The Protective Effects of Ganoderic Acids from Ganoderma lucidum Fruiting Body on Alcoholic Liver Injury and Intestinal Microflora Disturbance in Mice with Excessive Alcohol Intake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemical Reagents
2.2. Preparation of GA by Organic Solvent Extraction
2.3. Compound Composition Analysis of GA by HPLC-QTOF MS
2.4. Animals and Experimental Design
2.5. Sample Collection and Preparation
2.6. Biochemical Assays of the Serum and Liver Samples
2.7. Pathological Analysis of Liver
2.8. High-Throughput Sequencing of Intestinal Microbiome
2.9. UPLC-QTOF MS Analysis of Liver Metabolome
2.10. Quantitative RT-PCR
2.11. Statistical Analysis
3. Results
3.1. HPLC-QTOF/MS Analysis of the Compound Composition of GA
3.2. Effects of GA Intervention on the Body Growth Performance
3.3. Effects of GA Intervention on Serum Biochemical Parameters
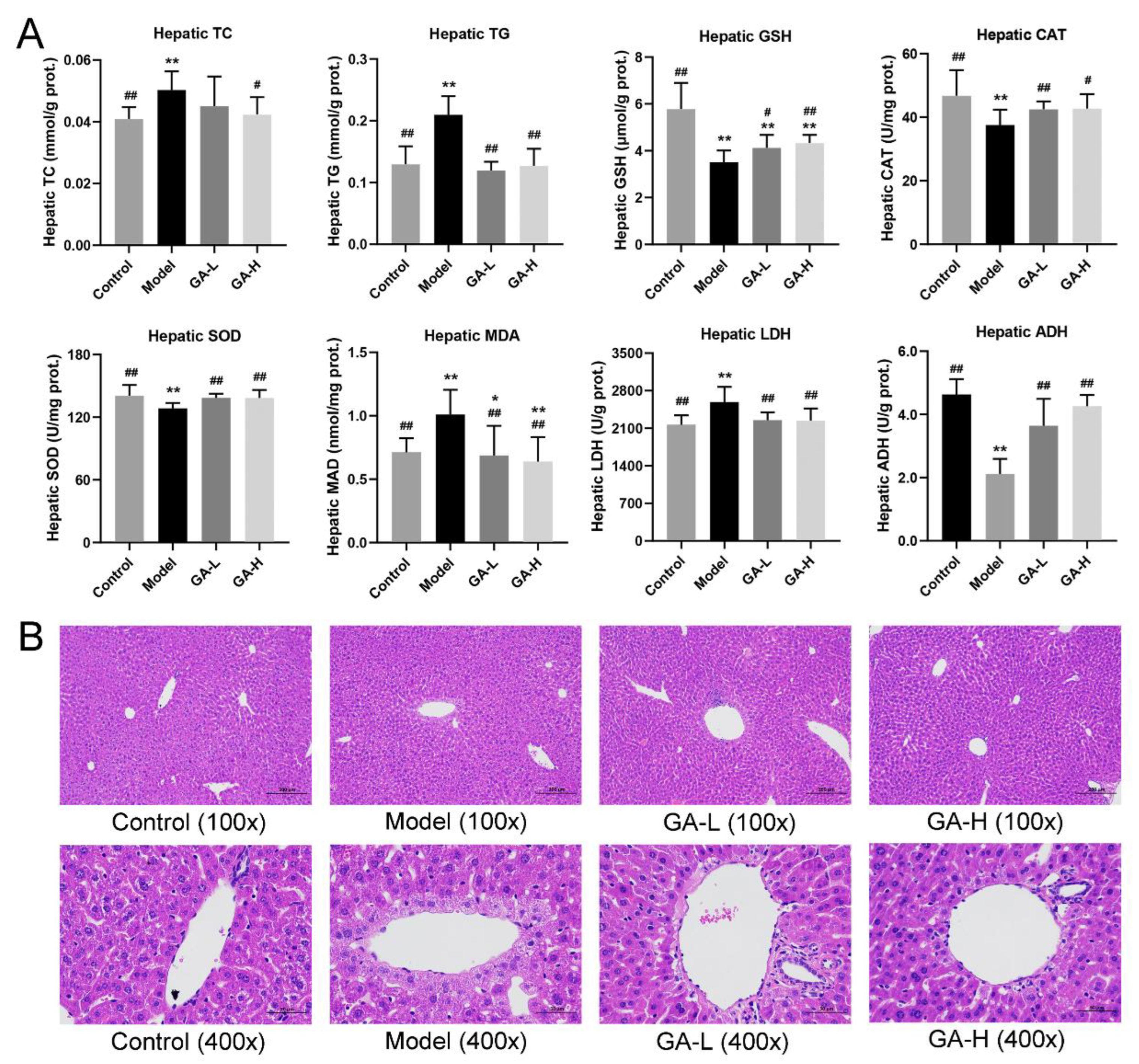
3.4. Effects of GA on Liver Biochemical Parameters and Histopathological Features
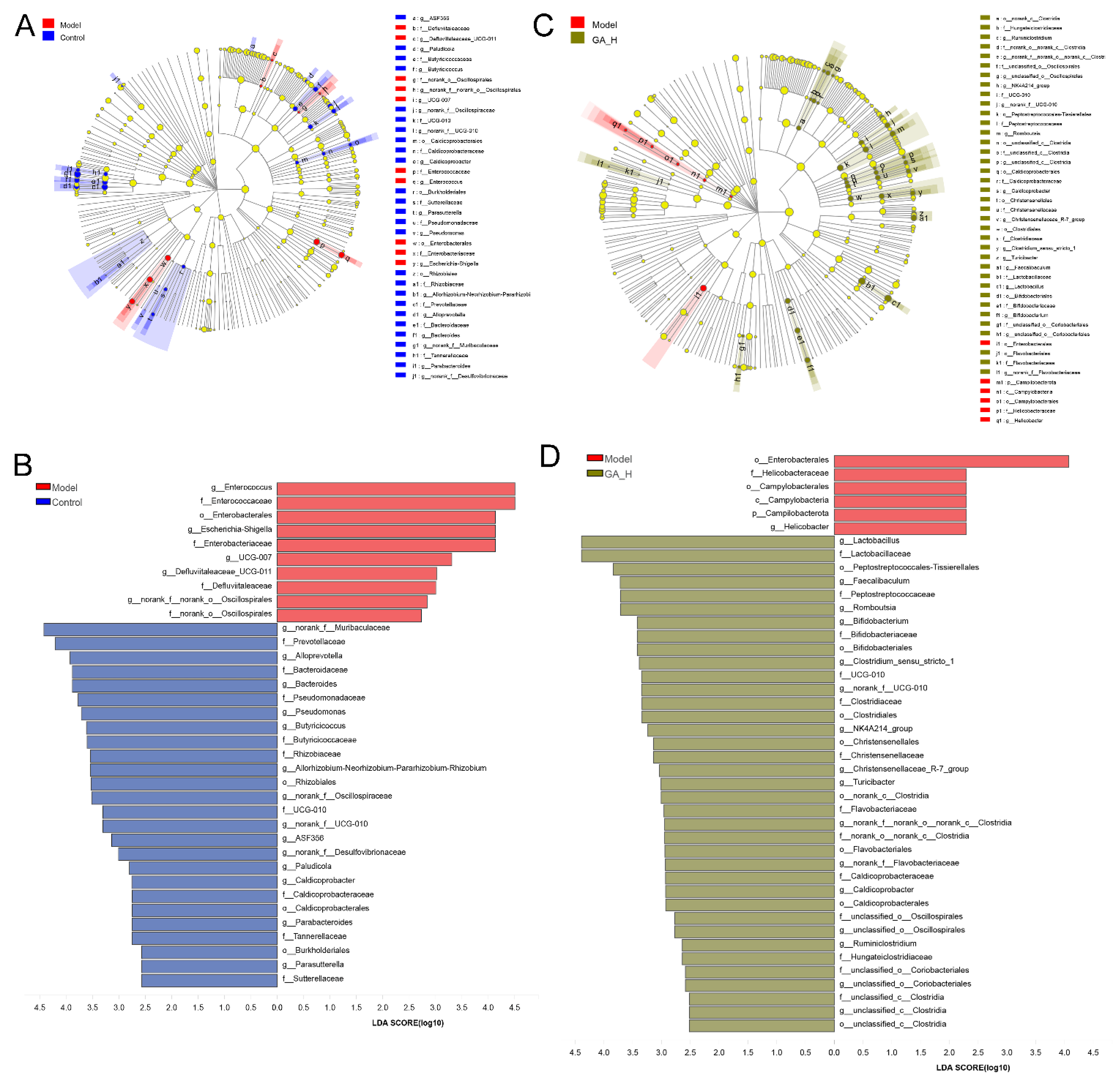
3.5. Effects of GA Intervention on the Composition of Intestinal Microbiota
3.6. Correlations of the Key Microbial Phylotypes with the Biochemical Parameters
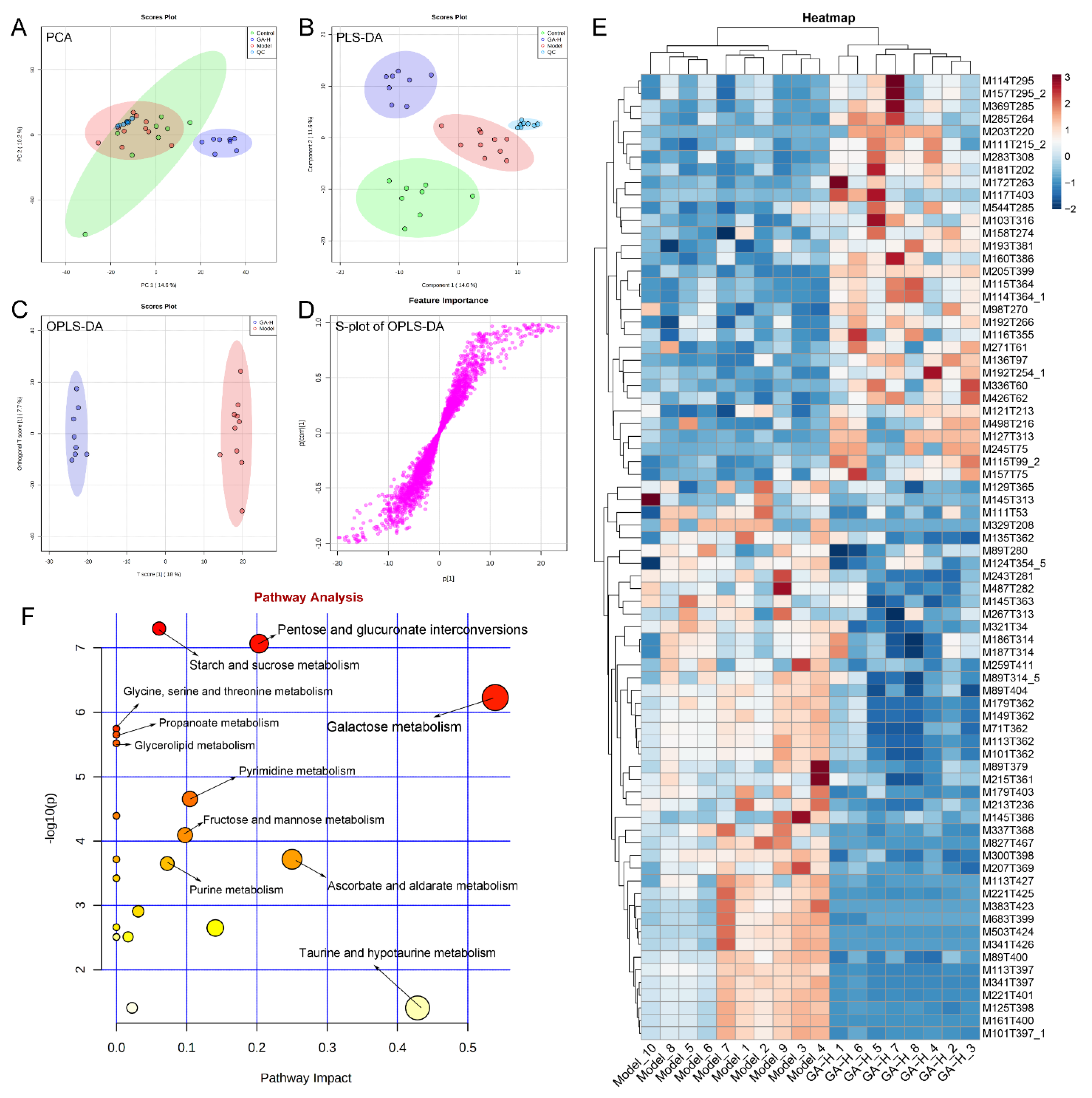
3.7. Effects of GA Intervention on Liver Metabolome in Mice with Excessive Alcohol Intake
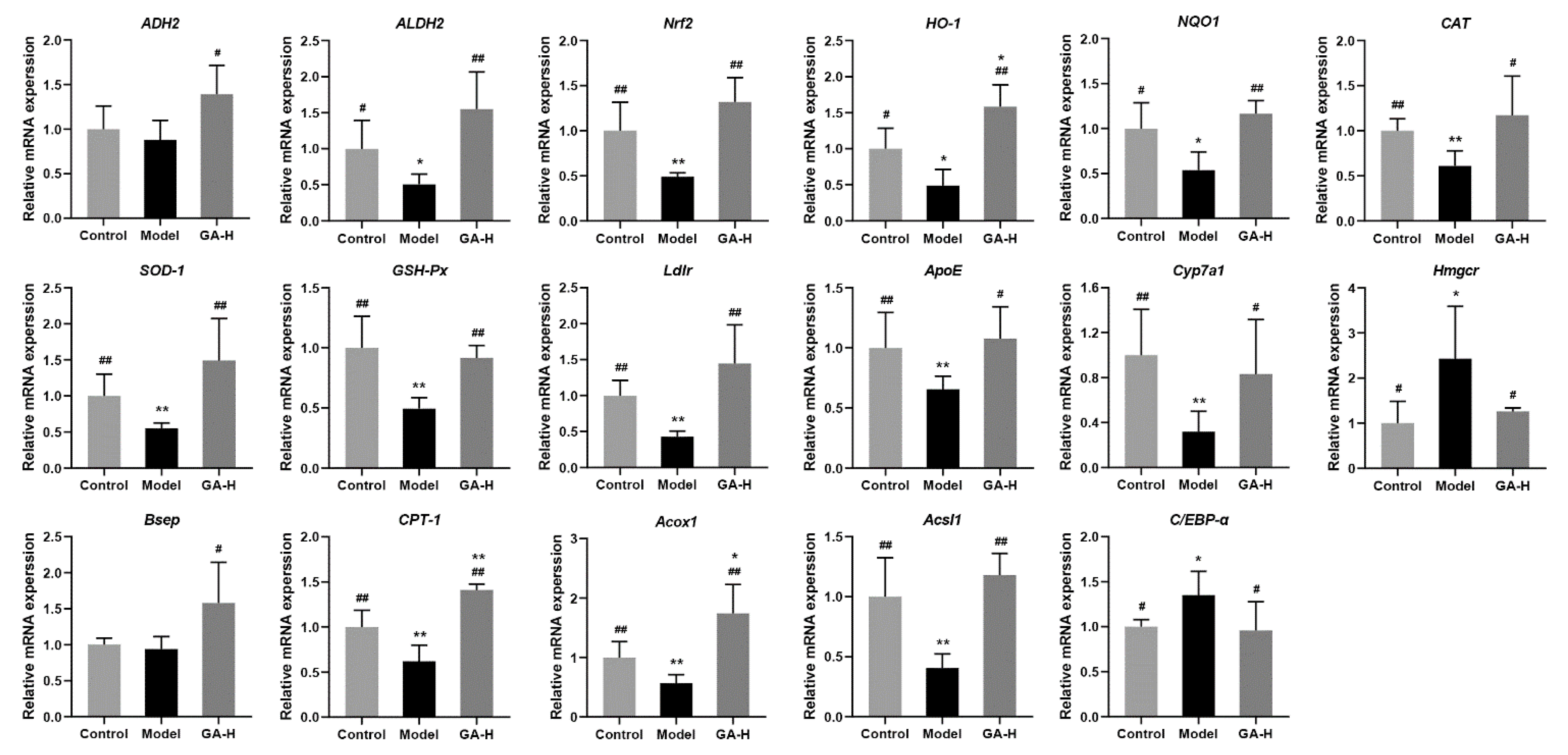
3.8. Effects of GA Intervention on Liver mRNA Levels in Mice with Excessive Alcohol Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Namachivayam, A.; Gopalakrishnan, A.V. A review on molecular mechanism of alcoholic liver disease. Life Sci. 2021, 274, 119328. [Google Scholar] [CrossRef]
- Teschke, R. Alcoholic liver disease: Alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Sun, W.; Cui, W.; Jia, L.; Zhang, J. A polysaccharide of PFP-1 from Pleurotus geesteranus attenuates alcoholic liver diseases via Nrf2 and NF-kappa B signaling pathways. Food Funct. 2021, 12, 4591–4605. [Google Scholar] [CrossRef]
- Sang, T.; Guo, C.; Guo, D.; Wu, J.; Wang, Y.; Wang, Y.; Chen, J.; Chen, C.; Wu, K.; Na, K.; et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 2021, 256, 117594. [Google Scholar] [CrossRef]
- Guo, W.; Pan, Y.; Li, L.; Li, T.; Liu, B.; Lv, X. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018, 9, 3419–3431. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guo, J.; Liu, B.; Lu, J.; Chen, M.; Liu, B.; Bai, W.; Rao, P.; Ni, L.; Lv, X. Ganoderic acid A from Ganoderma lucidum ameliorates lipid metabolism and alters gut microbiota composition in hyperlipidemic mice fed a high-fat diet. Food Funct. 2020, 11, 6818–6833. [Google Scholar] [CrossRef] [PubMed]
- Abulizi, A.; Hu, L.; Ma, A.; Shao, F.; Zhu, H.; Lin, S.; Shao, G.; Xu, Y.; Ran, J.; Li, J.; et al. Ganoderic acid alleviates chemotherapy-induced fatigue in mice bearing colon tumor. Acta Pharmacol. Sin. 2021, 42, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.H.; Cho, J.Y.; Bin Sadiq, N.; Kim, J.; Lee, B.; Hamayun, M.; Lee, T.S.; Kim, H.S.; Park, S.H.; Nho, C.W.; et al. Optimization of antioxidant, anti-diabetic, and anti-inflammatory activities and ganoderic acid content of differentially dried Ganoderma lucidum using response surface methodology. Food Chem. 2021, 335, 127645. [Google Scholar] [CrossRef]
- Chung, D.; Yang, M.; Li, Y.; Chen, W.; Hung, C.; Wang, C. Ganoderma lucidum repress injury of ethanol-induced steatohepatitis via anti-inflammation, anti-oxidation and reducing hepatic lipid in C57BL/6J mice. J. Funct. Foods 2017, 33, 314–322. [Google Scholar] [CrossRef]
- Kim, H.; Choi, S.E.; Jeong, W. Oxidative stress and glutamate excretion in alcoholic steatosis: Metabolic synapse between hepatocyte and stellate cell. Clin. Mol. Hepatol. 2020, 26, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 785. [Google Scholar] [CrossRef]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Lang, S.; Duan, Y.; Zhang, X.; Gao, B.; Chopyk, J.; Schwanemann, L.K.; Ventura-Cots, M.; Bataller, R.; Bosques-Padilla, F.; et al. Intestinal virome in patients with alcoholic hepatitis. Hepatology 2020, 72, 2182–2196. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Du, Y.; Xu, F.; Zhou, K.; Wang, Z.; Al-Dalali, S.; Wang, Y.; Li, X.; Ma, Y.; Xie, Y.; et al. Oligopeptides from Jinhua ham prevent alcohol-induced liver damage by regulating intestinal homeostasis and oxidative stress in mice. Food Funct. 2021, 12, 10053–10070. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Solis-Urra, P.; Rodriguez-Rodriguez, F.; Olivares-Arancibia, J.; Navarro-Oliveros, M.; Abadia-Molina, F.; Alvarez-Mercado, A.I. The gut barrier, intestinal microbiota, and liver disease: Molecular mechanisms and strategies to manage. Int. J. Mol. Sci. 2020, 21, 8351. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Han, J.; Ye, M.; Ma, X.; Shen, X.; Xue, B.; Che, Q. Identification of major compounds in rat bile after oral administration of total triterpenoids of Ganoderma lucidum by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 63, 29–39. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, W.; Wang, Z.; Shi, F. Effects of chronic ethanol intake on the development of immune organs in the mammalian. J. Domest. Anim. Ecol. 2008, 29, 65–67. [Google Scholar] [CrossRef]
- Allen, F.; Greiner, R.; Wishart, D. Competitive fragmentation modeling of ESI-MS/MS spectra for putative metabolite identification. Metabolomics 2015, 11, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Kourkoumpetis, T.; Sood, G. Pathogenesis of alcoholic liver disease: An update. Clin. Liver Dis. 2019, 23, 71. [Google Scholar] [CrossRef]
- Lamas-Paz, A.; Hao, F.; Nelson, L.J.; Teresa Vazquez, M.; Canals, S.; Gomez Del Moral, M.; Martinez-Naves, E.; Nevzorova, Y.A.; Javier Cubero, F. Alcoholic liver disease: Utility of animal models. World J. Gastroenterol. 2018, 24, 5063–5075. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yang, H.; Yan, M.; Feng, R.; Deng, L. Protective effects of lemon extract and silymarin against acute alcoholic liver injury in rats. J. Southwest Med. Univ. 2021, 44, 105–109. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B.; Fu, R. Alcoholic hepatic and renal lesion and expression of laminin, collagen III in kidney. World Chin. J. Dig. 2001, 9, 1134–1138. [Google Scholar] [CrossRef]
- Hu, Q.; Li, S.; Qiao, E.; Tang, Z.; Jin, E.; Jin, G.; Gu, Y. Effects of boron on structure and antioxidative activities of spleen in rats. Biol. Trace Elem. Res. 2014, 158, 73–80. [Google Scholar] [CrossRef]
- Feng, H.; Dai, W.; Bai, S.; Shen, C.; Wen, W.; Cui, S.; Cui, Y.; Zhang, R.; Peng, W. Protective dffect of Ficus pandurata extract on alcohol-induced acute liver injury base on pyroptosis. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 125–131. [Google Scholar] [CrossRef]
- Bode, C.; Kugler, V.; Bode, J.C. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 1987, 4, 8–14. [Google Scholar] [CrossRef]
- Bruellman, R.; Llorente, C. A perspective of intestinal immune-microbiome interactions in alcohol-associated liver disease. Int. J. Biol. Sci. 2021, 17, 307–327. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Liu, J.; Chen, M.; Huang, M.; Huang, G.; Chen, X.; Du, Q.; Su, J.; Lin, R. Ethanol extract of Centella asiatica alleviated dextran sulfate sodium-induced colitis: Restoration on mucosa barrier and gut microbiota homeostasis. J. Ethnopharmacol. 2021, 267, 113445. [Google Scholar] [CrossRef]
- He, B.; Hoang, T.K.; Wang, T.; Ferris, M.; Taylor, C.M.; Tian, X.; Luo, M.; Tran, D.Q.; Zhou, J.; Tatevian, N.; et al. Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A(2A) receptors. J. Exp. Med. 2017, 214, 107–123. [Google Scholar] [CrossRef]
- Hsieh, F.; Lee, C.; Chai, C.; Chen, W.; Lu, Y.; Wu, C. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr. Metab. 2013, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.; Stehle, P.; Pacini, G.; et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: A proof of concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, N.; Sun, Y.; Wu, S.; Tian, W.; Lai, Y.; Li, P.; Du, B. Supplementation of Bacillus sp. DU-106 reduces hypercholesterolemia and ameliorates gut dysbiosis in high-fat diet rats. Appl. Microbiol. Biotechnol. 2021, 105, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Kuprys, P.V.; Cannon, A.R.; Shieh, J.; Iftekhar, N.; Park, S.K.; Eberhardt, J.M.; Ding, X.; Choudhry, M.A. Alcohol decreases intestinal ratio of Lactobacillus to Enterobacteriaceae and induces hepatic immune tolerance in a murine model of DSS-colitis. Gut Microbes 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Xue, G.; Lai, S.; Li, X.; Zhang, W.; You, J.; Chen, H.; Qian, Y.; Gao, P.; Liu, Z.; Liu, Y. Efficient bioconversion of organic wastes to high optical activity of L-lactic acid stimulated by cathode in mixed microbial consortium. Water Res. 2018, 131, 1–10. [Google Scholar] [CrossRef]
- Jung, S.; Hwang, J.; Park, E.; Lee, S.; Chung, Y.; Chung, M.; Lim, S.; Lim, T.; Ha, Y.; Park, B.; et al. Regulation of alcohol and acetaldehyde metabolism by a mixture of Lactobacillus and Bifidobacterium species in human. Nutrients 2021, 13, 1875. [Google Scholar] [CrossRef]
- Xu, B.; Yan, Y.; Yin, B.; Zhang, L.; Qin, W.; Niu, Y.; Tang, Y.; Zhou, S.; Yan, X.; Ma, L. Dietary glycyl-glutamine supplementation ameliorates intestinal integrity, inflammatory response, and oxidative status in association with the gut microbiota in LPS-challenged piglets dagger. Food Funct. 2021, 12, 3539–3551. [Google Scholar] [CrossRef]
- Duarte, V.D.S.; Dos Santos Cruz, B.C.; Tarrah, A.; Dias, R.S.; Dias Moreira, L.D.P.; Lemos, W.J.F., Jr.; Fidelis Silva, L.C.; Santana, G.R.; de Oliveira, L.L.; Gouveia Peluzio, M.C.; et al. Chemoprevention of dmh-induced early colon carcinogenesis in male balb/c mice by administration of Lactobacillus paracasei dta81. Microorganisms 2020, 8, 1994. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; Ye, J.; Lv, L.; Yang, L.; Bian, X.; Wu, W.; Wu, J.; Shi, D.; Wang, Q.; et al. New strain of Pediococcus pentosaceus alleviates ethanol-induced liver injury by modulating the gut microbiota and short-chain fatty acid metabolism. World J. Gastroenterol. 2020, 26, 6224–6240. [Google Scholar] [CrossRef]
- Calderon-Perez, L.; Llaurado, E.; Companys, J.; Pla-Paga, L.; Pedret, A.; Rubio, L.; Gosalbes, M.J.; Yuste, S.; Sola, R.; Valls, R.M. Interplay between dietary phenolic compound intake and the human gut microbiome in hypertension: A cross-sectional study. Food Chem. 2021, 344, 15. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, Y.; Chen, L.; Qi, Y.; He, J.; Hu, M.; Zhang, Y.; Fan, L.; Yang, T.; Wang, L.; et al. The effects of cigarettes and alcohol on intestinal microbiota in healthy men. J. Microbiol. 2020, 58, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Lanthier, N.; Rodriguez, J.; Nachit, M.; Hiel, S.; Trefois, P.; Neyrinck, A.M.; Cani, P.D.; Bindels, L.B.; Thissen, J.; Delzenne, N.M. Microbiota analysis and transient elastography reveal new extra-hepatic components of liver steatosis and fibrosis in obese patients. Sci. Rep. 2021, 11, 569. [Google Scholar] [CrossRef]
- Ruuskanen, M.O.; Aberg, F.; Mannisto, V.; Havulinna, A.S.; Meric, G.; Liu, Y.; Loomba, R.; Vazquez-Baeza, Y.; Tripathi, A.; Valsta, L.M.; et al. Links between gut microbiome composition and fatty liver disease in a large population sample. Gut Microbes 2021, 13, 1888673. [Google Scholar] [CrossRef]
- Li, Z.; Jia, R.; Wu, J.; Lin, L.; Ou, Z.; Liao, B.; Zhang, L.; Zhang, X.; Song, G.; Zhao, M. Sargassum fusiforme polysaccharide partly replaces acarbose against type 2 diabetes in rats. Int. J. Biol. Macromol. 2021, 170, 447–458. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, C.; Pan, P.; Echeveste, C.E.; Dong, A.; Oshima, K.; Yearsley, M.; Yu, J.; Wang, L. Dysregulated free fatty acid receptor 2 exacerbates colonic adenoma formation in apc(min/+) mice: Relation to metabolism and gut microbiota composition. J. Cancer Prev. 2021, 26, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, L.; Ciocan, D.; Hugot, C.; Spatz, M.; Dupeux, M.; Houron, C.; Lievin-Le Moal, V.; Puchois, V.; Ferrere, G.; Trainel, N.; et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut 2021, 70, 1299–1308. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, M.; Guo, W.; Li, T.; Liu, B.; Bai, W.; Ai, L.; Rao, P.; Ni, L.; Lv, X. Monascus purpureus-fermented common buckwheat protects against dyslipidemia and non-alcoholic fatty liver disease through the regulation of liver metabolome and intestinal microbiome. Food Res. Int. 2020, 136, 109511. [Google Scholar] [CrossRef]
- Cabrera, D.; Arab, J.P.; Arrese, M. UDCA, NorUDCA, and TUDCA in liver diseases: A review of their mechanisms of action and clinical applications. Handb. Exp. Pharmacol. 2019, 256, 237–264. [Google Scholar] [CrossRef]
- Ma, J.; Meng, X.; Liu, Y.; Yin, C.; Zhang, T.; Wang, P.; Park, Y.K.; Jung, H.W. Effects of a rhizome aqueous extract of Dioscorea batatas and its bioactive compound, allantoin in high fat diet and streptozotocin-induced diabetic mice and the regulation of liver, pancreas and skeletal muscle dysfunction. J. Ethnopharmacol. 2020, 259, 112926. [Google Scholar] [CrossRef]
- Tobwala, S.; Khayyat, A.; Fan, W.; Ercal, N. Comparative evaluation of N-acetylcysteine and N-acetylcysteineamide in acetaminophen-induced hepatotoxicity in human hepatoma HepaRG cells. Exp. Biol. Med. 2015, 240, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Pyruvate is an endogenous anti-inflammatory and anti-oxidant molecule. Med. Sci. Monit. 2006, 12, A79–A84. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zhang, Y.; Liu, H.; Li, P.; Zhang, H.; Cheng, G. Fortunellin protects against high fructose-induced diabetic heart injury in mice by suppressing inflammation and oxidative stress via AMPK/Nrf-2 pathway regulation. Biochem. Biophys. Res. Commun. 2017, 490, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Veskovic, M.; Mladenovic, D.; Milenkovic, M.; Tosic, J.; Borozan, S.; Gopcevic, K.; Labudovic-Borovic, M.; Dragutinovic, V.; Vucevic, D.; Jorgacevic, B.; et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur. J. Pharmacol. 2019, 848, 39–48. [Google Scholar] [CrossRef]
- Said, H.M.; Ortiz, A.; Ma, T.Y.; McCloud, E. Riboflavin uptake by the human-derived liver cells Hep G2: Mechanism and regulation. J. Cell. Physiol. 1998, 176, 588–594. [Google Scholar] [CrossRef]
- Sakurai, T.; Miyazawa, S.; Furuta, S.; Hashimoto, T. Riboflavin deficiency and β-oxidation systems in rat liver. Lipids 1982, 17, 598–604. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, H.; Kim, S.; Park, J. Liposomal borrelidin for treatment of metastatic breast cancer. Drug Deliv. Transl. Res. 2018, 8, 1380–1388. [Google Scholar] [CrossRef]
- Kong, B.S.; Im, S.J.; Lee, Y.J.; Cho, Y.H.; Do, Y.R.; Byun, J.W.; Ku, C.R.; Lee, E.J. Vasculoprotective effects of 3-Hydroxybenzaldehyde against VSMCs proliferation and ECs inflammation. PLoS ONE 2016, 11, e0149394. [Google Scholar] [CrossRef]
- Feng, S.; Wang, H.; Wang, Y.; Sun, R.; Xie, Y.; Zhou, Z.; Wang, H.; Aa, J.; Zhou, F.; Wang, G. Apatinib induces 3-hydroxybutyric acid production in the liver of mice by peroxisome proliferator-activated receptor alpha activation to aid its antitumor effect. Cancer Sci. 2019, 110, 3328–3339. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Sawa, T.; Akaike, T.; Maeda, H. Tumor-targeted delivery of polyethylene glycol-conjugated D-amino acid oxidase for antitumor therapy via enzymatic generation of hydrogen peroxide. Cancer Res. 2002, 62, 3138–3143. [Google Scholar]
- Kim, M.W.; Kang, J.H.; Jung, H.J.; Park, S.Y.; Phan, T.; Namgung, H.; Seo, S.Y.; Yoon, Y.S.; Oh, S.H. Allyl isothiocyanate protects acetaminophen-induced liver injury via NRF2 activation by decreasing spontaneous degradation in hepatocyte. Nutrients 2020, 12, 3585. [Google Scholar] [CrossRef]
- Kershenobich, D.; García-Tsao, G.; Saldana, S.A.; Rojkind, M. Relationship between blood lactic acid and serum proline in alcoholic liver cirrhosis. Gastroenterology 1981, 80, 1012–1015. [Google Scholar] [CrossRef]
- Manna, S.K.; Patterson, A.D.; Yang, Q.; Krausz, K.W.; Li, H.; Idle, J.R.; Fornace, A.J., Jr.; Gonzalez, F.J. Identification of Noninvasive Biomarkers for Alcohol-Induced Liver Disease Using Urinary Metabolomics and the Ppara-null Mouse. J. Proteome Res. 2010, 9, 4176–4188. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Lin, L.; Peng, Y.; Yuan, D.; Zhang, S.; Gong, Z.; Xiao, W. L-Theanine attenuates liver aging by inhibiting advanced glycation end products in D-galactose-induced rats and reversing an imbalance of oxidative stress and inflammation. Exp. Gerontol. 2020, 131, 110823. [Google Scholar] [CrossRef]
- Kitahara, Y.; Takeuchi, M.; Miura, K.; Mine, T.; Matsui, T.; Yamagishi, S. Glyceraldehyde-derived advanced glycation end products (AGEs). A novel biomarker of postprandial hyperglycaemia in diabetic rats. Clin. Exp. Med. 2008, 8, 175–177. [Google Scholar] [CrossRef]
- Mitidieri, E.; Vellecco, V.; Brancaleone, V.; Vanacore, D.; Manzo, O.L.; Martin, E.; Sharina, I.; Krutsenko, Y.; Monti, M.C.; Morretta, E.; et al. Involvement of 3′,5′-cyclic inosine monophosphate in cystathionine gamma-lyase-dependent regulation of the vascular tone. Br. J. Pharm. 2021, 178, 3765–3782. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Zhu, L.; Yan, L.; Lu, D.; Zhang, Q.; Zhao, M.; Li, Z. Never deem lightly the “less harmful” low-molecular-weight PAH, NPAH, and OPA—Disturbance of the immune response at real environmental levels. Chemosphere 2017, 168, 568–577. [Google Scholar] [CrossRef]
- Heidari, R.; Rasti, M.; Yeganeh, B.S.; Niknahad, H.; Saeedi, A.; Najibi, A. Sulfasalazine-induced renal and hepatic injury in rats and the protective role of taurine. Bioimpacts 2016, 6, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.F.; Liang, Y.C.; Lin, J.K. Inhibition of 1,2,4-benzenetriol-generated active oxygen species and induction of phase II enzymes by green tea polyphenols. Chem. Biol. Interact. 1995, 98, 283–301. [Google Scholar] [CrossRef]
- Kuo, P.C.; Abe, K.Y. Interleukin 1-induced production of nitric oxide inhibits benzenetriol-mediated oxidative injury in rat hepatocytes. Gastroenterology 1995, 109, 206–216. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, Y.; Li, L.; Xu, L.; Tang, Z.; Qi, Y.; Yin, L.; Peng, J. MicroRNA-128-3p aggravates doxorubicin-induced liver injury by promoting oxidative stress via targeting Sirtuin-1. Pharmacol. Res. 2020, 146, 104276. [Google Scholar] [CrossRef] [PubMed]
- Bains, J.S.; Singh, J.; Kamboj, S.S.; Nijjar, K.K.; Agrewala, J.N.; Kumar, V.; Kumar, A.; Saxena, A.K. Mitogenic and anti-proliferative activity of a lectin from the tubers of Voodoo lily (Sauromatum venosum). Biochim. Biophys. Acta Gen. Subj. 2005, 1723, 163–174. [Google Scholar] [CrossRef]
- Walter, A.; Korth, U.; Hilgert, M.; Hartmann, J.; Weichel, O.; Hilgert, M.; Fassbender, K.; Schmitt, A.; Klein, J. Glycerophosphocholine is elevated in cerebrospinal fluid of Alzheimer patients. Neurobiol. Aging 2004, 25, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Syme, C.; Czajkowski, S.; Shin, J.; Abrahamowicz, M.; Leonard, G.; Perron, M.; Richer, L.; Veillette, S.; Gaudet, D.; Strug, L.; et al. Glycerophosphocholine Metabolites and Cardiovascular Disease Risk Factors in Adolescents: A Cohort Study. Circulation 2016, 134, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Wang, H.L.; Zhong, G.Y.; Zhu, J.X. Molecular mechanism and research progress on pharmacology of traditional Chinese medicine in liver injury. Pharm. Biol. 2018, 56, 594–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieber, C.S. Metabolism of alcohol. Clin. Liver Dis. 2005, 9, 1–35. [Google Scholar] [CrossRef]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of keap1-nrf2-are pathway as potential preventive and therapeutic agents. Clin. Liver Dis. 2012, 32, 687–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Li, L.; Zhang, Y.; Zhang, L.; Lu, J.; Guo, S.; Liang, N.; Ge, J.; Zhu, M.; Tao, Y.; et al. Acetaldehyde dehydrogenase 2 interactions with LDLR and AMPK regulate foam cell formation. J. Clin. Investig. 2019, 129, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Golforoush, P.; Yellon, D.M.; Davidson, S.M. Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic Res. Cardiol. 2020, 115, 73. [Google Scholar] [CrossRef]
- Lv, X.; Chen, M.; Huang, Z.; Guo, W.; Ai, L.; Bai, W.; Yu, X.; Liu, Y.; Rao, P.; Ni, L. Potential mechanisms underlying the ameliorative effect of Lactobacillus paracasei FZU103 on the lipid metabolism in hyperlipidemic mice fed a high-fat diet. Food Res. Int. 2021, 139, 109956. [Google Scholar] [CrossRef]
- Donepudi, A.C.; Ferrell, J.M.; Boehme, S.M.; Chiang, J. Deficiency of cholesterol 7 alpha hydroxylase (Cyp7a1) in bile acid synthesis exacerbates alcohol-induced liver injury in mice. Hepatology 2017, 661, 1048A. [Google Scholar] [CrossRef]
- Olofsson, L.E.; Orho-Melander, M.; William-Olsson, L.; Sjoholm, K.; Sjostrom, L.; Groop, L.; Carlsson, B.; Carlsson, L.M.S.; Olsson, B. CCAAT/Enhancer binding protein alpha (C/EBP alpha) in adipose tissue regulates genes in lipid and glucose metabolism and a genetic variation in C/EBP alpha is associated with serum levels of triglycerides. J. Clin. Endocrinol. Metab. 2008, 93, 4880–4886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doh, K.O.; Kim, Y.W.; Park, S.Y.; Lee, S.K.; Park, J.S.; Kim, J.Y. Interrelation between long-chain fatty acid oxidation rate and carnitine palmitoyltransferase 1 activity with different isoforms in rat tissues. Life Sci. 2005, 77, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, J.; Winter, M.J.; Lange, A.; Cumming, R.; Owen, S.F.; Tyler, C.R. Effects of the lipid regulating drug clofibric acid on PPAR alpha-regulated gene transcript levels in common carp (Cyprinus carpio) at pharmacological and environmental exposure levels. Aquat. Toxicol. 2015, 161, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Gene | Forward Primer (5′−3′) | Reverse Primer (5′−3′) |
|---|---|---|
| Ldlr | ATGCTGGAGATAGAGTGGAGTT | CCGCCAAGATCAAGAAAG |
| Cyp7a1 | CCTTGGGACGTTTTCCTGCT | GCGCTCTTTGATTTAGGAAG |
| Acox1 | GCCTGCTGTGTGGGTATGTCATT | GTCATGGGCGGGTGCAT |
| ADH2 | AACGGTGAGAAGTTCCCAAAA | ACGACCCCCAGCCTAATACA |
| ALDH2 | ATCCTCGGCTACATCAAATCG | GTCTTTTACGTCCCCGAACAC |
| SOD-1 | TTG GCC GTA CAA TGG TGG | CGC AAT CCC AAT CAC TCC AC |
| GSH-Px | GGGACCCTGAGACTTAGAGC | AATCCGTACTAGCGCTCACA |
| CAT | TCA CCC ACG ATA TCA CCA GA | AGC TGA GCC TGA CTC TCC |
| Nrf2 | GGGACCCTGAGACTTAGAGC | AATCCGTACTAGCGCTCACA |
| HO-1 | AACAAGCAAKCCCAGTCTATGC | AGGTAGCGGGTATATGCGTGGGCC |
| NQO1 | AACAAGCAAKCCCAGTCTATGC | AGGTAGCGGGTATATGCGTGGGCC |
| Acl1 | CACTTCTTGCCTCGTTCCAC | GTCGTCCCGCTCTATGACAC |
| CPT-1 | TCCATGCATACCAAAGTGGA | TGGTAGGAGAGCAGCACCTT |
| ApoE | AKCCGCTTCTGGGATTACCT | TCAGTGCCGTCAGTTCTTGTG |
| C/EBP-α | GAACAGCAACGAGTACCGGGTA | GCCATGGCCTTGACCAAGGAG |
| Hmgcr | TGCTGGTGCTATCAAAGG | GCAGATGGGATGACTCGA |
| Bsep | TCTGACTCAGTGATTCTTCGCA | CCCATAAACATCAGCCAGTTGT |
| 18S | AGTCCCTGCCCTTTGTACACA | CGATCCCAGGGCCTCACTA |
| No | Rt (min) | UVλmax (nm) | Formula | Assigned Identity | Precursor ion [M-H]− | Fragment Ions | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 7.89 | 257 | C30H46O8 | Ganoderic acid L | 533.3101 | 515.2899, 497.2899, 405.2756, 129.0531, 87.0479 | [17] |
| 2 | 8.56 | 255 | C30H46O8 | 12-hydroxyganoderic acid C2 | 533.3024 | 515.2899, 497.2874, 485.2865, 467.2719, 453.2908, 423.2751, 405.2756, 303.1559 | [18] |
| 3 | 10.39 | 256 | C30H40O8 | Elfvingic acid A | 527.2595 | 509.2453, 465.2562, 421.2668, 317.1699 | [18] |
| 4 | 14.09 | 255 | C30H44O8 | Ganoderic acid η | 531.2866 | 513.2777, 129.0527, 111.0425 | [17] |
| 5 | 19.29 | 256 | C30H46O7 | Ganoderic acid C2 | 517.3029 | 499.2965, 455.3075, 437.2979, 302.1813, 287.1616, 195.1028 | [17] |
| 6 | 20.95 | 254 | C30H44O8 | Ganoderic acid G | 531.2929 | 513.2783, 469.2913, 451.2769, 436.2610, 319.1892, 265.1389, 249.1467 | [18] |
| 7 | 22.14 | - | C30H38O8 | Ganosporeric acid A | 525.2464 | 507.2344, 451.2106, 129.0529, 495.1996, 229.1176 | [19] |
| 8 | 24.11 | 256 | C30H42O8 | Ganodoeric acid C6 | 529.2715 | 511.2592, 493.2432, 481.2115, 467.2702, 449.2608, 437.2232, 317.1724, 303.1524 | [18] |
| 9 | 27.05 | 249 | C30H42O7 | Ganoderenic acid B | 513.2810 | 451.2839, 436.2592, 287.1642, 249.1462 | [18] |
| 10 | 27.99 | - | C30H42O7 | Ganoderic acid Xi | 513.2764 | 495.2726, 465.2214, 451.2825, 383.2162, 331.1900, 235.1690, 151.1109, 73.0285 | [19] |
| 11 | 29.98 | 253 | C30H44O7 | Ganoderic acid B | 515.2975 | 497.2840, 453.2940, 438.2717, 420.2620, 303.1926, 263.1626, 249.1457, 195.1362 | [18] |
| 12 | 31.98 | 263 | C30H42O7 | Ganoderic acid AM1 | 513.2782 | 495.2653, 451.2793, 436,2567, 421.2316, 249.1460 | [18] |
| 13 | 33.11 | 254 | C32H46O9 | Ganoderenic acid K | 571.2791 | 553.2698, 538.2423, 511.2628, 467.2710, 303.1897, 265.1393 | [18] |
| 14 | 34.48 | 256 | C32H46O9 | Ganoderic acid K | 573.3030 | 555.2904, 511.2988, 469.2920, 451.2807, 302.1843, 265.1405 | [18] |
| 15 | 37.86 | 253 | C30H44O7 | Ganoderic acid A | 515.2923 | 497.2807, 453.2919, 435.2819, 195.0978 | [17] |
| 16 | 38.65 | 254 | C32H44O9 | Ganoderic acid H | 571.2862 | 553.2768, 511.2671, 467.2768, 437.2306, 423.2668, 303.1578 | [18] |
| 17 | 41.61 | 250 | C27H36O6 | Lucidenic acid F | 455.2348 | 425.1831, 395.2175, 383.2162, 301.1748, 247.1287, 149.0581 | [18] |
| 18 | 42.14 | - | - | 12-hydroxy-3,7,11,15,23-pentaoxo-lanost-8-en-26-oic acid | 527.2552 | 509.2541, 465.2643, 435.2168, 301.1433 | [18] |
| 19 | 43.23 | 254 | C30H40O7 | Ganoderic acid E | 511.2671 | 493.2484, 449.2638, 434.2381, 285.1442 | [17] |
| 20 | 45.26 | 256 | C30H42O8 | 12-hydroxyganoderic acid D | 529.2702 | 511.2629, 493.2510, 449.2618, 434.2406, 301.1764 | [18] |
| 21 | 47.54 | 256 | C30H42O7 | Ganoderic acid D | 513.2782 | 495.2657, 451.2789, 301.1766, 283.1649, 247.1302, 193.1199 | [18] |
| 22 | 50.92 | 260 | C32H42O9 | Ganoderic acid F | 511.2602 | 493.2537, 449.2638, 434.2404, 247.1307, 149.0509 | [18] |
| 23 | 54.92 | 250 | C32H42O9 | 12-acetoxyganoderic acid F | 569.2656 | 511.2525, 509.2514, 479.2054, 465.2617, 435.2144 | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.-J.; Huang, Z.-R.; You, S.-Z.; Guo, W.-L.; Zhang, F.; Liu, B.; Lv, X.-C.; Lin, Z.-X.; Liu, P.-H. The Protective Effects of Ganoderic Acids from Ganoderma lucidum Fruiting Body on Alcoholic Liver Injury and Intestinal Microflora Disturbance in Mice with Excessive Alcohol Intake. Foods 2022, 11, 949. https://doi.org/10.3390/foods11070949
Cao Y-J, Huang Z-R, You S-Z, Guo W-L, Zhang F, Liu B, Lv X-C, Lin Z-X, Liu P-H. The Protective Effects of Ganoderic Acids from Ganoderma lucidum Fruiting Body on Alcoholic Liver Injury and Intestinal Microflora Disturbance in Mice with Excessive Alcohol Intake. Foods. 2022; 11(7):949. https://doi.org/10.3390/foods11070949
Chicago/Turabian StyleCao, Ying-Jia, Zi-Rui Huang, Shi-Ze You, Wei-Ling Guo, Fang Zhang, Bin Liu, Xu-Cong Lv, Zhan-Xi Lin, and Peng-Hu Liu. 2022. "The Protective Effects of Ganoderic Acids from Ganoderma lucidum Fruiting Body on Alcoholic Liver Injury and Intestinal Microflora Disturbance in Mice with Excessive Alcohol Intake" Foods 11, no. 7: 949. https://doi.org/10.3390/foods11070949
APA StyleCao, Y.-J., Huang, Z.-R., You, S.-Z., Guo, W.-L., Zhang, F., Liu, B., Lv, X.-C., Lin, Z.-X., & Liu, P.-H. (2022). The Protective Effects of Ganoderic Acids from Ganoderma lucidum Fruiting Body on Alcoholic Liver Injury and Intestinal Microflora Disturbance in Mice with Excessive Alcohol Intake. Foods, 11(7), 949. https://doi.org/10.3390/foods11070949










