Multiple Technologies Combined to Analyze the Changes of Odor and Taste in Daokou Braised Chicken during Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. GC-MS Analysis
2.2. GC-IMS Analysis
2.3. E-Nose Analysis
2.4. E-Tongue Analysis
2.5. Data Treatment
3. Results and Discussion
3.1. Analysis of GC-MS
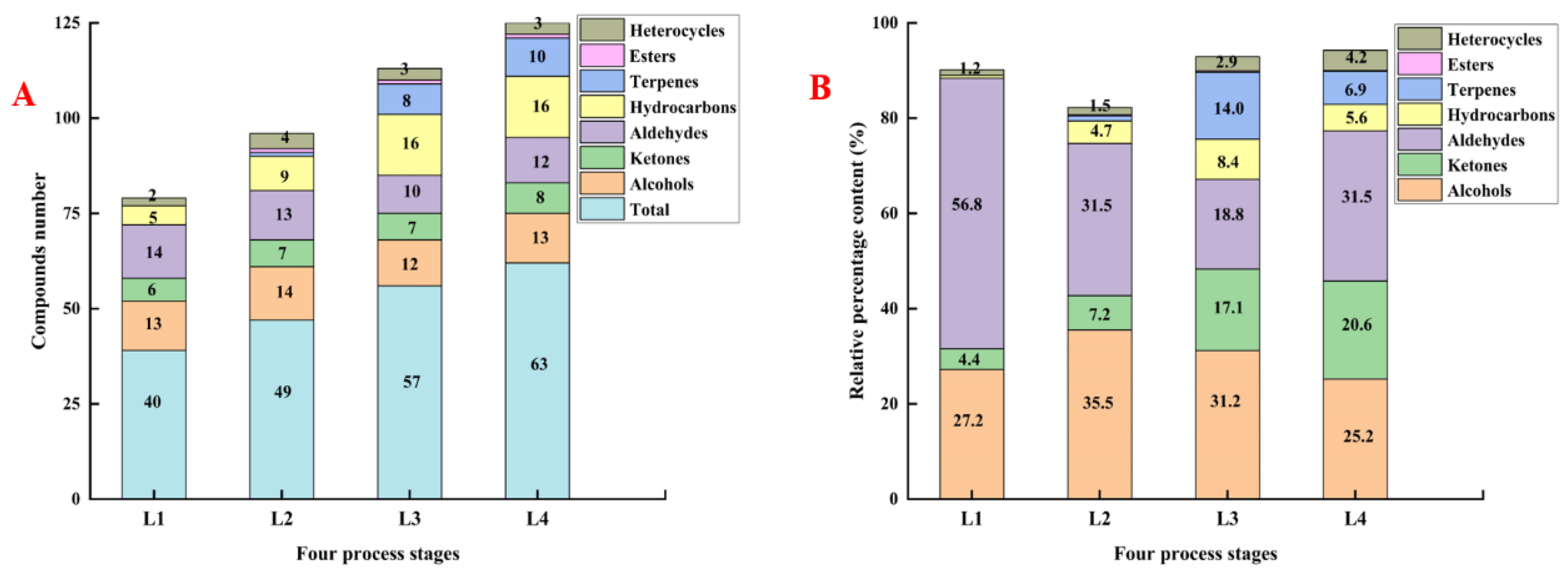
3.1.1. Volatile Compounds in Different Processing Stages
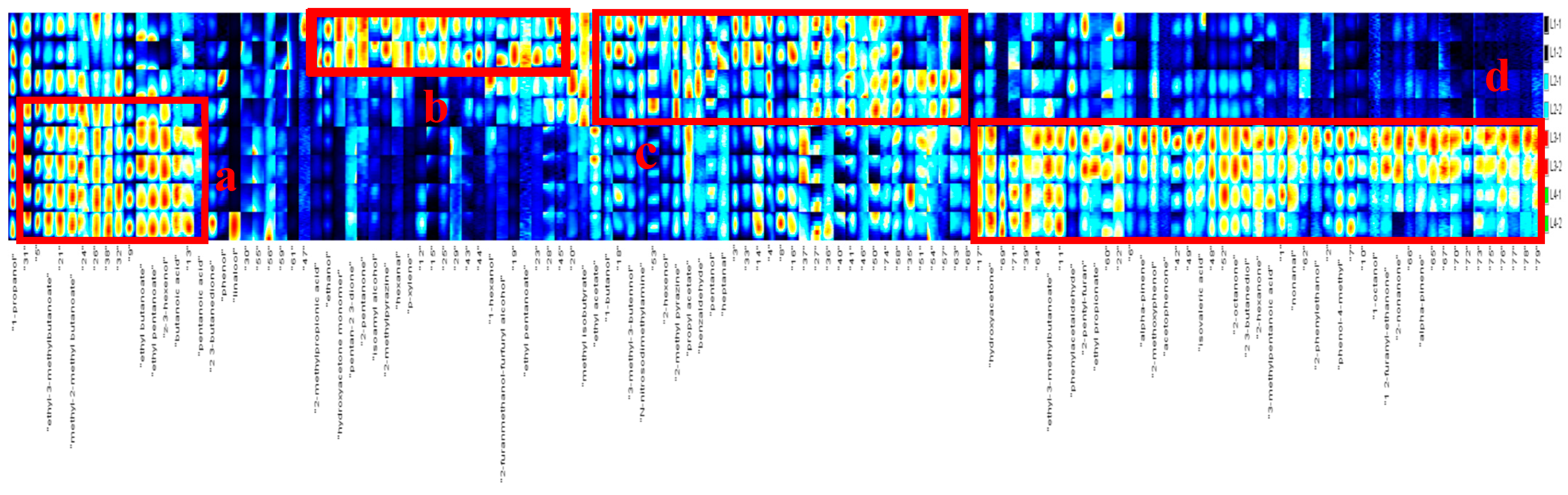
3.1.2. Analysis of Heat Map of Volatile Compounds
3.2. Analysis of GC-IMS
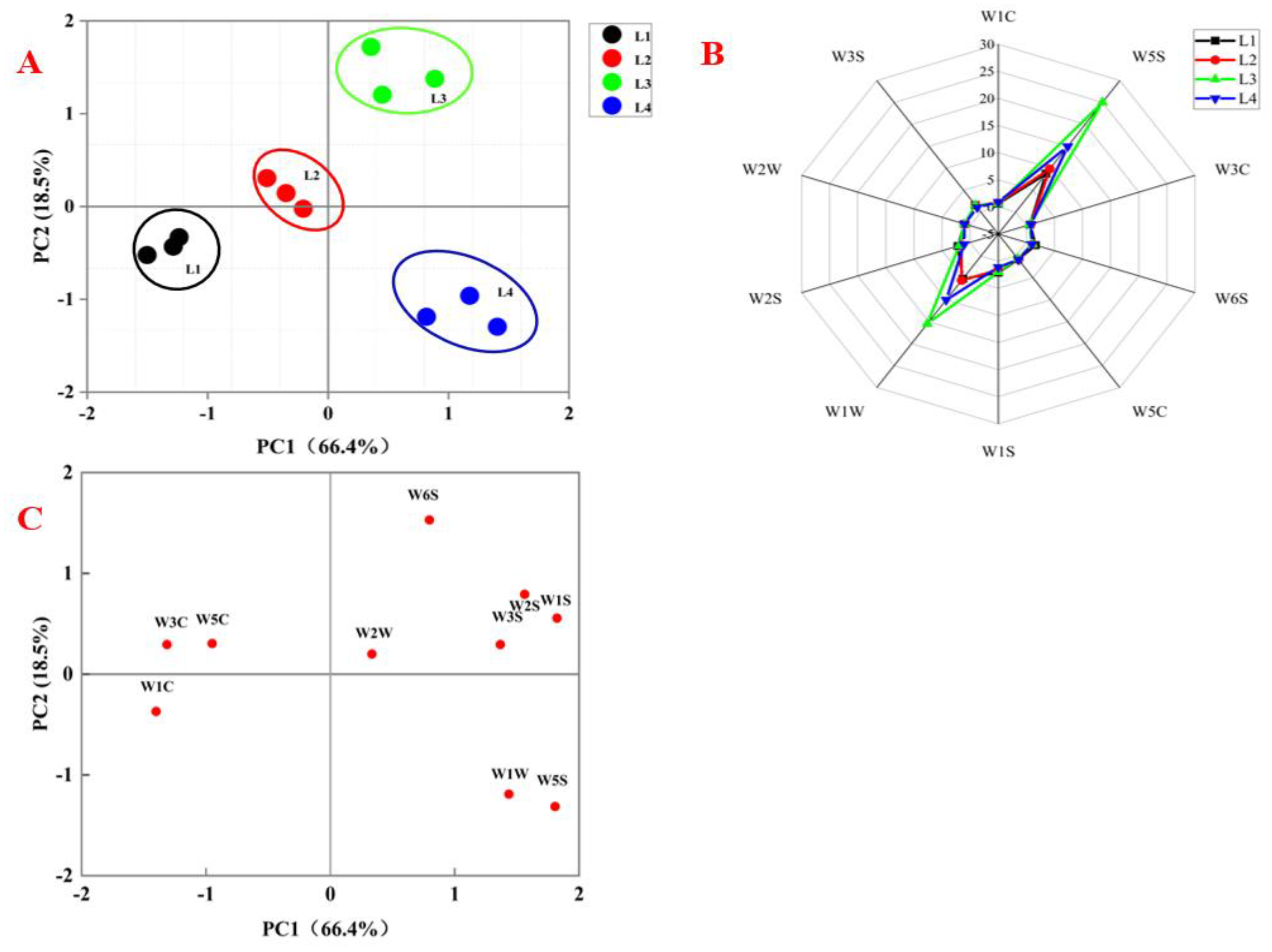
3.2.1. Topographic Analysis
3.2.2. Fingerprints Analysis
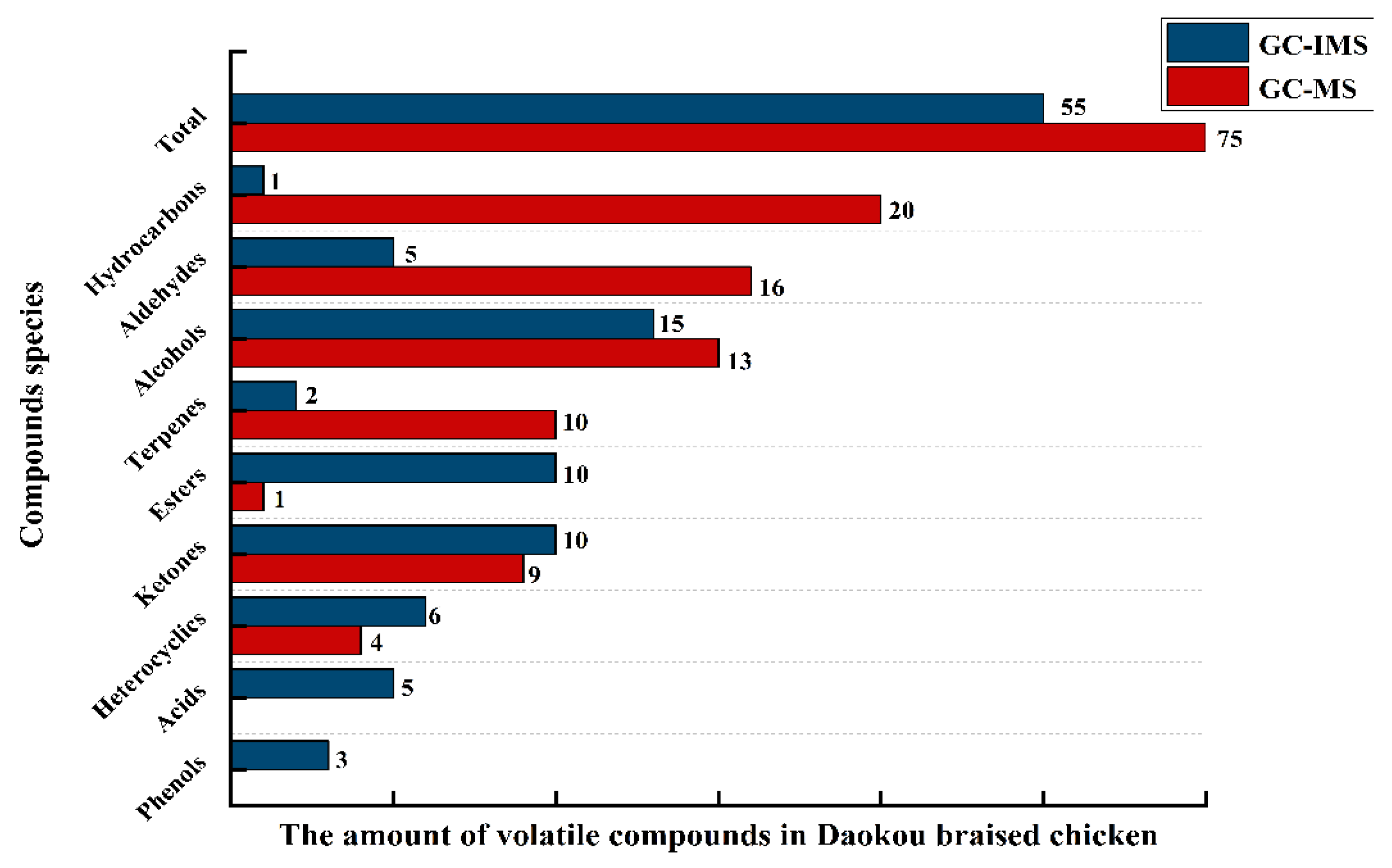
3.3. Comparison of GC-MS and GC-IMS
3.4. Analysis of E-Nose
3.5. E-Tongue Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravana, P.S.; Getachew, A.T.; Ahmed, R.; Cho, Y.-J.; Lee, Y.-B.; Chun, B.-S. Optimization of phytochemicals production from the ginseng by-products using pressurized hot water: Experimental and dynamic modelling. Biochem. Eng. J. 2016, 113, 141–151. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, C.; Hou, L.; Liu, J.; Jiang, S.; Liu, Q. Extraction of Oleoresin from Dao-Kou Roasted Chicken Flavor Spice Blends Using Supercritical Carbon Dioxide. Food Anal. Methods 2016, 10, 900–909. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, G.; Yin, X.; Ge, C.; Liao, G. Effects of different cooking methods on free fatty acid profile, water-soluble compounds and flavor compounds in Chinese Piao chicken meat. Food Res. Int. 2021, 149, 110696. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Toldra, F.; Mora, L. Quantification and in silico analysis of taste dipeptides generated during dry-cured ham processing. Food Chem. 2022, 370, 130977. [Google Scholar] [CrossRef]
- Yao, W.; Cai, Y.; Liu, D.; Chen, Y.; Li, J.; Zhang, M.; Chen, N.; Zhang, H. Analysis of flavor formation during production of Dezhou braised chicken using headspace-gas chromatography-ion mobility spec-trometry (HS-GC-IMS). Food Chem. 2022, 370, 130989. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, F.; Chen, H.; Huang, M.; Xie, J.; Chen, F.; Sun, B. Analysis of volatiles in Dezhou Braised Chicken by comprehensive two-dimensional gas chromatography/high resolution-time of flight mass spectrometry. LWT—Food Sci. Technol. 2015, 60, 1235–1242. [Google Scholar] [CrossRef]
- Liu, D.; Li, S.; Wang, N.; Deng, Y.; Sha, L.; Gai, S.; Liu, H.; Xu, X. Evolution of Taste Compounds of Dezhou-Braised Chicken during Cooking Evaluated by Chemical Analysis and an Electronic Tongue System. J. Food Sci. 2017, 82, 1076–1082. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT 2021, 140, 110764. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Zhang, J.; Xu, L.; Zhou, H.; Wei, K.; Peng, L.; Zhang, J.; Liu, Z.; Wei, X. Integration of non-targeted metabolomics and E-tongue evaluation reveals the chemical variation and taste characteristics of five typical dark teas. LWT 2021, 150, 111875. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Jia, X.; Zhao, Q.; Ma, Q.; Yu, Y.; Tang, C.; Zhang, J. Characterization of the Flavor Precursors and Flavor Fingerprints in Grazing Lambs by Foodomics. Foods 2022, 11, 191. [Google Scholar] [CrossRef]
- Xia, C.; He, Y.; Cheng, S.; He, J.; Pan, D.; Cao, J.; Sun, Y. Free fatty acids responsible for characteristic aroma in various sauced-ducks. Food Chem. 2021, 343, 128493. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Martin-Gomez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs. spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace—Gas chromatography-ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Aheto, J.H.; Huang, X.; Zheng, K.; Dai, C.; Wang, C.; Bai, J.W. An evaluation of biochemical, structural and volatile changes of dry-cured pork using a combined ion mobility spectrometry, hyperspectral and confocal imaging approach. J. Sci. Food Agric. 2021, 101, 5972–5983. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Duan, Z.; Dong, S.; Dong, Y.; Gao, Q. Geographical origin identification of two salmonid species via flavor compound analysis using headspace-gas chromatography-ion mobility spectrometry combined with electronic nose and tongue. Food Res. Int. 2021, 145, 110385. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Zhu, Z.; Lei, Y.; Huang, S.; Huang, M. Effect of ageing time on the flavour compounds in Nanjing water-boiled salted duck detected by HS-GC-IMS. LWT 2021, 155, 112870. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Tian, X.; Li, Z.J.; Chao, Y.Z.; Wu, Z.Q.; Zhou, M.X.; Xiao, S.T.; Zeng, J.; Zhe, J. Evaluation by electronic tongue and headspace-GC-IMS analyses of the flavor compounds in dry-cured pork with different salt content. Food Res. Int. 2020, 137, 109456. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1099, 46–55. [Google Scholar] [CrossRef]
- Sun, L.; Chen, J.; Li, M.; Liu, Y.; Zhao, G. Effect of Star Anise (Illicium verum) on the Volatile Compounds of Stewed Chicken. J. Food Process Eng. 2014, 37, 131–145. [Google Scholar] [CrossRef]
- Yin, X.; Lv, Y.; Wen, R.; Wang, Y.; Chen, Q.; Kong, B. Characterization of selected Harbin red sausages on the basis of their flavour profiles using HS-SPME-GC/MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108345. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, J.; Wang, X.; Wang, R.; Ren, F.; Zhang, Q.; Shan, Y.; Ding, S. Characterization of Volatile Component Changes in Jujube Fruits during Cold Storage by Using Headspace-Gas Chromatography-Ion Mobility Spectrometry. Molecules 2019, 24, 3904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariutti, L.R.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Canedo, A.; Martinez-Onandi, N.; Gaya, P.; Nunez, M.; Picon, A. Effect of high-pressure processing and chemical composition on lipid oxidation, aminopeptidase activity and free amino acids of Serrano dry-cured ham. Meat Sci. 2021, 172, 108349. [Google Scholar] [CrossRef] [PubMed]
- MacNELL, J.; Dimick, P. Poultry product quality. 1. Compositional changes during cooking of turkey roasts. J. Food Sci. 1970, 35, 184–186. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Mottram, D.S. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 2009, 31, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, X.; Dai, Z. Comparison of nonvolatile and volatile compounds in raw, cooked, and canned yellowfin tuna (Thunnus albacores). J. Food Process. Preserv. 2019, 43, 14111. [Google Scholar] [CrossRef]
- Wall, K.R.; Kerth, C.R.; Miller, R.K.; Alvarado, C. Grilling temperature effects on tenderness, juiciness, flavor and volatile aroma compounds of aged ribeye, strip loin, and top sirloin steaks. Meat Sci. 2019, 150, 141–148. [Google Scholar] [CrossRef]
- Tian, P.; Zhan, P.; Tian, H.; Wang, P.; Lu, C.; Zhao, Y.; Ni, R.; Zhang, Y. Analysis of volatile compound changes in fried shallot (Allium cepa L. var. aggregatum) oil at different frying temperatures by GC-MS, OAV, and multivariate analysis. Food Chem. 2021, 345, 128748. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Liao, P.; Chen, L.; Sun, J.; Sun, B.; Zhao, D.; Wang, B.; Li, H. HS-SPME Combined with GC-MS/O to Analyze the Flavor of Strong Aroma Baijiu Daqu. Foods 2022, 11, 116. [Google Scholar] [CrossRef]
- Wang, D.D.; Tang, Y.; Chen, G.; Li, H.; Ming, J.Y.; Cai, D.F.; Wang, Y.; Wu, Y.; Zhang, Q.S. Dynamic analysis of volatile components of salted radish during different fermentation processes. Food Sci. 2020, 41, 146–154. [Google Scholar]
- Xu, J.; Zhang, M.; Wang, Y.; Bhandari, B. Novel Technologies for Flavor Formation in the Processing of Meat Products: A Review. Food Rev. Int. 2021, 1–25. [Google Scholar] [CrossRef]
- Hwang, H.S.; Ball, J.C.; Doll, K.M.; Anderson, J.E.; Vermillion, K. Investigation of polymers and alcohols produced in oxidized soybean oil at frying temperatures. Food Chem. 2020, 317, 126379. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tao, L.; Zhang, T.; Zhang, J.; Wu, T.; Luan, D.; Ni, L.; Wang, X.; Zhong, J. Effect of four types of thermal processing methods on the aroma profiles of acidity regulator-treated tilapia muscles using E-nose, HS-SPME-GC-MS, and HS-GC-IMS. LWT 2021, 147, 111585. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC x GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y.; Du, W.; Jin, L.; Ren, P.; Ren, F.; Xie, J.C. Comparative analysis of aroma compounds in Chinese traditional dry-rendered fat by HS/GC-IMS, SPME/GC-MS, and SPME/GC-O. J. Food Compos. Anal. 2022, 107, 104378. [Google Scholar] [CrossRef]
- Pu, D.; Zhang, H.; Zhang, Y.; Sun, B.; Ren, F.; Chen, H.; He, J. Characterization of the aroma release and perception of white bread during oral processing by gas chromatography-ion mobility spectrometry and temporal dominance of sensations analysis. Food Res. Int. 2019, 123, 612–622. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef]
- Lantsuzskaya, E.V.; Krisilov, A.V.; Levina, A.M. Structure of the cluster ions of ketones in the gas phase according to ion mobilityspectrometry and ab initio calculations. Rus. J. Phys. Chem. A 2015, 89, 1838–1842. [Google Scholar] [CrossRef]
- Liu, D.; Bai, L.; Feng, X.; Chen, Y.P.; Zhang, D.; Yao, W.; Zhang, H.; Chen, G.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar] [CrossRef]
- Zoldan, S.M.; Braga, G.d.S.; Fonseca, F.J.; Carrão-Panizzi, M.C. Electronic tongue system to evaluate flavor of soybean (Gly-cine Max (L.) Merrill) genotypes. Braz. Arch. Biol. Technol. 2014, 57, 797–802. [Google Scholar] [CrossRef] [Green Version]




| NO. | Compounds | Relative Percentage Content (%) | |||
|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | ||
| 1 | (Z)-3-Nonen-2-ol | - | 0.02 ± 0.00 b | 0.07 ± 0.01 a | 0.06 ± 0.01 a |
| 2 | 1-Penten-3-ol | 0.05 ± 0.01 b | 0.09 ± 0.00 b | - | 0.52 ± 0.58 a |
| 3 | 3-Methyl-1-butanol | 0.34 ± 0.04 b | 0.28 ± 0.06 b | 1.89 ± 0.52 a | - |
| 4 | 1-Pentanol | 1.84 ± 0.13 | 3.37 ± 0.79 | 2.78 ± 0.86 | 2.16 ± 0.47 |
| 5 | 1-Hexanol | 8.59 ± 0.35 b | 7.88 ± 0.74 b | 11.57 ± 1.05 a | 7.92 ± 2.17 b |
| 6 | 1-Octen-3-ol | 7.20 ± 0.05 b | 11.80 ± 0.34 a | 7.65 ± 1.32 b | 9.25 ± 0.01 a,b |
| 7 | 1-Heptanol | 2.39 ± 0.23 a | 2.00 ± 0.08 a | 0.85 ± 0.02 b | 0.63 ± 0.27 b |
| 8 | 2,6-Dimethyl-4-heptanol | - | 0.24 ± 0.01 | 0.77 ± 0.18 | 0.29 ± 0.25 |
| 9 | 2,4-Dimethyl-cyclohexanol | 0.35 ± 0.14 b | 0.91 ± 0.12 a | 0.84 ± 0.06 a | 0.38 ± 0.06 b |
| 10 | 1-Octanol | 3.82 ± 0.49 a | 2.88 ± 0.21 b | 1.07 ± 0.08 c | 0.81 ± 0.00 c |
| 11 | (E)-2-Octen-1-ol | 1.22 ± 0.04 b | 2.50 ± 0.47 a | 1.18 ± 0.05 b | 1.52 ± 0.13 b |
| 12 | 1-Nonen-4-ol | 0.55 ± 0.00 c | 2.10 ± 0.49 a | 1.92 ± 0.31 a,b | 1.20 ± 0.06 b,c |
| 13 | 1-Nonanol | 0.74 ± 0.17 b | 1.25 ± 0.27 a | 0.66 ± 0.06 b | 0.31 ± 0.02 b |
| 14 | 2-Ethyl-1-hexanol | 0.17 ± 0.05 | - | - | - |
| 15 | (E, Z)-2,4-Decadien-1-ol | 0.06 ± 0.01 c | 0.21 ± 0.01 a | - | 0.15 ± 0.03 b |
| Total alcohols | 27.28 ± 0.23 b | 35.50 ± 3.34 a | 31.21 ± 4.34 a,b | 25.16 ± 1.96 b | |
| 16 | 2-Butanone | - | - | - | 0.14 ± 0.02 |
| 17 | 2,3-Butanedione | - | - | 2.52 ± 0.10 | 2.86 ± 0.72 |
| 18 | 2,3-Pentanedione | - | 0.06 ± 0.01 b | 0.29 ± 0.02 a | 0.30 ± 0.04 a |
| 19 | 2,3-Heptanedione | 0.48 ± 0.52 | 0.10 ± 0.03 | 0.47 ± 0.11 | 0.81 ± 0.04 |
| 20 | 6-Methyl-2-Heptanone | 0.07 ± 0.04 | 0.11 ± 0.06 | - | - |
| 21 | 3-Hydroxy-2-butanone | 0.27 ± 0.08 b | 1.45 ± 0.21 b | 7.06 ± 0.43 a | 5.97 ± 1.51 a |
| 22 | 4-Octen-3-one | 0.17 ± 0.02 | 0.13 ± 0.02 | 0.21 ± 0.03 | 0.25 ± 0.05 |
| 23 | 2,3-Octanedione | 3.42 ± 0.78 c | 5.24 ± 0.49 b | 6.24 ± 0.02 b | 10.19 ± 0.18 a |
| 24 | 2-Nonanone | 0.06 ± 0.01 b | 0.13 ± 0.01 b | 0.37 ± 0.11 a | 0.11 ± 0.04 b |
| Total ketones | 4.43 ± 0.27 c | 7.21 ± 0.83 c | 17.13 ± 0.39 b | 20.58 ± 1.98 a | |
| 25 | 3-Methyl-butanal | - | - | - | 0.20 ± 0.02 |
| 26 | Pentanal | 1.53 ± 0.02 | 1.31 ± 0.06 | - | - |
| 27 | Hexanal | 26.62 ± 0.16 a | 17.21 ± 1.10 b | 9.36 ± 2.78 c | 18.71 ± 2.76 b |
| 28 | Heptanal | 4.30 ± 0.52 a | 2.02 ± 0.33 b | 2.12 ± 0.23 b | 2.07 ± 0.08 b |
| 29 | Octanal | 6.12 ± 0.29 a | 2.39 ± 0.22 b | 0.86 ± 0.02 c | 1.19 ± 0.45 c |
| 30 | Nonanal | 15.18 ± 0.22 a | 6.31 ± 0.53 b | 3.86 ± 0.05 b | 5.12 ± 1.75 b |
| 31 | 2-Octenal | 0.57 ± 0.14 | 0.79 ± 0.26 | 0.54 ± 0.09 | 0.56 ± 0.03 |
| 32 | Decanal | 0.70 ± 0.01 | 0.68 ± 0.13 | - | - |
| 33 | Benzaldehyde | 0.21 ± 0.04 | 0.19 ± 0.05 | 0.37 ± 0.04 | 1.18 ± 0.61 |
| 34 | 2-Nonenal | 0.32 ± 0.07 | 0.32 ± 0.10 | 0.26 ± 0.06 | 0.34 ± 0.06 |
| 35 | Z-4-Decenal | - | - | - | 0.44 ± 0.05 |
| 36 | Undecanal | 0.06 ± 0.01 b | 0.14 ± 0.01 b | 0.67 ± 0.23 a | 0.32 ± 0.13 a,b |
| 37 | (E, E)-2,4-Nonadienal | 0.10 ± 0.01 | 0.11 ± 0.01 | - | - |
| 38 | Trans-2-undecenal | 0.27 ± 0.06 | 0.13 ± 0.02 | - | - |
| 39 | (E, E)-2,4-decadienal | 0.33 ± 0.00 b | 0.43 ± 0.03 a | 0.24 ± 0.03 c | 0.27 ± 0.02 c |
| 40 | Tetradecanal | 0.47 ± 0.07 | - | 0.58 ± 0.29 | 1.00 ± 0.35 |
| Total aldehydes | 56.76 ± 0.35 a | 32.02 ± 2.34 b | 18.82 ± 2.93 c | 31.51 ±6.13 b | |
| 41 | Decane | - | - | 0.11 ± 0.01 | 0.08 ± 0.01 |
| 42 | Methyl-benzene | 0.23 ± 0.15 c | 0.60 ± 0.08 b | 1.05 ± 0.17 a | 1.02 ± 0.04 a |
| 43 | Undecane | 0.07 ± 0.01 b | 0.13 ± 0.04 a,b | 0.22 ± 0.04 a | - |
| 44 | Ethylbenzene | 0.06 ± 0.01 c | 0.12 ± 0.01 c | 0.36 ± 0.04 a | 0.24 ± 0.03 b |
| 45 | 1,3-Dimethyl-benzene | - | - | - | 0.21 ± 0.01 |
| 46 | (Z)-3-Dodecene | - | - | 1.15 ± 0.57 | - |
| 47 | Dodecane | - | 0.12 ± 0.02 b | 0.42 ± 0.02 a | 0.32 ± 0.05 a |
| 48 | 1-Ethyl-4-methyl-benzene | - | - | - | 0.20 ± 0.01 |
| 49 | 1-Ethyl-2-methyl-benzene | - | - | 0.17 ± 0.04 | - |
| 50 | 1-Ethyl-3-methyl-benzene | - | - | - | 0.11 ± 0.01 |
| 51 | 4,7-Methano-1H-indene, octahydro- | - | - | 0.19 ± 0.01 | 0.21 ± 0.01 |
| 52 | 1,2,3-Trimethyl-benzene | - | - | 0.12 ± 0.01 | 0.07 ± 0.01 |
| 53 | Tridecane | - | 0.56 ± 0.18 | 0.38 ± 0.25 | 0.44 ± 0.01 |
| 54 | Tetradecane | - | 0.66 ± 0.06 | 0.57 ± 0.04 | 0.53 ± 0.07 |
| 55 | 3-Ethyl-2-methyl-1,3-hexadiene | 0.16 ± 0.07 b | 0.37 ± 0.04 a | 0.17 ± 0.01 b | 0.18 ± 0.02 b |
| 56 | Nonadecane | - | - | 1.25 ± 0.06 | - |
| 57 | (2RS,3SR,4RS.5RS)-2,3: 4,5-Diepoxyhexan | - | 2.13 ± 0.05 | 1.80 ± 0.22 | 1.60 ± 0.19 |
| 58 | Heptadecane | 0.04 ± 0.01 b | 0.07 ± 0.01 b | 0.39 ± 0.13 a,b | 0.23 ± 0.06 a |
| 59 | 4-Decene, 2,2-dimethyl-, (E)- | - | - | - | 0.26 ± 0.10 |
| 60 | Octadecane | - | - | 0.16 ± 0.06 | 0.10 ± 0.01 |
| Total hydrocarbons | 0.55 ± 0.09 c | 4.73 ± 0.25 b | 8.47 ± 1.10 a | 5.62 ± 0.12 b | |
| 61 | Limonene | - | - | 0.73 ± 0.01 | 0.72 ± 0.11 |
| 62 | 1,8-Cineole | - | 1.05 ± 0.11 c | 11.28 ± 0.45 a | 4.38 ± 1.49 b |
| 63 | Trans-p-mentha-1(7),8-dien-2-ol | - | - | - | 0.06 ± 0.01 |
| 64 | Linalool | - | - | - | 0.23 ± 0.06 |
| 65 | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)- | - | - | 0.13 ± 0.01 | 0.21 ± 0.01 |
| 66 | α-Terpineol | - | - | 0.08 ± 0.00 | 0.10 ± 0.01 |
| 67 | β-Fenchol | - | - | 0.80 ± 0.11 | 0.60 ± 0.15 |
| 68 | Anethole | - | - | 0.23 ± 0.04 | 0.14 ± 0.02 |
| 69 | Trans-geraniol | - | - | 0.42 ± 0.02 | 0.25 ± 0.01 |
| 70 | (E)-Cinnamaldehyde | - | - | 0.43 ± 0.01 | 0.28 ± 0.01 |
| Total terpenes | - | 1.05 ± 0.18 c | 14.07 ± 0.07 a | 6.93 ± 0.31 b | |
| 71 | Acetic acid, hexyl ester | - | 0.27 ± 0.07 | 0.28 ± 0.04 | 0.20 ± 0.06 |
| Total esters | - | 0.27 ± 0.07 | 0.28 ± 0.04 | 0.20 ± 0.06 | |
| 72 | 2-Pentyl-furan | 0.29 ± 0.08 d | 1.09 ± 0.12 c | 1.38 ± 0.07 b | 1.98 ± 0.18 a |
| 73 | 2-Furanmethanol, 5-ethenyltetrahydro | - | 0.27 ± 0.07 b | 1.32 ± 0.21 a | 2.10 ± 0.47 a |
| 74 | Oxime-,methoxy-phenyl- | 0.66 ± 0.49 a | 0.14 ± 0.01 b | 0.21 ± 0.07 b | 0.19 ± 0.03 b |
| 75 | N,N-Dibutylformamide | - | 0.07 ± 0.01 | - | - |
| Total heterocycles | 1.20 ± 0.25 d | 1.54 ± 0.26 c | 2.94 ± 0.52 b | 4.22 ± 0.45 a | |
| a | b | c | d |
|---|---|---|---|
| Ethyl-3-methyl butanoate | 2-Methylpropionic acid | 1-Butanol | Hydroxyacetone |
| Methyl-2-methyl butanoate | Ethanol | 3-Methyl-3-butennol | Ethyl-3-methylbutanoate |
| Ethyl butanoate | Hydroxyacetone monomer | N-nitrosodimethylamine | Phenylacetaldehyde |
| Ethyl pentanoate | Pentan-2 3-dione | 2-Hexenol | 2-Pentyl-furan |
| Z-3-hexenol | 2-Pentanone | 2-Methyl pyrazine | Ethyl propionate |
| Butanoic acid | Isoamyl alcohol | Propyl acetate | Alpha-pinene |
| Pentanoic acid | 2-Methylpyrazine | Benzaldehyde | 2-Methoxyphenol |
| Hexanal | Pentanol | Acetophenone | |
| P-xylene | Heptanal | Isovaleric acid | |
| Hexanol | 2-Octanone | ||
| 2-Furanmethanol-furfuryl alcohol | 2 3-Butanediol | ||
| Ethyl pentanoate | 2-Hexanone | ||
| 3-Methylpentanoic acid | |||
| nonanal | |||
| 2-Phenylethanol | |||
| Phenol-4-methyl | |||
| 1-Octanol | |||
| 1 2-Furanyl-ethanone | |||
| 2-Nonanone | |||
| Alpha-pinene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, F.; Sun, L.; Zhao, G.; Li, M.; Zhu, C. Multiple Technologies Combined to Analyze the Changes of Odor and Taste in Daokou Braised Chicken during Processing. Foods 2022, 11, 963. https://doi.org/10.3390/foods11070963
Zhan F, Sun L, Zhao G, Li M, Zhu C. Multiple Technologies Combined to Analyze the Changes of Odor and Taste in Daokou Braised Chicken during Processing. Foods. 2022; 11(7):963. https://doi.org/10.3390/foods11070963
Chicago/Turabian StyleZhan, Feili, Lingxia Sun, Gaiming Zhao, Miaoyun Li, and Chaozhi Zhu. 2022. "Multiple Technologies Combined to Analyze the Changes of Odor and Taste in Daokou Braised Chicken during Processing" Foods 11, no. 7: 963. https://doi.org/10.3390/foods11070963
APA StyleZhan, F., Sun, L., Zhao, G., Li, M., & Zhu, C. (2022). Multiple Technologies Combined to Analyze the Changes of Odor and Taste in Daokou Braised Chicken during Processing. Foods, 11(7), 963. https://doi.org/10.3390/foods11070963






