google Scholar]
- Wright, A.; Pietrangelo, C.; Macnaughton, A. Influence of simulated upper intestinal parameters on the efficiency of beta carotene micellarisation using an in vitro model of digestion. Food Chem. 2007, 107, 1253–1260. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, R.; Ye, A.; Singh, H.; Zhong, F. Effects of calcium on lipid digestion in nanoemulsions stabilized by modified starch: Implications for bioaccessibility of β -carotene. Food Hydrocoll. 2017, 73, 184–193. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Controlled release of beta-carotene in beta-lactoglobulin-dextran-conjugated nanoparticles’ in vitro digestion and transport with caco-2 monolayers. J. Agric. Food Chem. 2014, 62, 8900–8907. [Google Scholar] [CrossRef] [PubMed]
- Tzoumaki, M.V.; Moschakis, T.; Scholten, E.; Biliaderis, C.G. In vitro lipid digestion of chitin nanocrystal stabilized o/w emulsions. Food Funct. 2013, 4, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, C.; Zhang, R.; Gao, Y.; McClements, D.J. Controlling the potential gastrointestinal fate of beta-carotene emulsions using interfacial engineering: Impact of coating lipid droplets with polyphenol-protein-carbohydrate conjugate. Food Chem. 2017, 221, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on beta-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Z.; Miao, P. One-pot synthesis of GSH-Capped CdTe quantum dots with excellent biocompatibility for direct cell imaging. Heliyon 2018, 4, e00576. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Cui, X.; Zhu, M. 3D-printable supramolecular hydrogels with shear-thinning property: Fabricating strength tunable bioink via dual crosslinking. Bioact. Mater. 2020, 5, 808–818. [Google Scholar] [CrossRef]
- Feng, X.; Ma, L.; Dai, H. The study on stability of food-grade pickering emulsion and the loading of β-carotene. Food Ferment. Ind. 2021, 47, 18–25. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, L.; Yi, J.; Zhang, Y.; Yokoyama, W. Development of beta-carotene-loaded organogel-based nanoemulsion with improved in vitro and in vivo bioaccessibility. J. Agric. Food Chem. 2017, 65, 6188–6194. [Google Scholar] [CrossRef]
- Yu, H.; Shi, K.; Liu, D.; Huang, Q. Development of a food-grade organogel with high bioaccessibility and loading of curcuminoids. Food Chem. 2012, 131, 48–54. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, R.; Shoemaker, C.F.; Yang, X.; Zhong, F.; Huang, Q. Stability and bioaccessibility of beta-carotene in nanoemulsions stabilized by modified starches. J. Agric. Food Chem. 2013, 61, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Narain, P.K.; DeMaria, E.J.; Heuman, D.M. Cholesterol enhances membrane-damaging properties of model bile by increasing the intervesicular-intermixed micellar concentration of hydrophobic bile salts. J. Surg. Res. 1999, 84, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Penn, A.H.; Altshuler, A.E.; Small, J.W.; Taylor, S.F.; Dobkins, K.R.; Schmid-Schoenbein, G.W. Effect of digestion and storage of human milk on free fatty acid concentration and cytotoxicity. J. Pediatr. Gastr. Nutr. 2014, 59, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, M.; Berner, A.Z.; Chervet, N.; Chassard, C.; Lacroix, C. Comparison of the caco-2, ht-29 and the mucus-secreting ht29-mtx intestinal cell models to investigate salmonella adhesion and invasion. J. Microbiol. Meth. 2013, 94, 274–279. [Google Scholar] [CrossRef]
- Campbell, N.B.; Ruaux, C.G.; Shifflett, D.E.; Steiner, J.M.; Williams, D.A.; Blikslager, A.T. Physiological concentrations of bile salts inhibit recovery of ischemic-injured porcine ileum. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G399–G407. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.-M.; Liu, P.-D.; Chen, Z.-J.; Li, X.-Y.; Huang, R.; Liu, G.-D.; Dong, R.-S.; Chen, J. Encapsulation of a Desmodium intortum Protein Isolate Pickering Emulsion of β-Carotene: Stability, Bioaccesibility and Cytotoxicity. Foods 2022, 11, 936. https://doi.org/10.3390/foods11070936
Tang X-M, Liu P-D, Chen Z-J, Li X-Y, Huang R, Liu G-D, Dong R-S, Chen J. Encapsulation of a Desmodium intortum Protein Isolate Pickering Emulsion of β-Carotene: Stability, Bioaccesibility and Cytotoxicity. Foods. 2022; 11(7):936. https://doi.org/10.3390/foods11070936
Chicago/Turabian StyleTang, Xue-Mei, Pan-Dao Liu, Zhi-Jian Chen, Xin-Yong Li, Rui Huang, Guo-Dao Liu, Rong-Shu Dong, and Jian Chen. 2022. "Encapsulation of a Desmodium intortum Protein Isolate Pickering Emulsion of β-Carotene: Stability, Bioaccesibility and Cytotoxicity" Foods 11, no. 7: 936. https://doi.org/10.3390/foods11070936
APA StyleTang, X.-M., Liu, P.-D., Chen, Z.-J., Li, X.-Y., Huang, R., Liu, G.-D., Dong, R.-S., & Chen, J. (2022). Encapsulation of a Desmodium intortum Protein Isolate Pickering Emulsion of β-Carotene: Stability, Bioaccesibility and Cytotoxicity. Foods, 11(7), 936. https://doi.org/10.3390/foods11070936




