Quantitative Microbial Risk Assessment of Listeria monocytogenes and Enterohemorrhagic Escherichia coli in Yogurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prevalence and Initial Contamination Level in an Offline Market
2.2. Physicochemical and Microbiological Analyses of Yogurt
2.3. Strain Preparation
2.4. Sample Preparation and Inoculation
2.5. Development of Primary and Secondary Model
2.6. Validation
2.7. Development of Scenario from Market to Home
2.8. Estimation of Consumption Data of Yogurt
2.9. Hazard Characterization
2.10. Risk Characterization
2.11. Statistical Analysis
3. Results and Discussion
3.1. Prevalence and Intial Contamination Level in an On- an Offline Market
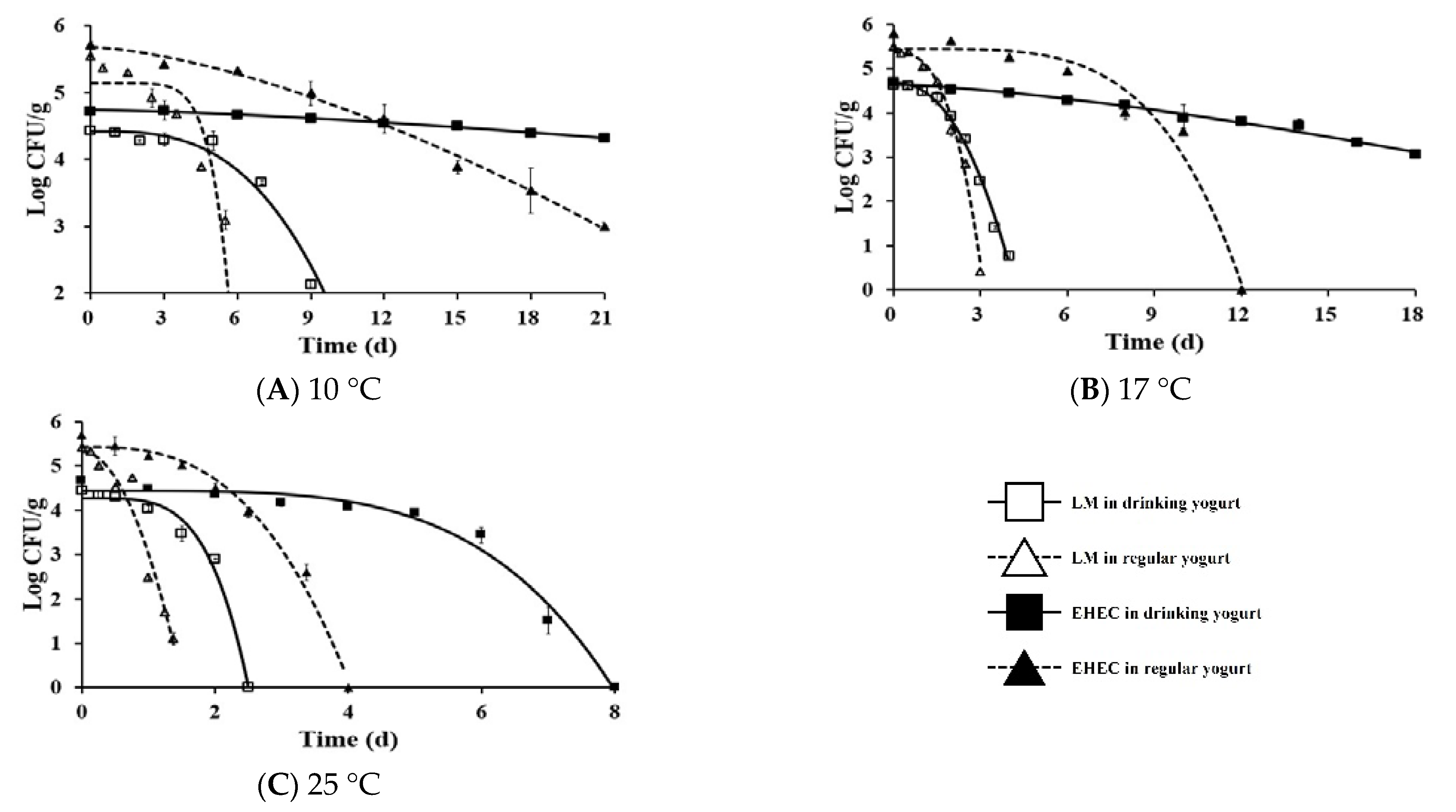
3.2. Development of Primary and Secondary Predictive Model
3.3. Validation
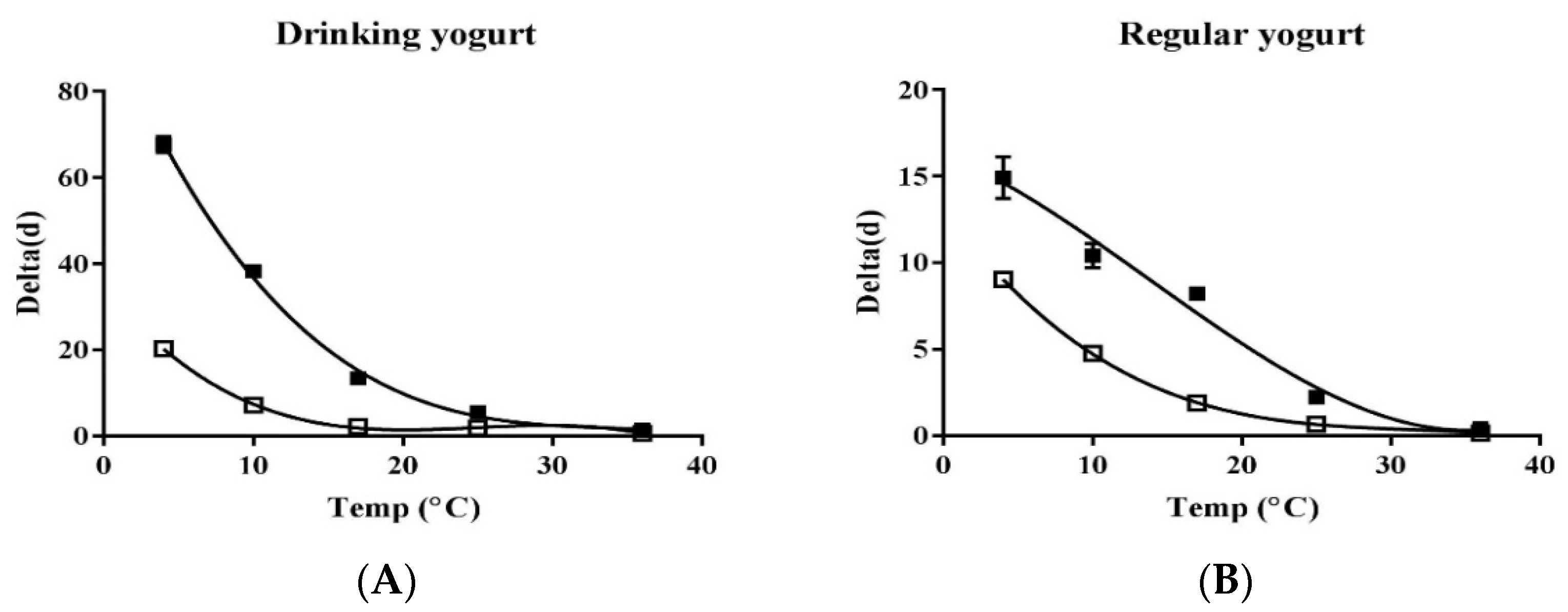
3.4. Change in Contamination Level of Listeria Monocytogenes and EHEC from Market to Home
3.5. Consumption Data of Yogurt
3.6. Hazard Characterization and Risk Characterization
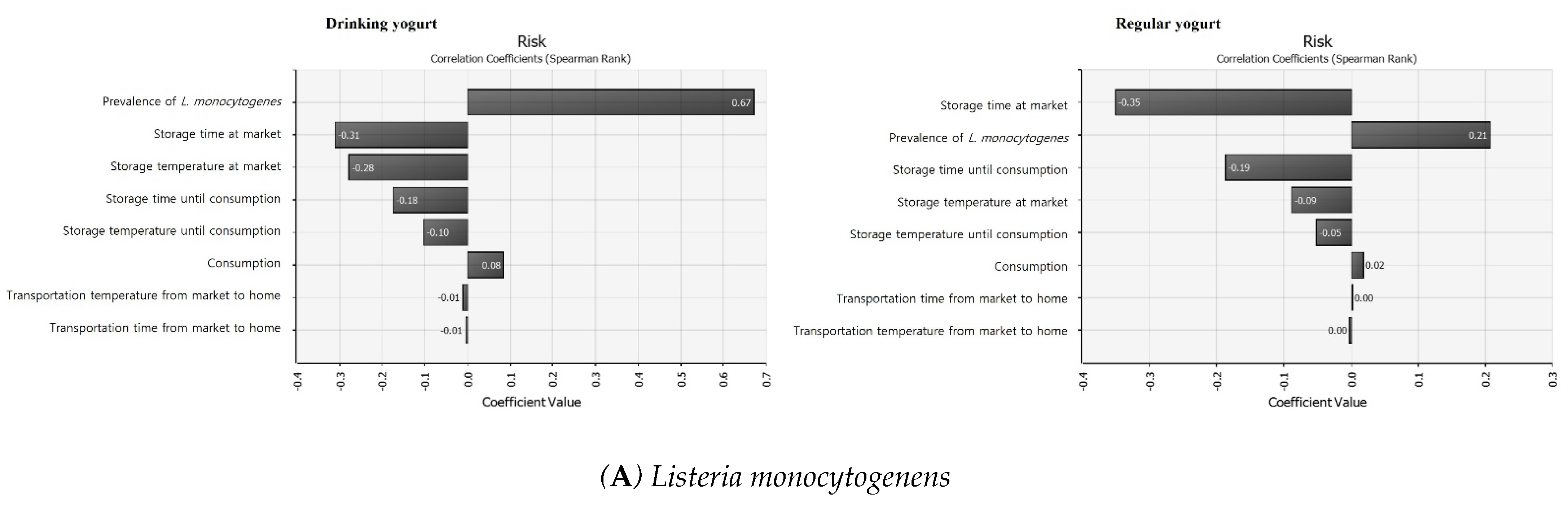
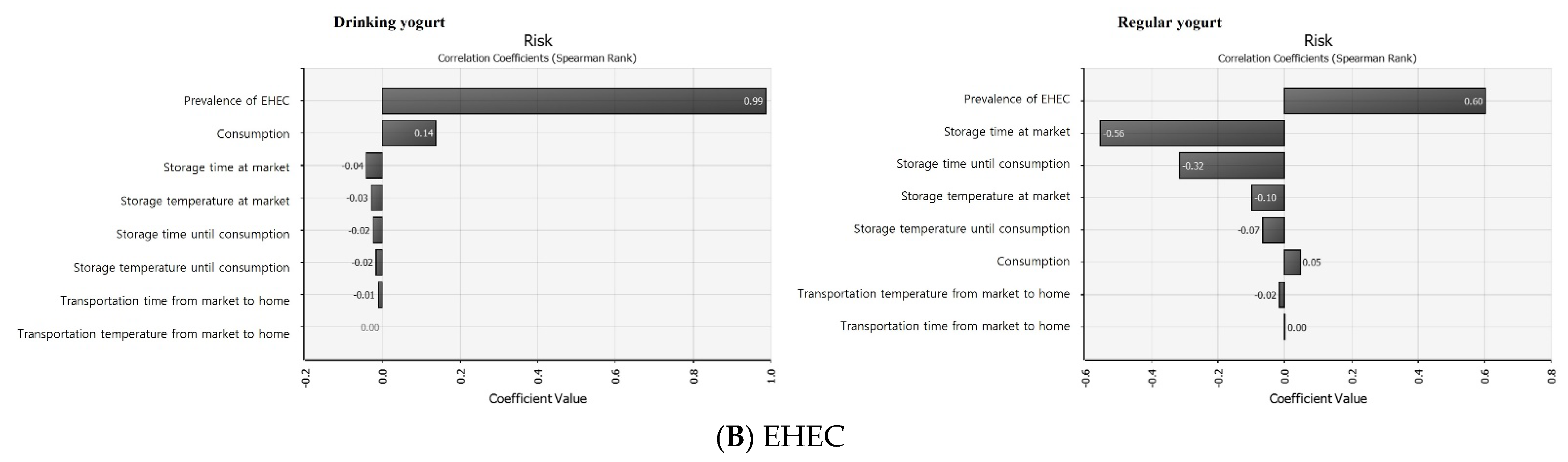
3.7. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamann, W.T.; Marth, E.H. Survival of Streptococcus thermophilus and Lactobacillus bulgaricus in commercial and experimental yogurts. J. Food Prot. 1984, 47, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Korea Health Supplements Association (KHSA). Consumer and Market Research Report. 2020. Available online: https://www.khsa.or.kr/user/info/InfoBoardUserView.do?_menuNo=374&boardSeqno=10039&postsSeqno=114886. (accessed on 18 August 2021).
- Korea Dairy Committee. Distribution Consumption Statistics (2010–2020). 2021. Available online: https://www.dairy.or.kr/kor/sub05/menu_01_5_1.php (accessed on 18 August 2021).
- Nyanzi, R.; Jooste, P.J.; Buys, E.M. Invited review: Probiotic yogurt quality criteria, regulatory framework, clinical evidence, and analytical aspects. J. Dairy Sci. 2021, 104, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Korea Disease Control and Prevention Agency (KDCA). The Prevalence of Pathogenic Escherichia coli Isolated by the Enteric Pathogens Active Surveillance Network (Enter-Net), 2010–2019. 2020. Available online: https://kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&act=view&list_no=368538 (accessed on 23 August 2021).
- Paton, J.C.; Paton, A.W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 1988, 11, 450–479. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Breidt, F.; Fratamico, P.; Oh, D.H. Acid resistance and molecular characterization of Escherichia coli O157: H7 and different non-O157 Shiga toxin-producing E. coli serogroups. J. Food Sci. 2015, 80, M2257–M2264. [Google Scholar] [CrossRef]
- Morgan, D.; Newman, C.P.; Hutchinson, D.N.; Walker, A.M.; Rowe, B.; Majid, F. Verotoxin producing Escherichia coli O 157 infections associated with the consumption of yoghurt. Epidemiol. Infect. 1993, 111, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Cutrim, C.S.; de Barros, R.F.; da Costa, M.P.; Franco, R.M.; Conte-Junior, C.A.; Cortez, M.A.S. Survival of Escherichia coli O157: H7 during manufacture and storage of traditional and low lactose yogurt. LWT 2016, 70, 178–184. [Google Scholar] [CrossRef]
- Moineau-Jean, A.; Guévremont, E.; Champagne, C.P.; Roy, D.; Raymond, Y.; LaPointe, G. Fate of Escherichia coli and Kluyveromyces marxianus contaminants during storage of Greek-style yogurt produced by centrifugation or ultrafiltration. Int. Dairy J. 2017, 72, 36–43. [Google Scholar] [CrossRef]
- Farber, J.M.; Peterkin, P. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef]
- Lado, B.; Yousef, A.E. Characteristics of Listeria monocytogenes Important to Food Processors. In Listeria, Listeriosis and Food Safety, 3rd ed.; Ryser, E.T., Marth, E.H., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Domınguez-Bernal, G.; Goebel, W.; Gonzaılez-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farver, J.M.; Ross, W.H.; Harwig, J. Health risk assessment of listeria monocytogenes in Canada. Int. J. Food Microbiol. 1996, 30, 145–156. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Listeria (Listeriosis). 2019. Available online: https://www.fda.gov/food/foodborne-pathogens/listeria-listeriosis (accessed on 28 July 2021).
- Ulusoy, B.H.; Chirkena, K. Two perspectives of Listeria monocytogenes hazards in dairy products: The prevalence and the antibiotic resistance. Food Qual. Saf. 2019, 3, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Lammerding, A.M. An overview of microbial food safety risk assessment. J. Food Prot. 1997, 60, 1420–1425. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Microbiological Risk Assessment: Guidance for Food. 2021. Available online: https://www.who.int/publications/i/item/9789240024892 (accessed on 15 August 2021).
- Codex. Principles and Guidelines for the Conduct of Microbiological Risk Assessment. 1999. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B30-1999%252FCXG_030e_2014.pdf (accessed on 14 July 2021).
- FAO/WHO. Food Safety Risk Analysis: A Guide for National Food Safety Authorities. 2006. Available online: http://apps.who.int/iris/bitstream/handle/10665/43718/9789251056042_eng.pdf;jsessionid=689C4D54F8646357776C37326910DD2A?sequence=1 (accessed on 14 August 2021).
- Makita, K.; Desissa, F.; Teklu, A.; Zewde, G.; Grace, D. Risk assessment of staphylococcal poisoning due to consumption of informally-marketed milk and home-made yoghurt in Debre Zeit, Ethiopia. Int. J. Food Microbiol. 2012, 153, 135–141. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Study on the Evaluation and Reduction of Risk of Listeria monocytogenes, Pathogenic Escherichia coli and Campylobacter spp. in Dairy Products; Ministry of Food and Drug Safety (MFDS): Cheongju-si, Korea, 2021.
- Ministry of Food and Drug Safety (MFDS). Food Code. Dairy Products. 2020. Available online: https://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=39 (accessed on 28 June 2021).
- Vose, D.J. The application of quantitative risk assessment to microbial food safety. J. Food Prot. 1998, 61, 640–648. [Google Scholar] [CrossRef]
- Sanaa, M.; Coroller, L.; Cerf, O. Risk assessment of listeriosis linked to the consumption of two soft cheeses made from raw milk: Camembert of Normandy and Brie of Meaux. Risk Anal. 2004, 24, 389–399. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Risk Assessment and reduction of Listeria monocytogenes in Agriculture, Livestock and Fishery Products. 2019. Available online: https://scienceon.kisti.re.kr/srch/selectPORSrchReport.do?cn=TRKO202000029896 (accessed on 4 August 2021).
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguérinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impe, J.F. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef]
- Mataragas, M.; Drosinos, E.H.; Vaidanis, A.; Metaxopoulos, I. Development of a predictive model for spoilage of cooked cured meat products and its validation under constant and dynamic temperature storage conditions. J. Food Sci. 2006, 71, M157–M167. [Google Scholar] [CrossRef]
- Jung, H. Consumer Survey and Hazard Analysis for the Improvement of Food Hygiene and Safety in Purchase. Master’s Thesis, Korea University, Seoul, Korea, 2011. [Google Scholar]
- Ministry of Food and Drug Safety (MFDS). Food Hygiene in the Home for Consumer Awareness Survey. 2009. Available online: https://scienceon.kisti.re.kr/commons/util/originalView.do?cn=TRKO201000014868&dbt=TRKO&rn= (accessed on 30 August 2021).
- Bahk, G.J. Statistical probability analysis of storage temperatures of domestic refrigerator as a risk factor of foodborne illness outbreak. Korean J. Food Sci. Technol. 2010, 42, 373–376. [Google Scholar]
- Park, J.H.; Cho, J.L.; Joo, I.S.; Heo, J.J.; Yoon, K.S. Estimation of Amount and Frequency of Consumption of 50 Domestic Livestock and Processed Livestock Products. J. Korean Soc. Food Sci Nutr. 2016, 45, 1177–1191. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations and the World Health Organization (FAO/WHO). Risk Assessment of Listeria Monocytogenes in Ready-to-Eat Foods: Technical Report. Microbiological Risk Assessment Series 5. 2004. Available online: https://apps.who.int/iris/handle/10665/42875 (accessed on 11 August 2021).
- Park, M.S.; Cho, J.L.; Lee, S.H.; Bahk, G.J. A study on dose-response models for foodborne disease pathogens. J. Food Hyg. Saf. 2014, 29, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Cheigh, H.S.; Park, K.Y. Biochemical, microbiological and nutritional aspects of kimchi (Korean fermented vegetable products). Crit. Rev. Food Sci. Nutr. 1994, 34, 175–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Han, J.; Bie, X.; Lu, Z.; Zhang, C.; Lv, F. Purification and characterization of plantaricin JLA-9: A novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a traditional Chinese fermented cabbage. J. Agric. Food Chem. 2016, 64, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, F.; Giarratana, F.; Caridi, A.; Sidari, R.; De Bruno, A.; Giuffrida, A. Lactic acid bacteria isolated from traditional Italian dairy products: Activity against Listeria monocytogenes and modelling of microbial competition in soft cheese. LWT 2021, 137, 110446. [Google Scholar] [CrossRef]
- Adamberg, K.; Kask, S.; Laht, T.M.; Paalme, T. The effect of temperature and pH on the growth of lactic acid bacteria: A pH-auxostat study. Int. J. Food Microbiol. 2003, 85, 171–183. [Google Scholar] [CrossRef]
- Choi, S.J.; Yang, S.Y.; Yoon, K.S. Lactic acid bacteria starter in combination with sodium chloride controls pathogenic Escherichia coli (EPEC, ETEC, and EHEC) in kimchi. Food Microbiol. 2021, 100, 103868. [Google Scholar] [CrossRef]
- Bachrouri, M.; Quinto, E.J.; Mora, M.T. Survival of Escherichia coli O157: H7 during storage of yogurt at different temperatures. J. Food Sci. 2002, 67, 1899–1903. [Google Scholar] [CrossRef]
- Szczawinski, J.; Szczawinska, M.E.; Lobacz, A.; Jackowska-Tracz, A. Modeling the effect of temperature on survival rate of Listeria monocytogenes in yogurt. Pol. J. Vet. Sci. 2016, 19. [Google Scholar] [CrossRef]
- Millet, L.; Saubusse, M.; Didienne, R.; Tessier, L.; Montel, M.C. Control of Listeria monocytogenes in raw-milk cheeses. Int. J. Food Microbiol. 2006, 108, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Guraya, R.; Frank, J.F.; Hassan, A.N. Effectiveness of salt, pH, and diacetyl as inhibitors for Escherichia coli O157: H7 in dairy foods stored at refrigeration temperatures. J. Food Prot. 1998, 61, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Faradonbeh, R.S.; Armaghani, D.J.; Monjezi, M. Development of a new model for predicting flyrock distance in quarry blasting: A genetic programming technique. Bull. Eng. Geol. Environ. 2016, 75, 993–1006. [Google Scholar] [CrossRef]
- Coşansu, S. Survival kinetics of heat-stressed Escherichia coli O157: H7 and Listeria monocytogenes cells as post-fermentation contaminants in kefir during refrigerated storage. LWT 2018, 98, 635–641. [Google Scholar] [CrossRef]
- Hu, C.H.; Ren, L.Q.; Zhou, Y.; Ye, B.C. Characterization of antimicrobial activity of three Lactobacillus plantarum strains isolated from Chinese traditional dairy food. Food Sci. Nutr. 2019, 7, 1997–2005. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.; Shimizu, K.; Nomoto, K.; Tanaka, R.; Hamabata, T.; Yamasaki, S.; Takeda, T.; Takeda, Y. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157: H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 2001, 68, 135–140. [Google Scholar] [CrossRef]
- Yang, E.; Fan, L.; Jiang, Y.; Doucette, C.; Fillmore, S. Antimicrobial activity of bacteriocin-producing lactic acid bacteria isolated from cheeses and yogurts. AMP Express. 2012, 2, 48. Available online: https://amb-express.springeropen.com/articles/10.1186/2191-0855-2-48 (accessed on 28 July 2021). [CrossRef] [Green Version]
- Gulmez, M.; Guven, A. Survival of Escherichia coli O157: H7, Listeria monocytogenes 4b and Yersinia enterocolitica O3 in different yogurt and kefir combinations as prefermentation contaminant. J. Appl. Microbiol. 2003, 95, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Afrin, S.; Hoque, M.A.; Sarker, A.K.; Satter, M.A.; Bhuiyan, M.N.I. Characterization and profiling of bacteriocin-like substances produced by lactic acid bacteria from cheese samples. Access Microbiol. 2021, 3, 000234. [Google Scholar] [CrossRef]
- Vose, D.J. Understanding and Using the Results of a Risk Analysis. Risk Analysis: A Quantitative Guide; John Wiley Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Akkaya, L.; Telli, R.; Sagdic, O. Growth-death kinetics of Listeria monocytogenes in strained yogurt. Int. J. Food Prop. 2009, 12, 705–712. [Google Scholar] [CrossRef]
- Kasımoğlu, A.; Akgün, S. Survival of Escherichia coli O157: H7 in the processing and post-processing stages of acidophilus yogurt. Int. J. Food Sci. Technol. 2004, 39, 563–568. [Google Scholar] [CrossRef]
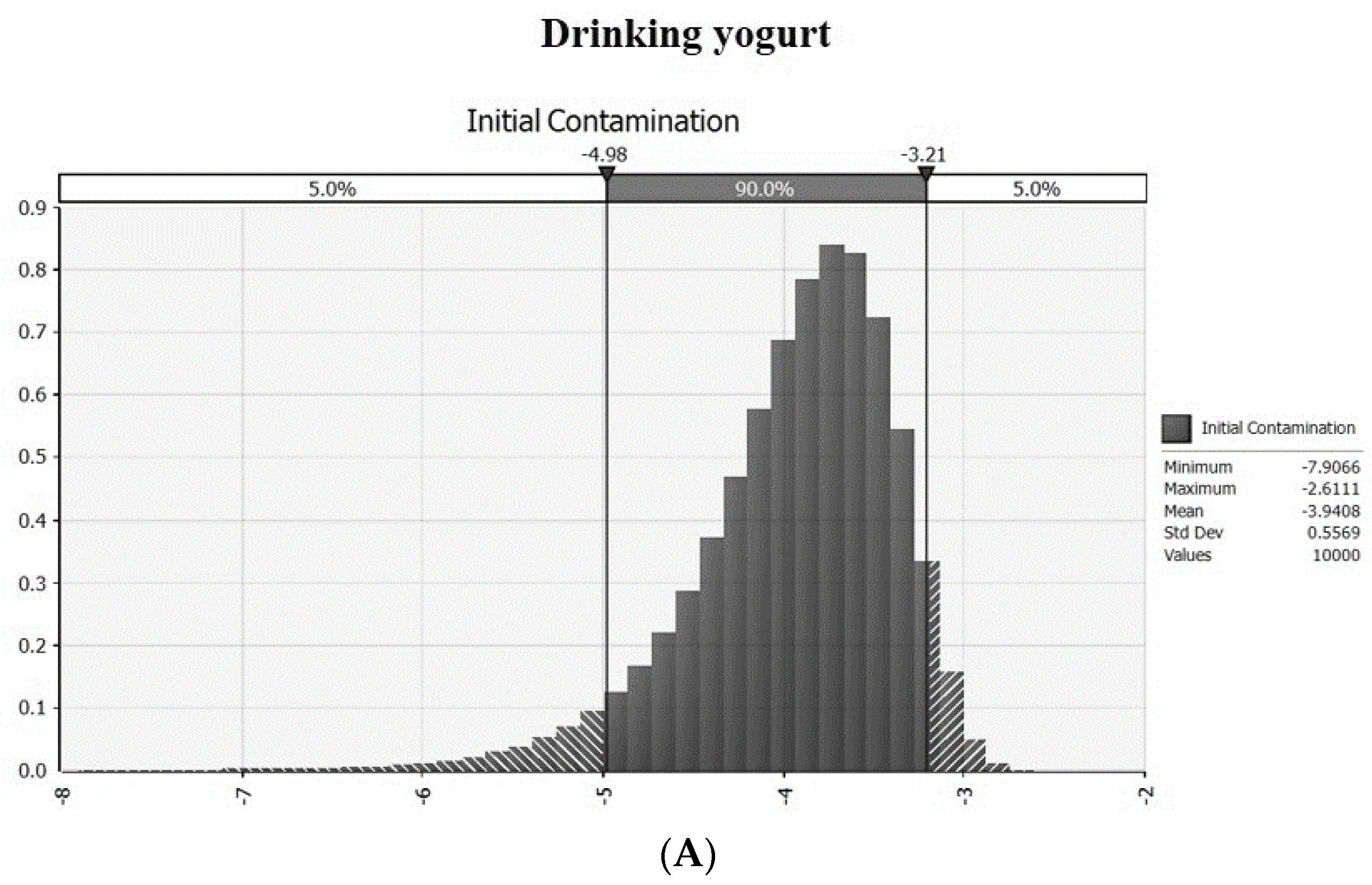
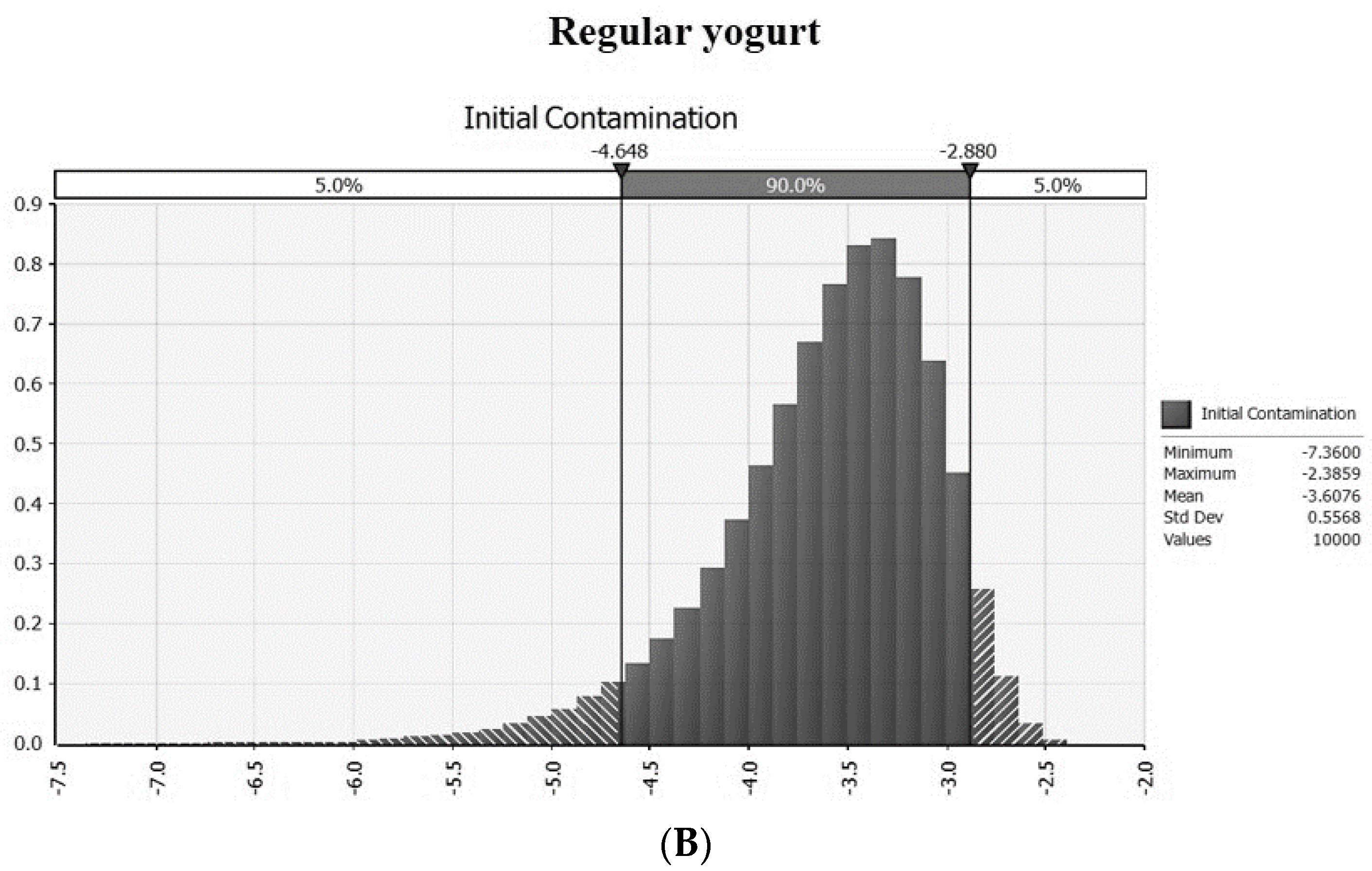
| Temperature (°C) | Pathogens | Drinking | Regular | ||
|---|---|---|---|---|---|
| Delta (day) 2 | p3 | Delta (day) | p | ||
| 4 | LM 4 | 20.31 ± 0.20 * | 0.73 | 9.04 ± 0.13 * | 1.07 ± 0.07 |
| EHEC 5 | 67.61 ± 1.92 * | 1.25 ± 0.01 | 14.93 ± 1.20 * | 1.12 ± 0.06 | |
| 10 | LM | 7.16 * | 3.1 ± 0.08 | 4.76 ± 0.08 * | 6.88 ± 0.44 |
| EHEC | 38.31 ± 0.37 * | 1.45 ± 0.01 | 10.41 ± 0.71 * | 1.45 ± 0.12 | |
| 17 | LM | 2.15 ± 0.01 * | 2.27 ± 0.03 | 1.89 ± 0.06 * | 3.32 ± 0.08 |
| EHEC | 13.42 * | 1.35 ± 0.08 | 8.21 ± 0.11 * | 4.17 ± 0.16 | |
| 25 | LM | 1.81 ± 0.03 * | 4.43 ± 0.21 | 0.66 * | 1.98 ± 0.02 |
| EHEC | 5.51 ± 0.12 * | 4.09 ± 0.21 | 2.23 ± 0.01 * | 2.84 ± 0.03 | |
| 36 | LM | 0.62 * | 2.83 ± 0.03 | 0.14 * | 1.17 ± 0.06 |
| EHEC | 1.42 * | 3.90 ± 0.05 | 0.42 ± 0.04 * | 2.57 ± 0.33 | |
| Symbol | Unit | Definition | Formula | Reference |
|---|---|---|---|---|
| Product | ||||
| PR | Prevalence of LM in drinking yogurt | =RiskBeta(1, 196) | MFDS [24] | |
| Prevalence of LM in regular yogurt | =RiskBeta(1, 91) | |||
| CL | CFU/g | Contamination level of LM | =−LN(1 − PR)/25 | Sanna et al. [27] |
| IC | log CFU/g | Initial contamination level | =Log(CL) | |
| Market | ||||
| MTime | h | Storage time in market of drinking yogurt | =RiskPert(0, 240, 312) | MFDS [24] |
| Storage time in market of regular yogurt | =RiskPert(0, 240, 480) | |||
| MTemp | °C | Storage temperature in market | =RiskPert(2.1, 7, 9.7) | |
| Death | ||||
| Delta | h | Drinking yogurt | =823.8 + (−100.8) × MTemp + 4.177 × MTemp2 + (−0.0556) × MTemp3 | This research |
| Regular yogurt | =315.1 + (−27.57) × MTemp + 0.8396 × MTemp2 + (−0.0087) × MTemp3 | |||
| p | Drinking yogurt | =2.67 (Fixed) | ||
| Regular yogurt | =2.882 (Fixed) | |||
| LM survival model | log CFU/g | C1 | =IC − (MTime/delta)p | |
| Transportation to home | ||||
| TTime | h | Storage time during transportation | =RiskPert(0.325, 0.984, 1.643) | Jung [32] |
| TTemp | °C | Storage temperature during transportation | =RiskPert(10, 18, 25) | |
| Death | ||||
| Delta | h | Drinking yogurt | =823.8 + (−100.8) × TTemp + 4.177 × TTemp2 + (−0.0556) × TTemp3 | This research |
| Regular yogurt | =315.1 + (−27.57) × TTemp + 0.8396 × TTemp2 + (−0.0087) × TTemp3 | |||
| p | Drinking yogurt | =2.67 (Fixed) | ||
| Regular yogurt | =2.882 (Fixed) | |||
| LM survival model | log CFU/g | C2 | =C1-(TTime/delta)p | |
| Home | ||||
| HTime | h | Storage time until consumption | =RiskPert(0, 60, 720) | MFDS [33] |
| HTemp | °C | Storage temperature until consumption | =RiskLogLogistic(−10.407, 13.616, 8.611) | Bahk [34] |
| Death | ||||
| Delta | h | Drinking yogurt | =823.8 + (−100.8) × HTemp + 4.177 × HTemp2 + (−0.0556) × HTemp3 | This research |
| Regular yogurt | =315.1 + (−27.57) × HTemp + 0.8396 × HTemp2 + (−0.0087) × HTemp3 | |||
| p | Drinking yogurt | =2.67 (Fixed) | ||
| Regular yogurt | =2.882 (Fixed) | |||
| LM survival model | log CFU/g | C3 | =C2 − (HTime/delta)p | |
| Consumption | ||||
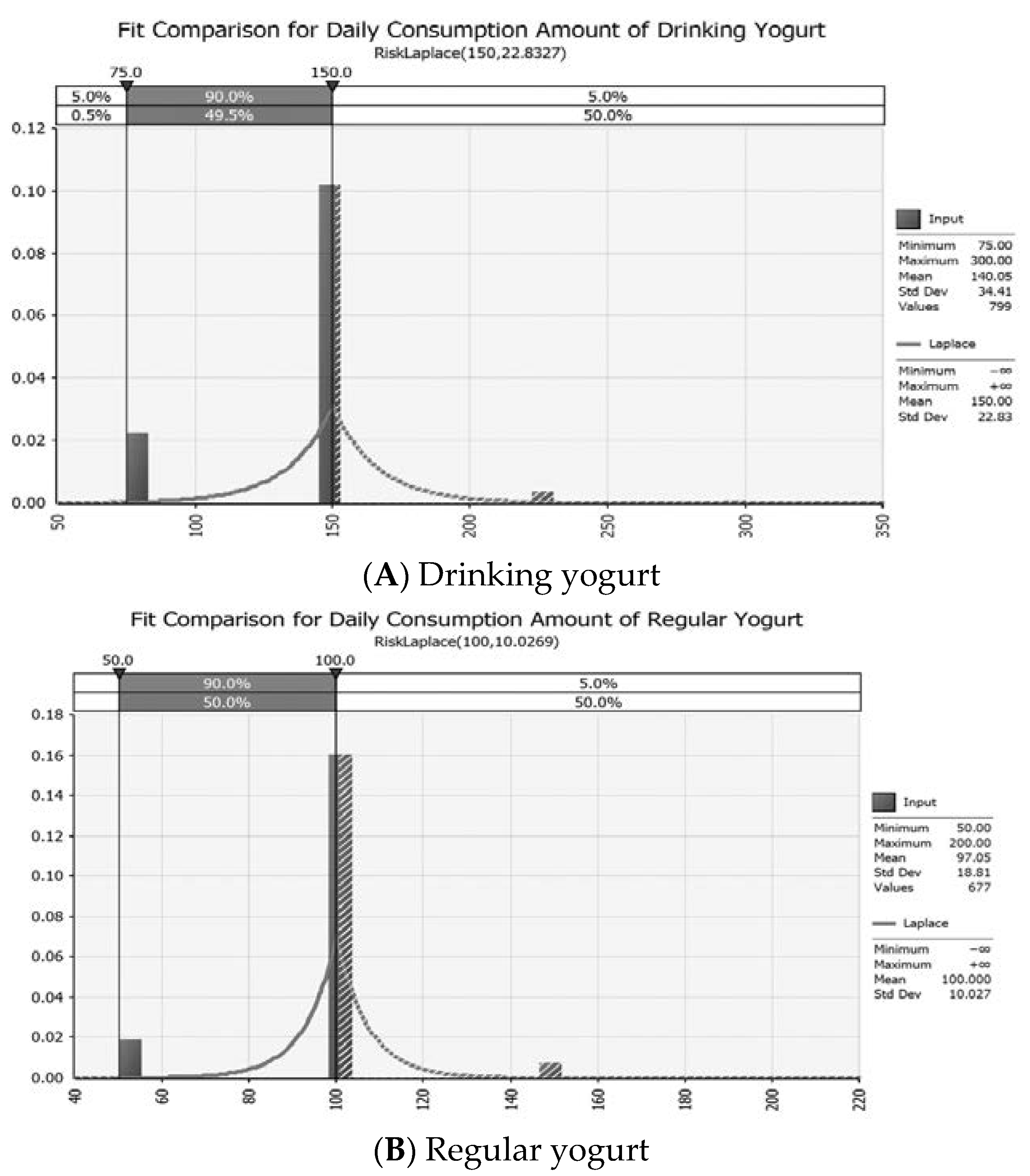
| Consume (Daily consumption average amount) | Drinking yogurt | =RiskLaplace(150, 22.833) | Park et al. [35] | |
| Regular yogurt | =RiskLaplace(100, 10.027) | |||
| Intake rate(Distribution for consumption frequency) | Drinking yogurt | =0.184(Fixed) | ||
| Regular yogurt | =0.146(Fixed) | |||
| Amount | Daily consumption average amount considered frequency | =Consume × Intake rate | ||
| Dose-Response model | ||||
| Dose(D) | LM amount | =10C3 × Amount | ||
| 1-EXP(-r × D) | Parameter of r | =1.06 × 10−12 (Susceptible population) | FDA/WHO [36] | |
| =2.37 × 10−14 (General population) | ||||
| Risk Characterization | ||||
| Risk | Probability of illness/person/day | =1 − exp(−r × D) | FDA/WHO [36] | |
| Symbol | Unit | Definition | Formula | Reference |
|---|---|---|---|---|
| Product | ||||
| PR | Prevalence of EHEC in drinking yogurt | =RiskBeta(1, 196) | MFDS [24] | |
| Prevalence of EHEC in regular yogurt | =RiskBeta(1, 91) | |||
| CL | CFU/g | Contamination level of EHEC | =−LN(1 − PR)/25 | Sanna et al. [27] |
| IC | log CFU/g | Initial contamination level | =Log(CL) | |
| Market | ||||
| MTime | h | Storage time in market of drinking yogurt | =RiskPert(0, 240, 312) | MFDS [24] |
| Storage time in market of regular yogurt | =RiskPert(0, 240, 480) | |||
| MTemp | °C | Storage temperature in market | =RiskPert(2.1, 7, 9.7) | |
| Death | ||||
| Delta | h | Drinking yogurt | =2347 + (−201.9) × MTemp + 6.044 × MTemp2 + (−0.0616) × MTemp3 | This research |
| Regular yogurt | =391.7 + (−8.478) × MTemp + (−0.4534) × MTemp2 + (−0.0109) × MTemp3 | |||
| p | Drinking yogurt | =2.406 (Fixed) | ||
| Regular yogurt | =2.429 (Fixed) | |||
| EHEC survival model | log CFU/g | C1 | =IC − (MTime/delta)p | |
| Transportation to home | ||||
| TTime | h | Storage time during transportation | =RiskPert(0.325, 0.984, 1.643) | Jung [32] |
| TTemp | °C | Storage temperature during transportation | =RiskPert(10, 18, 25) | |
| Death | ||||
| Delta | h | Drinking yogurt | =2347 + (−201.9) × TTemp + 6.044 × TTemp2 + (−0.0616) × TTemp3 | This research |
| Regular yogurt | =391.7 + (−8.478) × TTemp + (−0.4534) × TTemp2 + (−0.0109) × TTemp3 | |||
| p | Drinking yogurt | =2.406 (Fixed) | ||
| Regular yogurt | =2.429 (Fixed) | |||
| EHEC survival model | log CFU/g | C2 | =C1 − (TTime/delta)p | |
| Home | ||||
| HTime | h | Storage time until consumption | =RiskPert(0, 60, 720) | MFDS [33] |
| HTemp | °C | Storage temperature until consumption | =RiskLogLogistic(−10.407, 13.616, 8.611) | Bahk [34] |
| Death | ||||
| Delta | h | Drinking yogurt | =2347 + (−201.9) × HTemp + 6.044 × HTemp2 + (−0.0616) × HTemp3 | This research |
| Regular yogurt | =391.7 + (−8.478) × HTemp + (−0.4534) × HTemp2 + (−0.0109) × HTemp3 | |||
| p | Drinking yogurt | =2.406 (Fixed) | ||
| Regular yogurt | =2.429 (Fixed) | |||
| EHEC survival model | log CFU/g | C3 | =C2 − (HTime/delta)p | |
| Consumption | ||||
| Consume (Daily consumption average amount) | Drinking yogurt | =RiskLaplace(150, 22.833) | Park et al. [35] | |
| Regular yogurt | =RiskLaplace(100, 10.027) | |||
| Intake rate(Distribution for consumption frequency) | Drinking yogurt | =0.184(Fixed) | ||
| Regular yogurt | =0.146(Fixed) | |||
| Amount | Daily consumption average amount considered frequency | =Consume × Intake rate | ||
| Dose-Response model | ||||
| Dose(D) | EHEC amount | =10C3 × Amount | ||
| Model | Parameter of α | =0.49 | Park et al. [37] | |
| Parameter of β | =1.81 × 105 | |||
| Risk characterization | ||||
| Risk | Probability of illness/person/day | =1 − (1 + D/β)−α | Park et al. [37] | |
| Probability of Illness/Person/Day | |||||||
|---|---|---|---|---|---|---|---|
| Pathogens | Sample | Min | 25% | Mean | 95% | Max | |
| LM | Drinking | Susceptible population | 0 | 0 | 1.91 × 10−15 | 8.44 × 10−15 | 3.65 × 10−14 |
| General population | 0 | 0 | 0 | 0 | 0 | ||
| Regular | Susceptible population | 0 | 0 | 2.87 × 10−16 | 2.11 × 10−15 | 3.63 × 10−14 | |
| General population | 0 | 0 | 0 | 0 | 0 | ||
| EHEC | Drinking | 0 | 4.01 × 10−9 | 1.44 × 10−8 | 4.33 × 10−8 | 1.75 × 10−7 | |
| Regular | 0 | 4.39 × 10−10 | 5.09 × 10−9 | 2.12 × 10−8 | 9.45 × 10−8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.Y.; Yoon, K.S. Quantitative Microbial Risk Assessment of Listeria monocytogenes and Enterohemorrhagic Escherichia coli in Yogurt. Foods 2022, 11, 971. https://doi.org/10.3390/foods11070971
Yang SY, Yoon KS. Quantitative Microbial Risk Assessment of Listeria monocytogenes and Enterohemorrhagic Escherichia coli in Yogurt. Foods. 2022; 11(7):971. https://doi.org/10.3390/foods11070971
Chicago/Turabian StyleYang, So Young, and Ki Sun Yoon. 2022. "Quantitative Microbial Risk Assessment of Listeria monocytogenes and Enterohemorrhagic Escherichia coli in Yogurt" Foods 11, no. 7: 971. https://doi.org/10.3390/foods11070971
APA StyleYang, S. Y., & Yoon, K. S. (2022). Quantitative Microbial Risk Assessment of Listeria monocytogenes and Enterohemorrhagic Escherichia coli in Yogurt. Foods, 11(7), 971. https://doi.org/10.3390/foods11070971






