Changes in Histological Structure, Interleukin 12, Smooth Muscle Actin and Nitric Oxide Synthase 1. and 3. Expression in the Liver of Running and Non-Running Wistar Rats Supplemented with Bee Pollen or Whey Protein
Abstract
:1. Introduction
2. Material and Methods
3. Results
3.1. Morphological Changes
3.1.1. Dimensional Analysis of Histologically Significant Structures: Hepatocyte Nuclei and Central Veins
3.1.2. Collagen Deposition and Glycogen Content in Livers Analysis
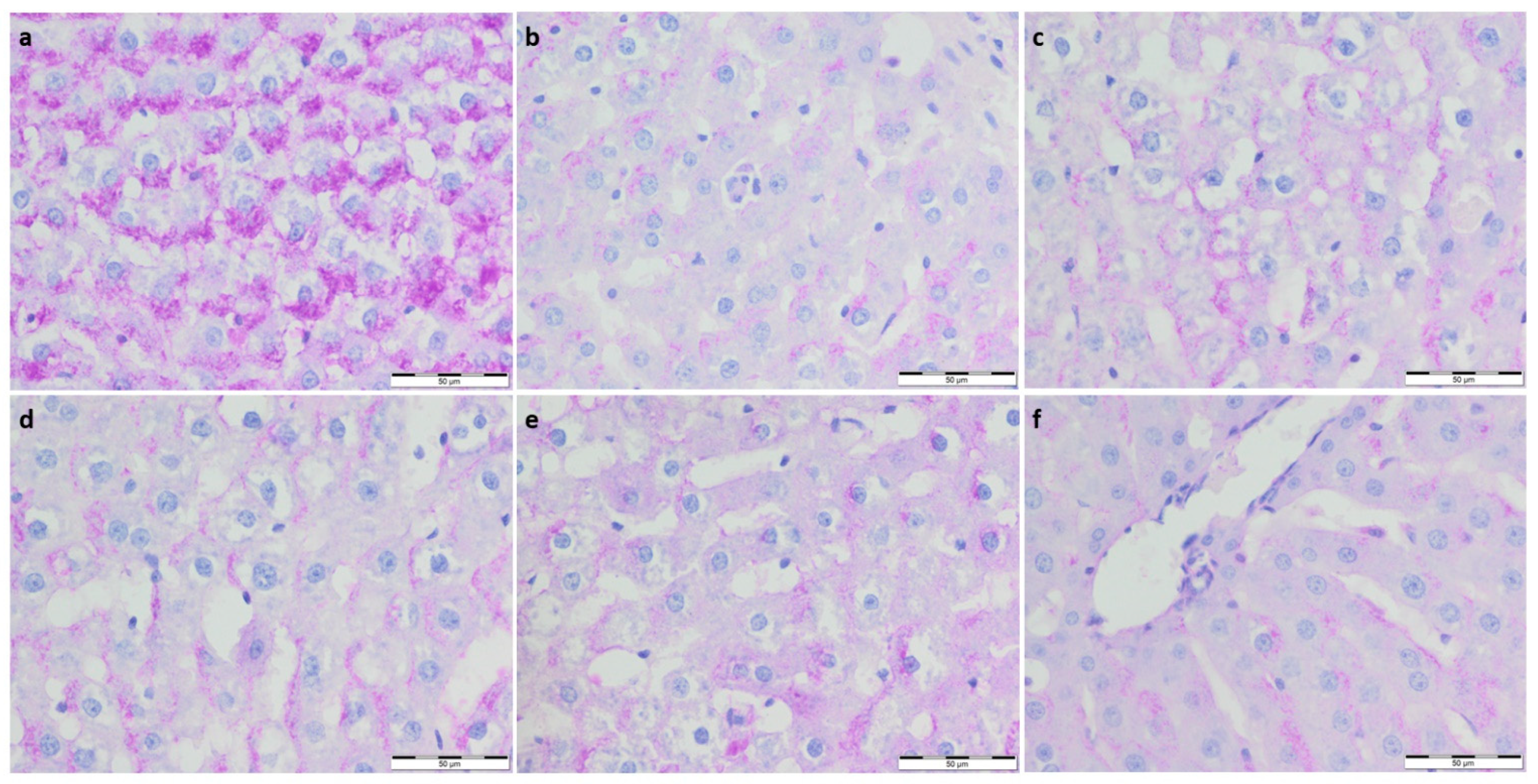
3.2. Immunohistochemistry Evaluation
3.2.1. IL-12 Expression
3.2.2. eNOS and nNOS Expression
3.2.3. α-SMA Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babińska, I.; Kleczek, K.; Szarek, J.; Makowski, W. Modulating Effect of Propolis and Bee Pollen on Chicken Breeding Parameters and Pathomorphology of Liver and Kidneys in the Course of Natural Infection with Salmonella Enteritidis. Bull. Veter-Inst. Pulawy 2012, 56, 3–8. [Google Scholar] [CrossRef]
- Haščík, P.; Elimam, I.O.E.; Garlík, J.; Bobko, M.; Kročko, M. Sensory evaluation for broiler meat after addition Slovak bee pollen in their feed mixture. Potravinarstvo Slovak J. Food Sci. 2013, 7, 107–110. [Google Scholar] [CrossRef]
- Kroyer, G.; Hegedus, N. Evaluation of bioactive properties of pollen extracts as functional dietary food supplement. Innov. Food Sci. Emerg. Technol. 2001, 2, 171–174. [Google Scholar] [CrossRef]
- Sforcin, J. Propolis and the immune system: A review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.; Green, C.; Doctor, V. Isolation and anticoagulant properties of polysaccharides of Typha Augustata and Daemonorops species. Thromb. Res. 1983, 32, 97–108. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, C.; Xu, D.; Huang, G.; Xu, Y.; Wang, Z.; Fang, S.; Chen, Y.; Gu, Y. The antiatherogenic effects of components isolated from pollen Typhae. Thromb. Res. 1990, 57, 957–966. [Google Scholar] [CrossRef]
- Burke, L.M.; Castell, L.M.; Stear, S.J.; Rogers, P.J.; Blomstrand, E.; Gurr, S.; Mitchell, N.; Stephens, F.B.; Greenhaff, P.L. BJSM reviews: A–Z of nutritional supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance Part 4. Br. J. Sports Med. 2009, 43, 1088–1090. [Google Scholar] [CrossRef]
- Shaldoum, F.; El-Kott, A.F.; Ouda, M.M.A.; Abd-Ella, E.M. Immunomodulatory effects of bee pollen on doxorubicin-induced bone marrow/spleen immunosuppression in rat. J. Food Biochem. 2021, 45, e13747. [Google Scholar] [CrossRef]
- Farrell, H.; Jimenez-Flores, R.; Bleck, G.; Brown, E.; Butler, J.; Creamer, L.; Hicks, C.; Hollar, C.; Ng-Kwai-Hang, K.; Swaisgood, H. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Davies, R.W.; Carson, B.P.; Jakeman, P.M. The Effect of Whey Protein Supplementation on the Temporal Recovery of Muscle Function Following Resistance Training: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 221. [Google Scholar] [CrossRef]
- Garthe, I.; Maughan, R.J. Athletes and Supplements: Prevalence and Perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, B.; Warzecha, M.; Tyrala, F.; Pieta, A. Prevalence of the use of effective ergogenic aids among professional athletes. Rocz. Panstw. Zakl. Hig. 2016, 67, 271–278. [Google Scholar] [PubMed]
- Morais, M.; Moreira, L.F.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Mărghitaş, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobis, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009, 115, 878–883. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, F.; Li, C.; Li, W.; Liao, W.; Li, Q.; Qin, G.; Liu, W.; Zhao, X. White Peony (Fermented Camellia sinensis) Polyphenols Help Prevent Alcoholic Liver Injury via Antioxidation. Antioxidants 2019, 8, 524. [Google Scholar] [CrossRef]
- Kume, H.; Okazaki, K.; Sasaki, H. Hepatoprotective Effects of Whey Protein on D-Galactosamine-Induced Hepatitis and Liver Fibrosis in Rats. Biosci. Biotechnol. Biochem. 2006, 70, 1281–1285. [Google Scholar] [CrossRef]
- Hamad, E.M.; Taha, S.H.; Dawood, A.-G.I.A.; Sitohy, M.Z.; Abdel-Hamid, M. Protective effect of whey proteins against nonalcoholic fatty liver in rats. Lipids Health Dis. 2011, 10, 57. [Google Scholar] [CrossRef]
- Al-Dhuayan, I. Possible Protective Role of Whey Protein on the Ratr’s Liver Tissues Treated with Nandrolone decanoate. Pak. J. Biol. Sci. 2018, 21, 262–274. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid.-Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef]
- Zarobkiewicz, M.K.; Sławiński, M.A.; Wawryk-Gawda, E.; Woźniakowski, M.M.; Kulak-Janczy, E.; Korzeniowska, S.; Jodłowska-Jędrych, B. Changes in histological structure and nitric oxide synthase expression in aorta of rats supplemented with bee pollen or whey protein. Appl. Physiol. Nutr. Metab. 2019, 44, 1150–1158. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-38629-6. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An Open Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Morera, P.; Castaño-González, I.; Travieso-Gonzalez, C.M.; Mompeo-Corredera, B.; Ortega-Santana, F. Quantification and Statistical Analysis Methods for Vessel Wall Components from Stained Images with Masson’s Trichrome. PLoS ONE 2016, 11, e0146954. [Google Scholar] [CrossRef]
- Jafari, S.M.S.; Hunger, R.E. IHC Optical Density Score: A New Practical Method for Quantitative Immunohistochemistry Image Analysis. Appl. Immunohistochem. Mol. Morphol. 2017, 25, e12–e13. [Google Scholar] [CrossRef] [PubMed]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Bhadoria, A.; Bhadoria, P.; Nagar, M.; Bahrioke, V. Effect of ethephon on the liver in albino rats: A histomorphometric study. Biomed. J. 2015, 38, 421–427. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Kabała-Dzik, A.; Kubina, R.; Jasik, K.; Kajor, M.; Wrześniok, D.; Stojko, J. Protective Effect of Polyphenol-Rich Extract from Bee Pollen in a High-Fat Diet. Molecules 2018, 23, 805. [Google Scholar] [CrossRef]
- Oda, M.; Yokomori, H.; Han, J.-Y. Regulatory mechanisms of hepatic microcirculation. Clin. Hemorheol. Microcirc. 2003, 29, 167–182. [Google Scholar]
- Baffy, G. Origins of Portal Hypertension in Nonalcoholic Fatty Liver Disease. Am. J. Dig. Dis. 2018, 63, 563–576. [Google Scholar] [CrossRef]
- Carpino, G.; Morini, S.; Corradini, S.G.; Franchitto, A.; Merli, M.; Siciliano, M.; Gentili, F.; Muda, A.O.; Berloco, P.; Rossi, M. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig. Liver Dis. 2005, 37, 349–356. [Google Scholar] [CrossRef]
- Zundler, S.; Neurath, M.F. Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev. 2015, 26, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, M.; Bajetta, E.; Canova, S.; Lotze, M.T.; Wesa, A.; Parmiani, G.; Anichini, A. Interleukin-12: Biological Properties and Clinical Application. Clin. Cancer Res. 2007, 13, 4677–4685. [Google Scholar] [CrossRef] [PubMed]
- Watford, W.T.; Moriguchi, M.; Morinobu, A.; O’Shea, J.J. The biology of IL-12: Coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003, 14, 361–368. [Google Scholar] [CrossRef]
- Ertek, S.; Cicero, A. State of the art paper Impact of physical activity on inflammation: Effects on cardiovascular disease risk and other inflammatory conditions. Arch. Med. Sci. 2012, 5, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, E.C.; Ronco, M.T. Role of nitric oxide in liver regeneration. Ann. Hepatol. 2012, 11, 636–647. [Google Scholar] [CrossRef]
- Abu-Amara, M.; Yang, S.Y.; Seifalian, A.; Davidson, B.; Fuller, B. The nitric oxide pathway—Evidence and mechanisms for protection against liver ischaemia reperfusion injury. Liver Int. 2012, 32, 531–543. [Google Scholar] [CrossRef]
- Leung, T.-M.; Tipoe, G.L.; Liong, E.C.; Lau, T.Y.; Fung, M.-L.; Nanji, A.A. Endothelial nitric oxide synthase is a critical factor in experimental liver fibrosis. Int. J. Exp. Pathol. 2008, 89, 241–250. [Google Scholar] [CrossRef]
- Costa, E.D.; Rezende, B.A.; Cortes, S.F.; Lemos, V.S. Neuronal Nitric Oxide Synthase in Vascular Physiology and Diseases. Front. Physiol. 2016, 7, 206. [Google Scholar] [CrossRef]
- Biecker, E.; Neef, M.; Sagesser, H.; Shaw, S.; Koshy, A.; Reichen, J. Nitric oxide synthase 1 is partly compensating for nitric oxide synthase 3 deficiency in nitric oxide synthase 3 knock-out mice and is elevated in murine and human cirrhosis. Liver Int. 2004, 24, 345–353. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Lu, Q. Separation and Characterization of Phenolamines and Flavonoids from Rape Bee Pollen, and Comparison of Their Antioxidant Activities and Protective Effects against Oxidative Stress. Molecules 2020, 25, 1264. [Google Scholar] [CrossRef]
- Eraslan, G.; Kanbur, M.; Silici, S.; Liman, B.C.; Altınordulu, Ş.; Sarıca, Z.S. Evaluation of protective effect of bee pollen against propoxur toxicity in rat. Ecotoxicol. Environ. Saf. 2009, 72, 931–937. [Google Scholar] [CrossRef] [PubMed]





| Group | Running | Supplementation | Group | Running | Supplementation |
|---|---|---|---|---|---|
| 1 Con-Sed | No | No | 2 Con-Run | Yes | No |
| 3 WP-Sed | No | Whey protein | 5 WP-Run | Yes | Whey protein |
| 4 BP-Sed | No | Bee pollen | 6 BP-Run | Yes | Bee pollen |
| Con-Sed (1) | Con-Run (2) | WP-Sed (3) | BP-Sed (4) | WP-Run (5) | BP-Run (6) | ap < 0.05 | |
|---|---|---|---|---|---|---|---|
| Nucleus diameter [μm] | 87.024 ± 12.85 | 101.1607 ± 12.03 | 95.9419 ± 123.47 | 104.7769 ± 13.97 | 100.4261 ± 14.37 | 101.3001 ± 16.69 | 1 vs. 5; 1 vs. 6 |
| Central vein diameter [μm] | 726.6297 ± 448.61 | 678.7059 ± 379.87 | 740.9134 ± 395.66 | 988.3653 ± 617.5406 | 845.2322 ± 522.1461 | 806.0831 ± 487.2394 | 1 vs. 3, 4, 5, 6; 2 vs. 3, 4; 3 vs. 4, 5, 6; 4 vs. 5, 6; 5 vs. 6 |
| Optical Density (OD) | Con-Sed (1) | Con-Run (2) | WP-Sed (3) | BP-Sed (4) | WP-Run (5) | BP-Run (6) | dp < 0.05 |
|---|---|---|---|---|---|---|---|
| SMA a | 1.235 ± 0.093 | 1.150 ± 0.079 | 1.253 ± 0.165 | 1.215 ± 0.182 | 1.239 ± 0.112 | 1.389 ± 0.274 | 2 vs. 6 |
| Il-12 a | 1.493 ± 0.315 | 1.2 ± 0.27 | 1.376 ± 0.217 | 1.334 ± 0.190 | 1.195 ± 0.237 | 1.250 ± 0.262 | 1 vs. 2 1 vs. 5 |
| nNOS a | 1.699 ± 0.133 | 1.512 ± 0.289 | 1.365 ± 0.46 | 1.246 ± 0.250 | 1.338 ± 0.321 | 1.26 ± 0.286 | 1 vs. 2, 3, 4, 5, 6 |
| eNOS a | 1.113 ± 0.186 | 1.301 ± 0.325 | 1.174 ± 0.258 | 1.099 ± 0.0388 | 1.232 ± 0.318 | 1.109 ± 0.177 | 1 vs. 2, 4 3 vs. 2; 2 vs. 6 |
| PAS b | 0.013 ± 0.048 | 0.011 ± 0.044 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.004 ± 0.002 | 0.003 ± 0.001 | 1 vs. 2, 3, 4, 5; 3 vs. 4, 5; 4 vs. 5; 5 vs. 6; |
| Masson c | 0.186 ± 0.087 | 0.227 ± 0.205 | 0.129 ± 0.04 | 0.193 ± 0.136 | 0.102 ± 0.021 | 0.146 ± 0.191 | 1 vs. 5; 2 vs. 5; 4 vs. 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, P.M.; Jasielski, P.P.; Zarobkiewicz, M.K.; Sławiński, M.A.; Wawryk-Gawda, E.; Jodłowska-Jędrych, B. Changes in Histological Structure, Interleukin 12, Smooth Muscle Actin and Nitric Oxide Synthase 1. and 3. Expression in the Liver of Running and Non-Running Wistar Rats Supplemented with Bee Pollen or Whey Protein. Foods 2022, 11, 1131. https://doi.org/10.3390/foods11081131
Jarosz PM, Jasielski PP, Zarobkiewicz MK, Sławiński MA, Wawryk-Gawda E, Jodłowska-Jędrych B. Changes in Histological Structure, Interleukin 12, Smooth Muscle Actin and Nitric Oxide Synthase 1. and 3. Expression in the Liver of Running and Non-Running Wistar Rats Supplemented with Bee Pollen or Whey Protein. Foods. 2022; 11(8):1131. https://doi.org/10.3390/foods11081131
Chicago/Turabian StyleJarosz, Piotr M., Patryk P. Jasielski, Michał K. Zarobkiewicz, Mirosław A. Sławiński, Ewelina Wawryk-Gawda, and Barbara Jodłowska-Jędrych. 2022. "Changes in Histological Structure, Interleukin 12, Smooth Muscle Actin and Nitric Oxide Synthase 1. and 3. Expression in the Liver of Running and Non-Running Wistar Rats Supplemented with Bee Pollen or Whey Protein" Foods 11, no. 8: 1131. https://doi.org/10.3390/foods11081131






