Abstract
To reveal the potential relationship between the bacterial community and quality attributes of vacuum-packaged peeled potatoes, the bacterial community dynamics, visual quality, organic acids, flavor and volatile organic compounds (VOCs) during 12 days of storage under 10 °C were studied, and a correlation analysis was performed between the bacterial community and VOCs. During the whole storage, the dominant bacteria changed from Ralstonia, Pseudomonas, Pantoea and Comamonas to Clostridia, Clostridium, Lacrimispora, Lactococcus and Leuconostoc. The visual quality and hardness deteriorated significantly on day 12; meanwhile, lactic and acetic acid content accumulated to 0.79 and 4.87 mg/g FW, respectively. Potatoes’ flavor deteriorated severely after 8 days, as evidenced by results of an electronic nose (e-nose). A total of 37 VOCs were detected, and the total content showed an increasing trend from 2164.85 to 10658.68 μg/kg during the whole storage. A correlation analysis showed that Enterobacteriaceae, Erwinia, Lacrimispora, Lactococcus, Serratia, Pantoea, Clostridium, Flavobacterium and Clostridia were positively correlated with the biosynthesis of VOCs. In addition, 10 spoilage markers were screened according to a variable importance in projection (VIP) ≥ 1. Ethanol, which was the most abundant spoilage marker, was significantly related to Enterobacteriaceae, Erwinia, Lacrimispora and Lactococcus. The results of this study have great practical significance for prolonging the shelf life of fresh-cut agricultural produce.
1. Introduction
The fresh-cut fruits and vegetables industry has rapidly grown due to consumers’ increasing demand for convenient foods with a fresh-like quality and high nutritive value. Raw peeled potatoes, as one of the fresh-cut produces that are rich in minerals, dietary fiber and other nutrients, have attracted widespread attention of households and the catering industry [1]. However, potatoes are susceptible to enzymatic browning after peeling, resulting in considerable degradation of sensory quality, safety and market values [2].
Different methods have been studied to prevent the surface browning of potatoes, including blanching, inert gas packaging, low temperatures, radiation treatment, acidification or reduction with antioxidants and the use of chelators or natural extracts [3,4,5]. However, these methods have not been widely applied in practical production due to disadvantages of inconvenient operation, high cost or being unfriendly to environment. Thus, there is an urgent need to study and develop proper techniques to inhibit browning.
Vacuum packaging is one of the most important techniques to prevent browning of fresh-cut fruits and vegetables, such as fresh-cut peaches [6], lotus roots [7,8] and potatoes [9], etc. It has also been reported to maintain better hardness, moisture content, microbial safety and visual quality in comparison with air packaging and other modified atmosphere packaging [9,10,11]. Nevertheless, vacuum packaging has an extremely low oxygen concentration that could promote the growth, fermentation and metabolism of anaerobic pathogens [12,13] and result in sensorial spoilage, particularly an off-odor, of fresh-cut produce [14]. The microbial load and sensorial quality are two main criteria to define the shelf life of fresh-cut fruits and vegetables. Extensive studies [15,16] have reported sensorial quality decay and microbial growth separately in fresh-cut produce, but the potential correlation between particular bacteria and quality decay have rarely been investigated. Some studies have reported the effects of vacuum packaging on soluble solids, enzyme activity and phenol content in fresh-cut potatoes [17]. However, no information has been published concerning the effects of vacuum packaging on the bacterial community, especially on the correlation between particular bacteria and the formation of flavor.
The objectives of this study were (1) to investigate shifts of the bacterial community and quality attributes in vacuum-packaged peeled potatoes during storage; (2) to reveal the potential relationship between quality attributes, especially flavor quality, and the bacterial community. The results of this research could be of great practical importance in developing effective intervention strategies to extend the shelf life of fresh-cut produce.
2. Materials and Methods
2.1. Plant Material
Fresh potatoes (Solanum tuberosum L. cv Netherlands 15) employed in this experiment were harvested in Zaozhuang, Shandong, China (34°80′ N, 117°33′ E). Potatoes with size uniformity, ground color and no diseases or insect pests were selected for experiment. The whole potatoes were cleaned, peeled manually (1–2 mm) and then washed to remove excess starch and other cellular constituents. Immediately after cleaning, potatoes were strained on a draining bucket to reduce the water on the potato surface, and then, the drained potatoes (6 in each bag) were vacuum-sealed using an automatic vacuum-packer (BEIBOO Automation Instrument Co., Ltd., Beijing, China) for 20 s using the EVOH film (91.18 µm film thickness) with an O2 permeability of 0.71 cm3/m2 24 h 0.1 MPa. During a period of 12 d of storage at 10 °C, samples were analyzed every 4 days.
2.2. DNA Extraction and PCR Amplification
The bacterial community’s DNA was extracted from potato samples using the Fast DNA SPIN Kit for soil (Omega Bio-tek, Norcross, GA, USA). The extracts were checked on 1% agarose gel, and their concentration and purity were determined by a NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, DC, USA). The hypervariable region 799F_1193R of the bacterial 16S rRNA gene was amplified using an ABI GeneAmp® 9700 PCR thermocycler (ABI, Carlsbad, CA, USA) (primer pairs: 799F (5’-AACMGGATTAGATACCCKG-3’) and 1193R (5’-ACGTCATCCCCACCTTCC-3’)). Specific PCR amplification was as follows: initial denaturation at 95 °C for 3 min; then, denaturation at 95 °C for 27 cycles for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 45 s and a single extension at 72 °C for 10 min and an end at 4 °C. A same procedure for 13 cycles was performed after the first amplification. The PCR mixtures contained 5 × TransStart FastPfu buffer 4 μL, 2.5 mM dNTPs 2 μL, forward and reverse primers (5 μM) 0.8 μL, TransStart FastPfu DNA Polymerase 0.4 μL, template DNA 10 ng and, finally, ddH2O up to 20 μL. PCR reactions were performed in triplicate. According to the manufacturer’s instructions, the PCR product was extracted from 2% agarose gel and purified by the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and then quantified by a Quantus™ Fluorometer (Promega, Madison, WI, USA).
2.3. Illumina MiSeq Sequencing and Processing of Sequencing Data
Purified amplicons were pooled in equimolar and paired-end sequenced on an Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina, San Diego, CA, USA), according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
The raw 16S rRNA gene sequencing reads were demultiplexed, quality-filtered by fastp version 0.20.0 [18] and merged by FLASH version 1.2.7 [19] with the following criteria: (a) Over the 50 bp sliding window, 300 bP readings were truncated at any site receiving an average quality score of <20. Truncated readings <50 bP and readings containing ambiguous characters were discarded; (b) Only overlapping sequences longer than 10 bp were assembled; (c) Primers were completely matched, and two nucleotides were allowed to be mismatched, and readings containing ambiguous bases were deleted. Operational taxonomic units (OTUs) with a 97% similarity cutoff [20,21] were clustered using UPARSE version 7.0.1090 (http://drive5.com/uparse/, accessed on 14 April 2022) [20], and chimeric sequences were identified and removed. The taxonomy of each OTU representative sequence was analyzed by RDP Classifier version 2.2 [22] against the 16S rRNA database using a confidence threshold of 70%.
2.4. Microbial Growth Analysis
Potatoes (15 g) were put in the sterile bag (S05D, Land Bridge Technology Co., Ltd., Beijing, China) and mixed with 135 mL NaCl solution (0.8%) using a beating homogenizer (BagMixer 400 W, Interscience Lab Inc., Hanover, MA, USA) for 2 min. The homogenized solution (1 mL) was serially diluted at a ratio of 1:10 with an NaCl solution. Next, 1 mL of the diluted suspensions were mixed with plant count agar and incubated at 37 ± 1 °C for 48 h for total aerobic mesophilics, and the total psychrotrophic bacteria were enumerated using a plant count agar by incubating plates at 28 ± 1 °C for 5 days. Mold and yeast counts were performed in a potato dextrose agar by incubation at 28 ± 1 °C for 5 days. Microbial counts were expressed as log CFU/g of tissue.
2.5. Visual Quality Assessment
For the surface color of potatoes within 180 min after opening the bag, this was evaluated using a CM-700d portable colorimeter (Konica Minolta, Inc., Tokyo, Japan). The time that browning started was recorded simultaneously. For the color properties (L*: brightness, a*: red-green color, b*: yellow-blue color), 10 potatoes were taken in each storage period, and five points were taken randomly for each potato. The average of the measured values of 50 points in total was taken as the color value.
2.6. Texture Analysis
Potatoes were cut into 1 cm3 cubes. The hardness of the potato was analyzed with a TA-XT Plus texture analyzer (Stable Micro Systems Ltd., Godalming, UK). A cylinder puncture probe with a diameter of 5 mm (p/5) was pressed down at 5 mm from contact of the samples at a constant speed of 0.50 mm/s. The hardness was determined by the maximum force (N) during pressing. For the texture measurement, 9 individual tubers from each of the storage days were tested.
2.7. Determination of pH and Organic Acids
The pH of the potatoes was measured using a pH meter (Metler-Toledo Instruments Co., Ltd., Shanghai, China). A 2 g sample was placed in a centrifugal tube, to which 3 mL of ultra-pure water was then added. Then, it was centrifuged for 15 min at 10,000 rpm after ultrasonic treatment for 30 min in a water bath. The supernatants were filtered through a 0.22 μm polytetrafluoroethylene membrane before being injected. Organic acids (lactic acid and acetic acid) were analyzed using a high-performance liquid chromatography system (Agilent Technologies, Palo Alto, CA, USA) equipped with a quaternary pump, an oven for controlling the column temperature (which was set at 25 °C), a UV-vis detector and a data acquisition system fitted with a XBridge® C18 column (4.6 mm × 250 mm, 5 μm). The mobile phase consisted of a 0.02 M solution of potassium dihydrogen phosphate buffer (pH 2.88) and methanol (98:2, v/v). The autosampler was adjusted to an injection volume of 20 μL. Isocratic elution was applied to the mobile phase with a flow rate of 0.6 mL/min. A wavelength of 210 nm was selected for quantification. The calibration curves for each compound were constructed using pure standards at different concentrations [23].
2.8. E-Nose
The flavor information of the samples was obtained by an e-nose instrument, which was composed of 10 sensors (W1C, W5S, W3C, W6S, W5C, W1S, W1W, W2S, W2W and W3S). A total of 10 g of potato paste was put into 30 mL headspace vials for 6 replicates per sampling day. The conditions of the e-nose system in this study were as follows: sampling time of 200 s, purging time of 100 s and airflow rate of 300 mL/min. All measurements were made at room temperature (25 °C). The average value of response (188–190 s) of each sensor was extracted for analysis.
2.9. Determination of VOCs Using Gas Chromatography-Mass Spectrometry (GC-MS)
The analysis of volatile organic compounds (VOCs) in potatoes was performed using headspace solid-phase microextraction (HS–SPME) combined with GC-MS (7890B GC, 5977B MS; Agilent Technologies, Santa Clara, CA, USA). Samples of 1.5 g were accurately weighed into 20 mL glass vials containing 3 mL of saturated salt water, and then, 20 μL 2-methyl-3-heptanone (0.816 × 10−2 μg/μL) was added to each vial as an internal standard. The vials were immediately placed in a heating block to equilibrate for 5 min at 40 °C, and then, a CAR/DVB/ PDMS SPME fiber (50/30 μm) (Stableflex™, Santa Clara, CA, USA) was exposed to the headspace for 30 min at 40 °C. Volatiles were desorbed from the SPME fiber at 250 °C for 5 min in the injector in splitless mode, and helium was used as the carrier gas at a flow rate of 1.0 mL/min. A DB-5 MS column (30 m × 0.25 mm, 0.25 μm film thickness; Agilent, Santa Clara, CA, USA) was used, and the column temperature was set at 40 °C (held for 3 min), increased to 150 °C at 5 °C/min and then increased to 250 °C at 10 °C/min (held for 10 min). MS was performed with an ion source temperature of 230 °C, a mass range of m/z 30–500 and an electron ionization energy of 70 eV.
2.10. Statistical Analysis
The data were analyzed by an analysis of variance (ANOVA) and Duncan’s test using the statistical products and services solution (version 17.0; SPSS in Chicago, IL, USA) with a significant level at p < 0.05. Results were expressed as the mean ± standard deviation. PLS-DA was performed using the SIMCA-P software (version 11.5; Umetrics, Umeå, Sweden). The Originpro 8 software (OriginLab Corporation, Northampton, MA, USA) was used for data plotting.
3. Results and Discussion
3.1. Bacterial Community
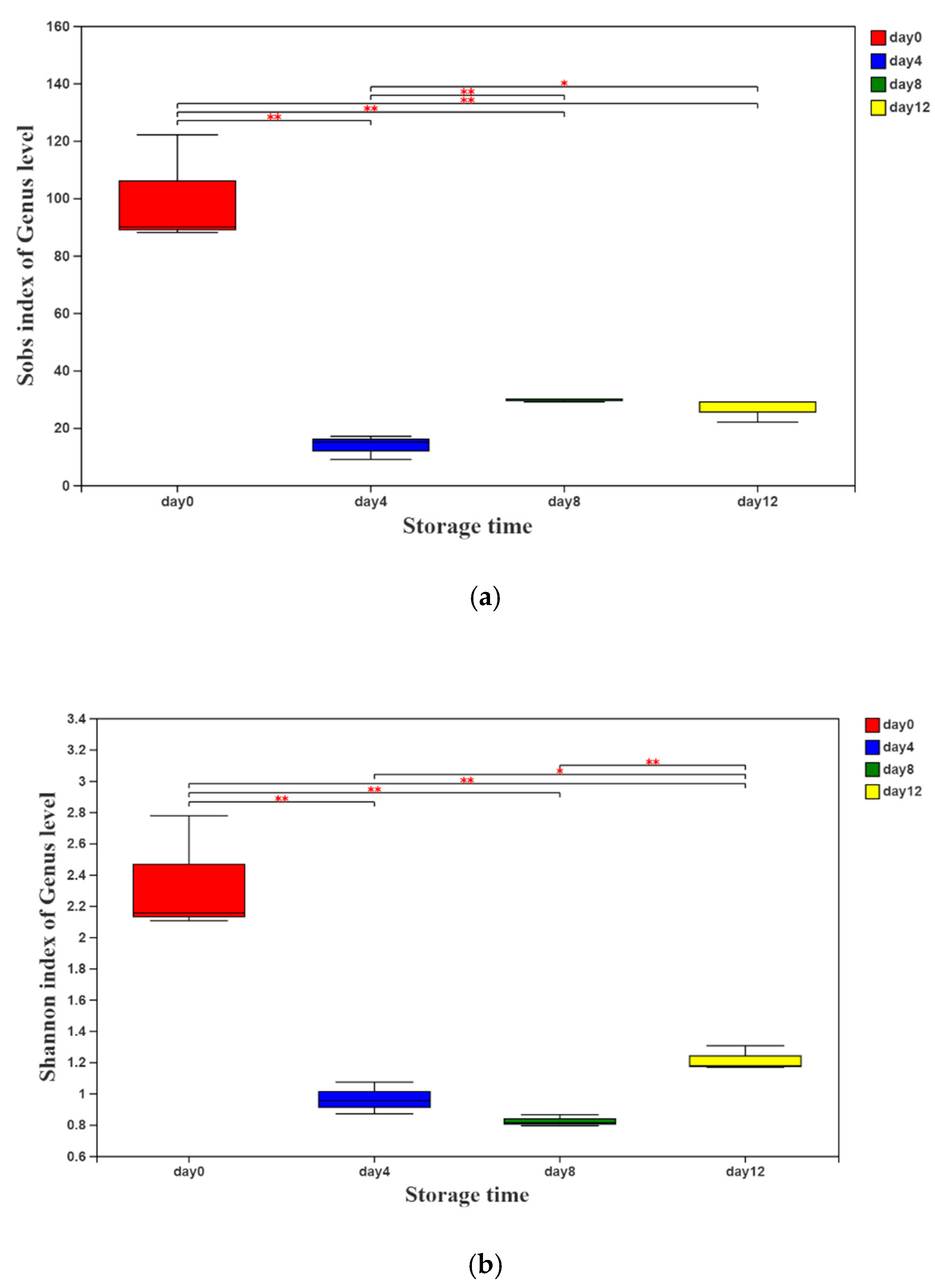
According to the sequencing results, the values of the α-diversity indices (Sobs and Shannon indices) are presented in Figure 1a,b. Both of the indices decreased sharply on day 4 and then increased slightly with the extension of storage time, indicating that the richness and diversity of the bacterial community in vacuum-packaged peeled potatoes decreased rapidly first and then increased gradually.
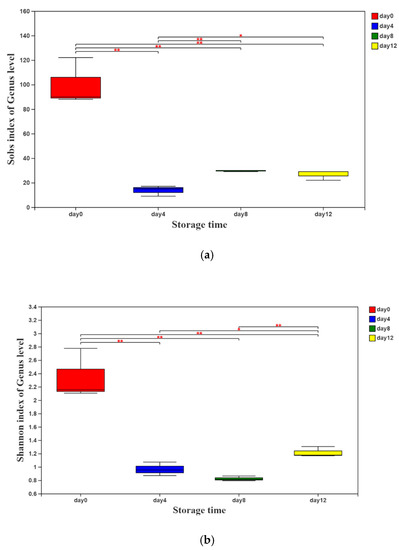
Figure 1.
Sobs (a) and Shannon (b) indices of the genus level of vacuum-packaged peeled potatoes during storage. The significant difference between the storage days, p ≤ 0.05, is marked as *, and p ≤ 0.01 is marked as **.
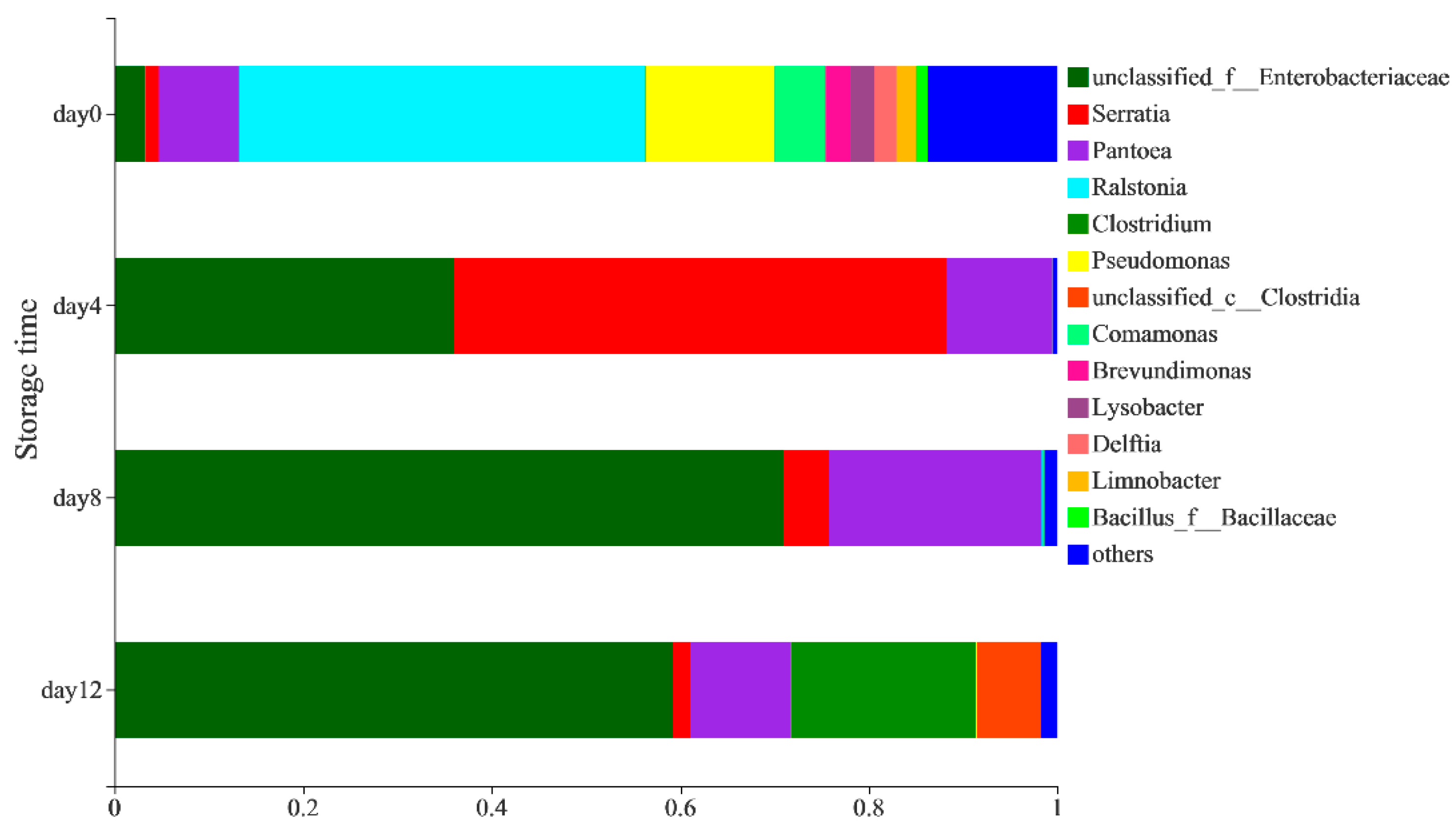
The distribution of the bacterial community at the genus level (Figure 2) showed that the bacterial community in peeled potatoes was mainly composed of Ralstonia (43.08%), Pseudomonas (13.66%), Pantoea (8.48%), Comamonas (5.37%), Enterobacteriaceae (3.24%), Brevundimonas (2.63%), Lysobacter (2.59%), Delftia (2.31%), Limnobacter (2.1%), Serratia (1.43%), Bacillus_f_Bacillaceae (1.2%) and others (13.77%) on day 0. The composition and abundance of the bacterial community in vacuum-packaged potatoes changed significantly during storage. A higher relative abundance of Serratia (52.27%), Pantoea (11.19%), Erwinia (0.4%) and Leuconostoc (0.03%) was observed in the samples on the 4th day, while the abundance of Ralstonia, Pseudomonas, Comamonas and other genera decreased (Supplementary Table S1). Paillart et al. [24] also found that the relative abundance of Leuconostoc spp. Increased. whereas Pseudomonas spp. decreased in fresh-cut lettuce during storage, which may have been due to the reduction of oxygen inside the package. On the 8th day, the abundance of Serratia (4.86%) decreased significantly, whereas the abundance of Enterobacteriaceae (70.95%) and Pantoea (22.51%) increased. On the 12th day, the presence of anaerobic bacteria such as Clostridia (6.81%), Clostridium (19.64%) and Lacrimispora (0.38%) (Supplementary Table S1) increased, which could have been due to the development of near-zero oxygen conditions [25]. Avci et al. [26] reported that Clostridium strains have the ability to produce ethanol through the fermentation of carbohydrate-rich sources, such as potatoes. Lactococcus and Leuconostoc were also detected on the 12th day (Supplementary Table S1), which had previously been observed in vacuum-packaged peeled potatoes by Lauridsen et al. [27] and may result in sensorial quality loss by producing organic acids in fresh-cut produce [24,28].
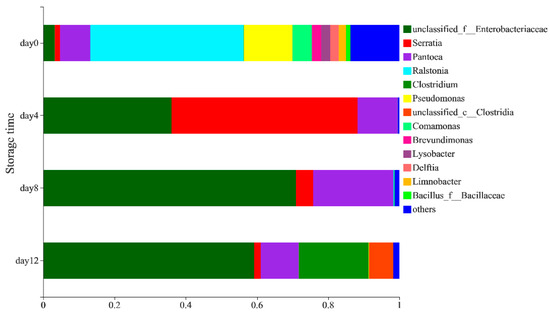
Figure 2.
The distribution of bacteria on a genus-level during the storage of vacuum-packaged peeled potatoes.
3.2. Microbial Quality
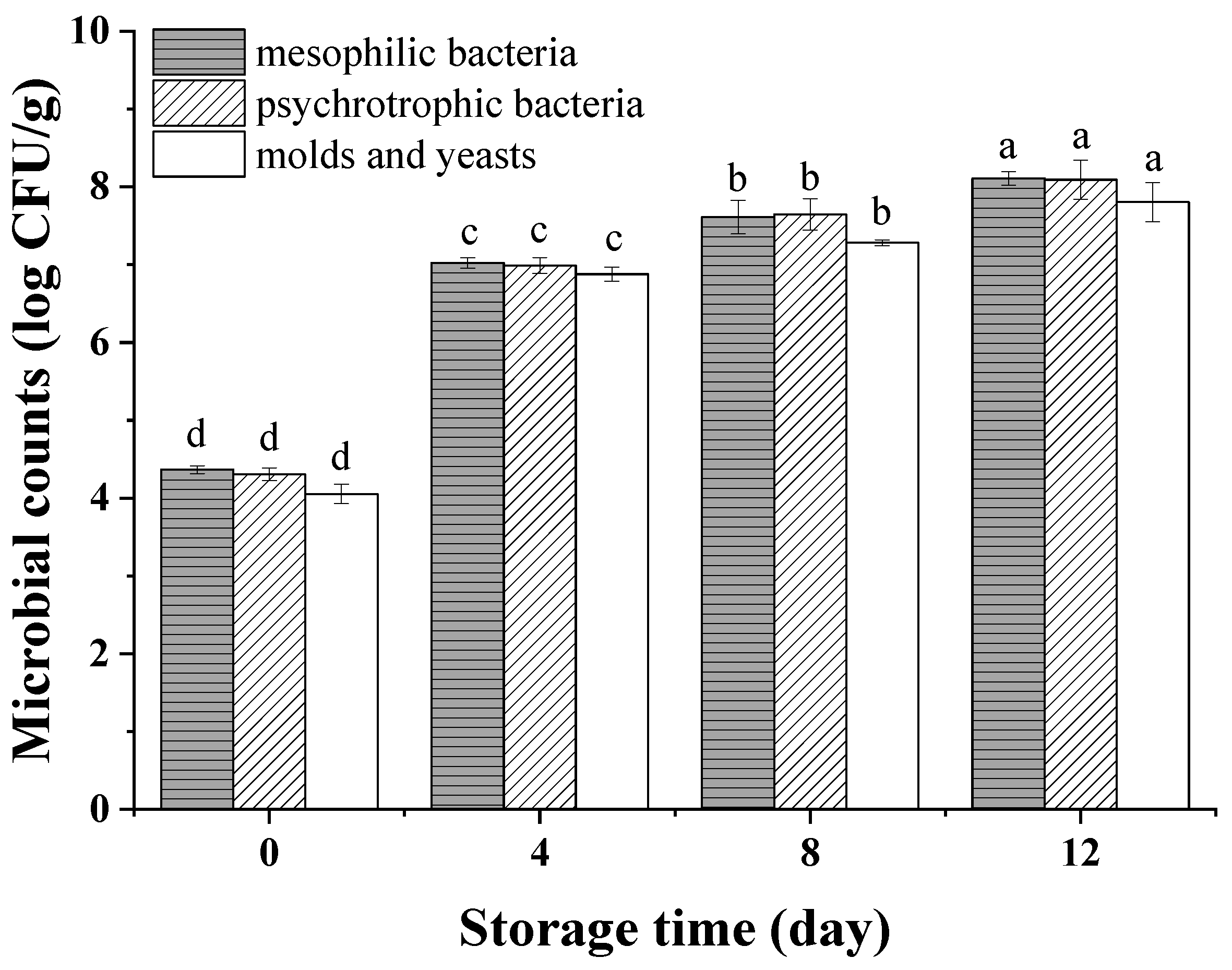
The initial counts of mesophilic, psychrophilic microorganisms and molds and yeasts on peeled potatoes were 4.36, 4.30 and 4.05 log CFU/g, respectively (Figure 3), which were similar to results of Oms-Oliu et al. [29], who found that these values were 4.09, 3.83 and 3.00 log CFU/g on fresh-cut mushrooms on day 0. The microbial counts increased rapidly from 0 to 4 days, and then, the growth slowed down during the subsequent storage. Waimaleongora-Ek et al. [30] also found a similar trend in fresh-cut sweet potatoes. This trend may have been due to the stable phase of microbial growth or the inhibition of some aerobic microbes by vacuum packaging [31]. Besides this, the slowing-down of microbial growth during the late storage period may also be caused by the increasing of organic acid. [32]. On the 8th day, the count of mesophilic bacteria increased to 7.61 log CFU/g, which is lower than the maximum acceptable contamination value (7.70 log CFU/g) required by the Applied and Environmental Microbiology of France for minimally processed products [33], indicating the potatoes maintained a qualified microbiology load until the 8th day. Moreover, the count of psychrophilic bacteria reached 7.64 log CFU/g, which is lower than 8 log CFU/g, which was reported to be insufficient to cause visual defects of minimally processed vegetables [34,35].
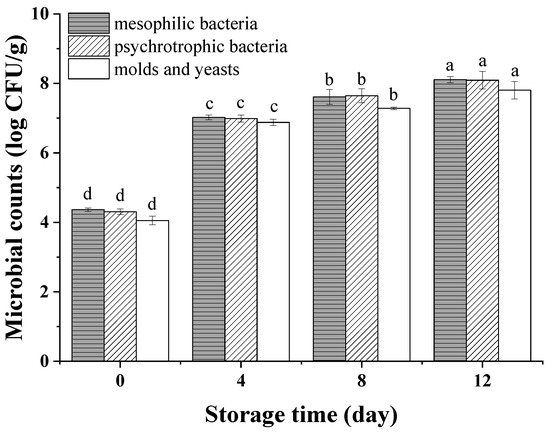
Figure 3.
Mesophilic bacteria, psychrotrophic bacteria and molds and yeasts of vacuum-packaged peeled potatoes during storage. The different letters in mesophilic, psychrophilic bacteria and molds and yeasts indicate significant differences (p < 0.05).
3.3. pH and Organic Acids
Changes in the pH and organic acids of vacuum-packaged peeled potatoes are shown in Table 1. The pH of potatoes continuously decreased from 5.91 to 5.60 during storage. The initial contents of lactic acid and acetic acid in raw potatoes were 0.04 and 2.06 mg/g FW, respectively, and they increased to 0.79 and 4.87 mg/g FW on the 12th day. Paillart et al. [28] also found that lactic acid and acetic acid accumulated significantly in fresh-cut lettuce during storage under modified atmosphere packaging, which was attributed to anaerobic fermentation caused by microorganisms. The production of lactic and acetic acid has been reported to be related to the microbial profile and metabolic pattern, and the occurrence of Leuconostoc and Lactococcus is likely to be the main reason for their production under anaerobic conditions [10,16,36,37]. Paillart et al. [24] found that organic acids, produced by lactic acid bacteria, were not only responsible for a sour off-odor, but also led to loss of membrane integrity, indicating that Leuconostoc and Lactococcus were correlated to the changes of flavor and texture.

Table 1.
Changes in pH, lactic acid, acetic acid content, hardness and browning time of vacuum-packaged potatoes during storage.
3.4. Visual Quality and Hardness
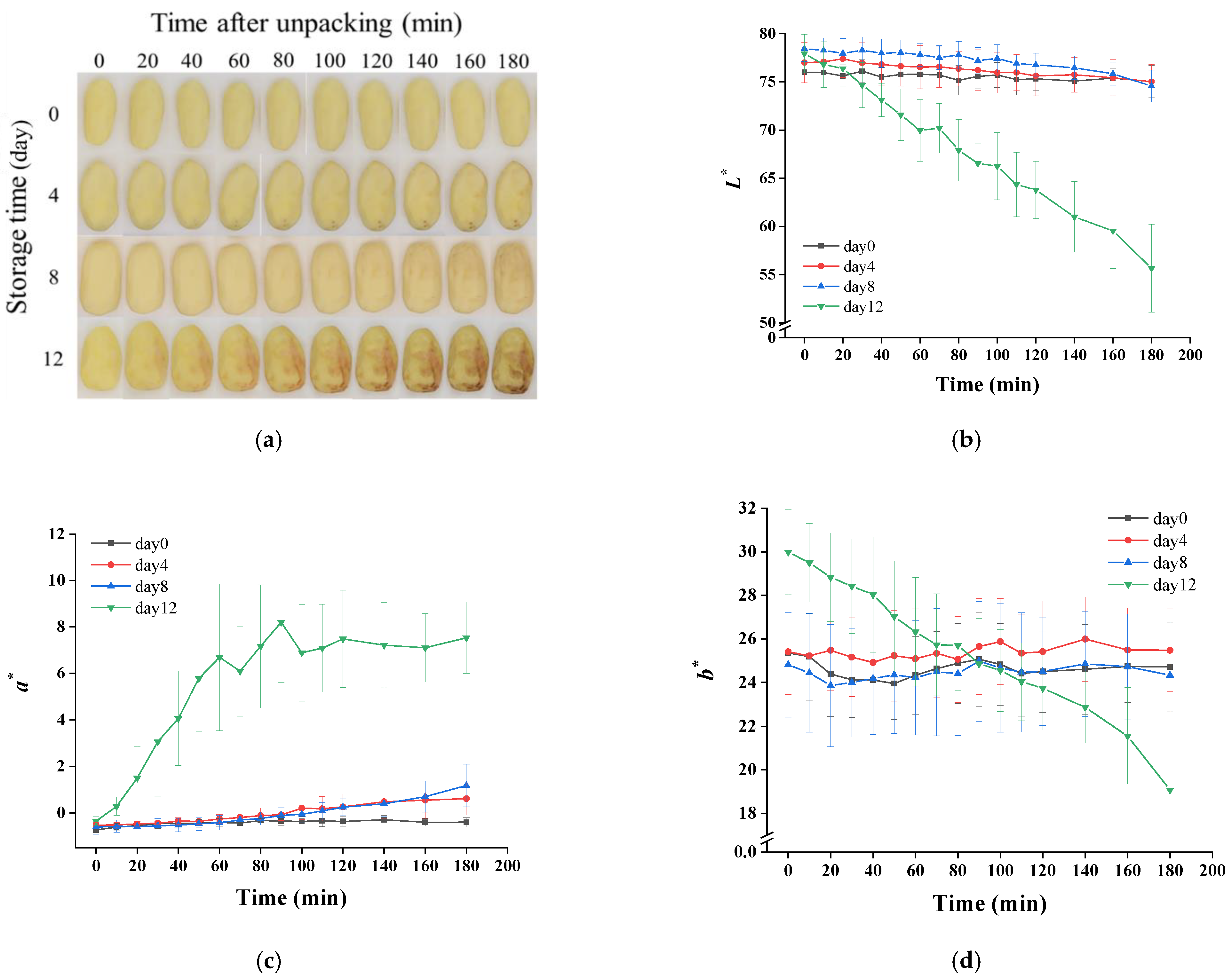
The color changes of potatoes after unpacking are shown in Figure 4. As exhibited in Figure 4a, a small part surfaces of samples from day 0 to day 8 changed gradually from yellow to brown, whereas a large surface area of the day 12 sample turned pink quickly and then dark brown. The starting time of discoloration for these samples is recorded in Table 1. The surface discoloration of the 0-, 4-, 8- and 12-day samples started at about 31.88, 35.50, 20.35 and 5.71 min, respectively. A delay of browning on day 4 was observed compared to day 0, which could have been due to the inhibition of phenylalanine ammonia lyase activity by the increase of organic acid [38]. The discoloration of day 12 samples happened significantly earlier than other samples, which was also found in the results of the color change measured by the colorimeter (Figure 4b–d). Lightness (L*), reddish–greenish (a*) and yellowish–bluish (b*) values also suggested a browning process of potatoes after opening the package. The samples of day 12 showed an obviously different trend compared to others. As presented in Figure 4b,d, a continual decrease was observed in the L* and b* values. In terms of a* values, day 12 showed an increasing trend firstly, and then it kept constant after 60 min. The aggravation of color change on the 12th day may have been due to cell damage, resulting in a mixing of enzymes with phenolic compounds, which was caused by microbial excessive proliferation and its metabolites’ production [28,38]. In addition, anaerobic bacteria (Clostridium) that multiplied during storage in this study have been reported to cause potato slimy rot, manifested by formation of pink pigments (aromatic polyketides metabolites), which can enable the anaerobic bacteria to survive when exposed to an oxygen-rich environment [39]. This could also explain why the potato turned pink rapidly after unpacking on day 12 compared with other samples.
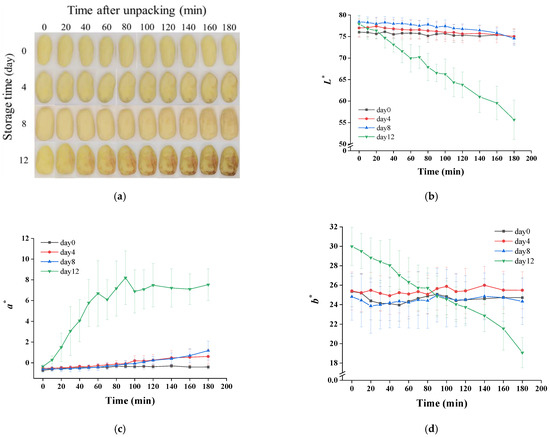
Figure 4.
Changes of visual quality (a) and the values of L* (b), a* (c) and b* (d) of vacuum-packaged peeled potatoes during storage within 180 min after unpacking.
The hardness of potatoes did not change significantly during the first 8 days (Table 1). As the storage was prolonged to 12 days, the potatoes softened obviously in comparison with other samples. The softening of potato tissues could have been due to the accumulation of organic acids, causing an increase of water-soluble pectin [28] or an elevation of pectin-degrading enzymes’ activities [40,41] and then resulting in pectin degradation in the cell wall. Additionally, pectin-decomposing bacteria (Erwinia, Clostridia) can secrete pectin-degrading enzymes and lead to pectin degradation [39,42].
3.5. E-Nose
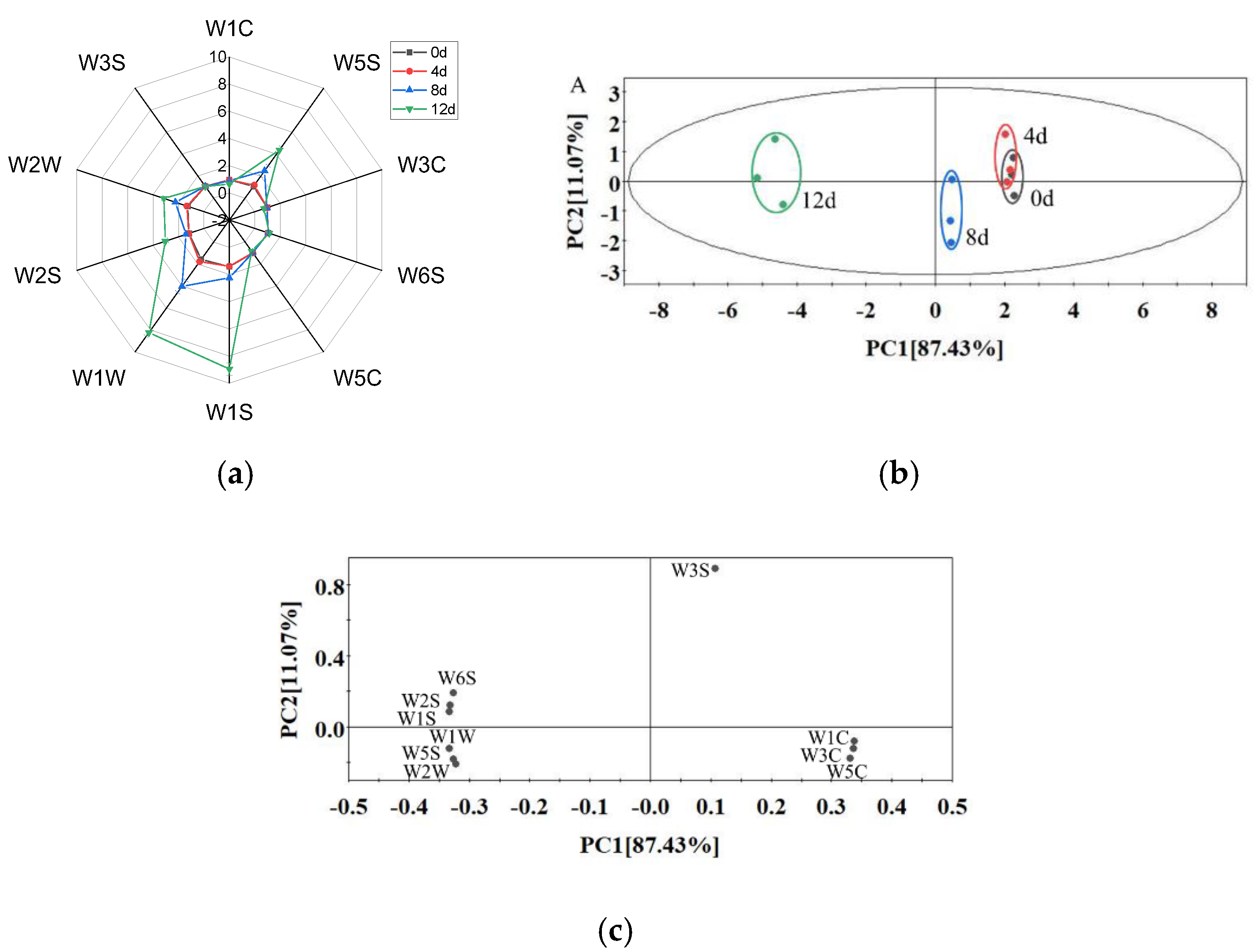
As shown in Figure 5a, the sensor response curve of the sample on day 4 almost overlapped with that of day 0, indicating that potatoes maintained a fresh-like flavor during the first 4 days. With the extension of storage time, the values of the W5S (highly sensitive to nitrogen oxides, furans), W1S (sensitive to hydrocarbons), W1W (sensitive to sulfides and pyrazine), W2S (sensitive to alcohols, aldehydes and ketones) and W2W (sensitive to organic sulfides) sensors increased significantly, which indicates that various compounds, such as nitrogen oxides, furans, sulfides, alcohols and aldehydes, were formed during storage. Chen et al. [43] also found that alcohols, aldehydes and sulfides increased during the storage of fresh-cut bell pepper.
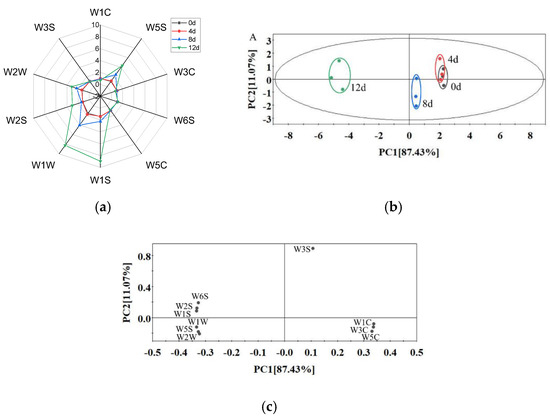
Figure 5.
Radar chart (a) and PCA score plot (b) and loading plot (c) from the E-nose data of vacuum-packaged peeled potato samples during storage.
A principal component analysis (PCA) was used to analyze the differences of the flavor profiles of samples from different storage periods (Figure 5b). PC1 and PC2 explained 87.43% and 11.07% of the total variance, respectively. The cumulative variance contribution rate of the first two principal components reached 98.5%, which explained a large fraction of the overall variability. As the storage period was prolonged, the distance of day 4–12 from day 0 became longer. The PCA profile of day 4 almost overlapped with day 0, while those of day 8 and day 12 were clearly separated from day 0–4, indicating that the flavor quality deteriorated significantly after 8 days of storage and that the e-nose could be used to discriminate the freshness of peeled potatoes. Furthermore, the off-odor may be caused by spoilage bacteria (Enterobacteriaceae, Erwinia, Leuconostoc and Lactococcus) due to fermenting sugar [24,28,44].
The loading plot (Figure 5c) showed a relationship among the variables of the e-nose sensors. The loading vectors of W3S, W1C, W3C and W5C had positive scores, and the other loading vectors had negative scores in the PC1 direction. The sensors of W3S, W6S, W2S and W1S had positive scores, and the remaining sensors had negative scores in the PC2 direction. All sensors were far from the origin, indicating that the response values of all sensors had effects on this PCA [45].
3.6. VOCs
Volatile organic compounds (VOCs) are closely related to the flavor quality of fresh-cut products, which greatly affects the consumers’ sensory evaluation. A total of 37 VOCs were detected in peeled potatoes during storage period, and they could be categorized as alcohols, aldehydes, ketones, esters, furans and hydrocarbons (Table 2), which was similar to the results of Dresow et al. [46]. Among these VOCs, alcohols, aldehydes and hydrocarbons, which accounted for the major proportion, showed significant upward trends and reached 5304.19 μg/kg, 3638.29 μg/kg and 1415.60 μg/kg at the end of storage, respectively. It is worth noting that esters were only detected in peeled potatoes on day 0, and the content was 17.92 μg/kg. Losses of esters, as an early response to the loss of freshness, have also been reported in fresh-cut cantaloupe [47,48]. In addition, some VOCs (ethanol, 1-pentanol, 3-methyl-1-butanol, 2-methyl-1-butanol, benzyl alcohol, hexanal, (E)-2-hexenal, heptanal, (E)-2-heptenal, (E, E)-2,4-heptadienal, (E)-2-octenal, decanal, 1-penten-3-one, 1-octen-3-one and tetradecane, 2-pentyl-furan) were also found in raw potatoes, as reported by previous studies [46,49,50].

Table 2.
The composition and content of VOCs of vacuum-packaged peeled potatoes during storage.
During the whole storage, total content of VOCs exhibited an increasing trend and reached 10,658.68 μg/kg at the end of storage (12 d), which was about 4.9-times that of day 0. This may have been because that spoilage microorganisms produced VOCs under anaerobic conditions by participating in carbohydrate metabolism, amino acid metabolism and other metabolic pathways [41]. Several studies have reported the relationship between microorganisms and the formation of VOCs. For instance, Erwinia has the ability to produce short chain alcohols and carbonyl compounds in raw potatoes [51]. Ethanol can originate from the hetero-fermentation of sugar metabolism by Leuconostoc [41]. 2-ethyl-1-hexanol may be produced by the action of mold [52]. 3-methyl-1-butanol and 2-methyl-1-butanol, which form off-odors with a fermentative character, are the products of amino acid metabolism under the action of Pantoea [35,53,54]. Furthermore, physical damage can result in the mixing of enzymes and nonvolatile precursors in cells to produce VOCs. For example, an intrinsic lipid attacked by enzymes in cut or sliced potatoes could produce a large number of aldehydes and alcohols, such as heptanal, 1-penten-3-one, 1-pentanol, 2,4-heptadienal and 2-pentyl furan [46].
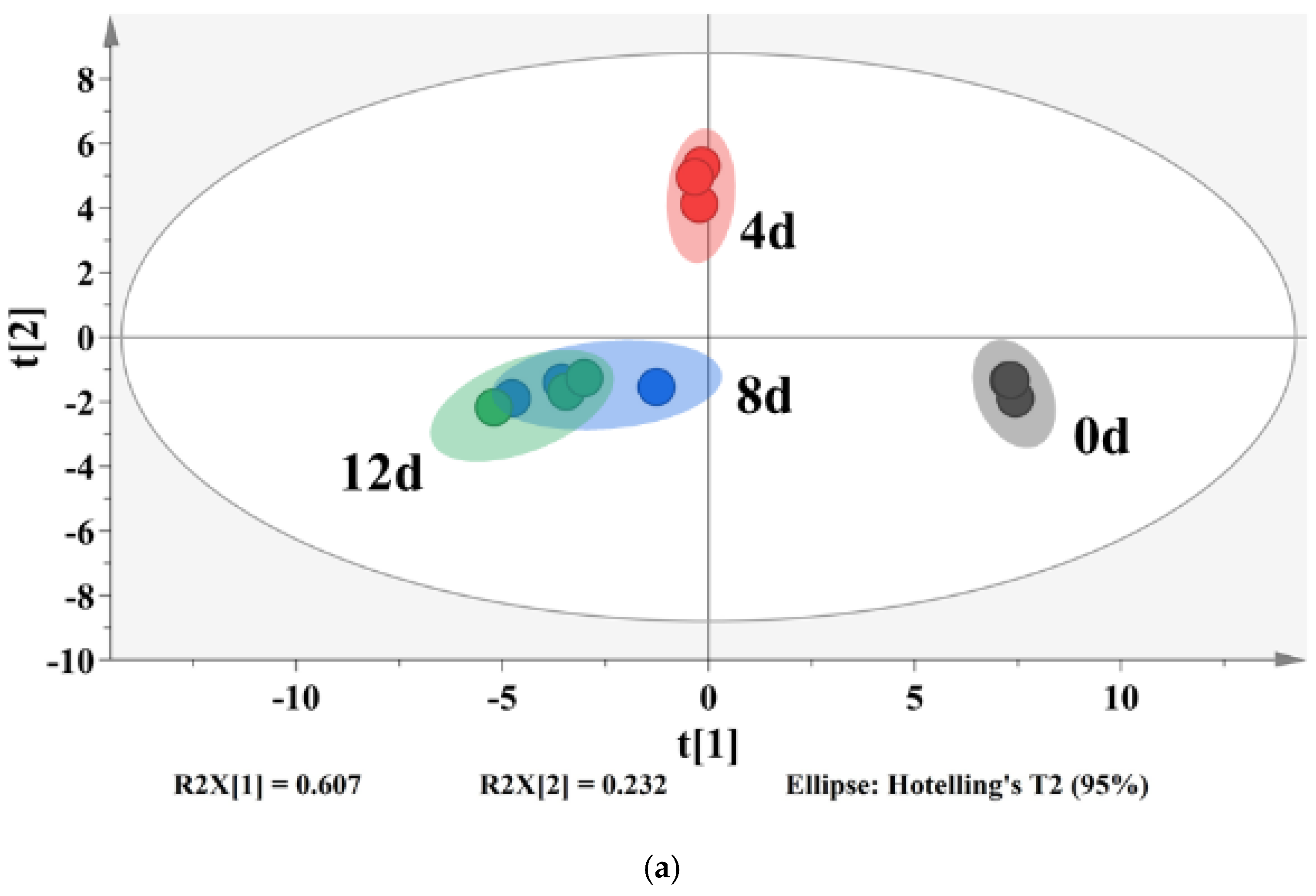
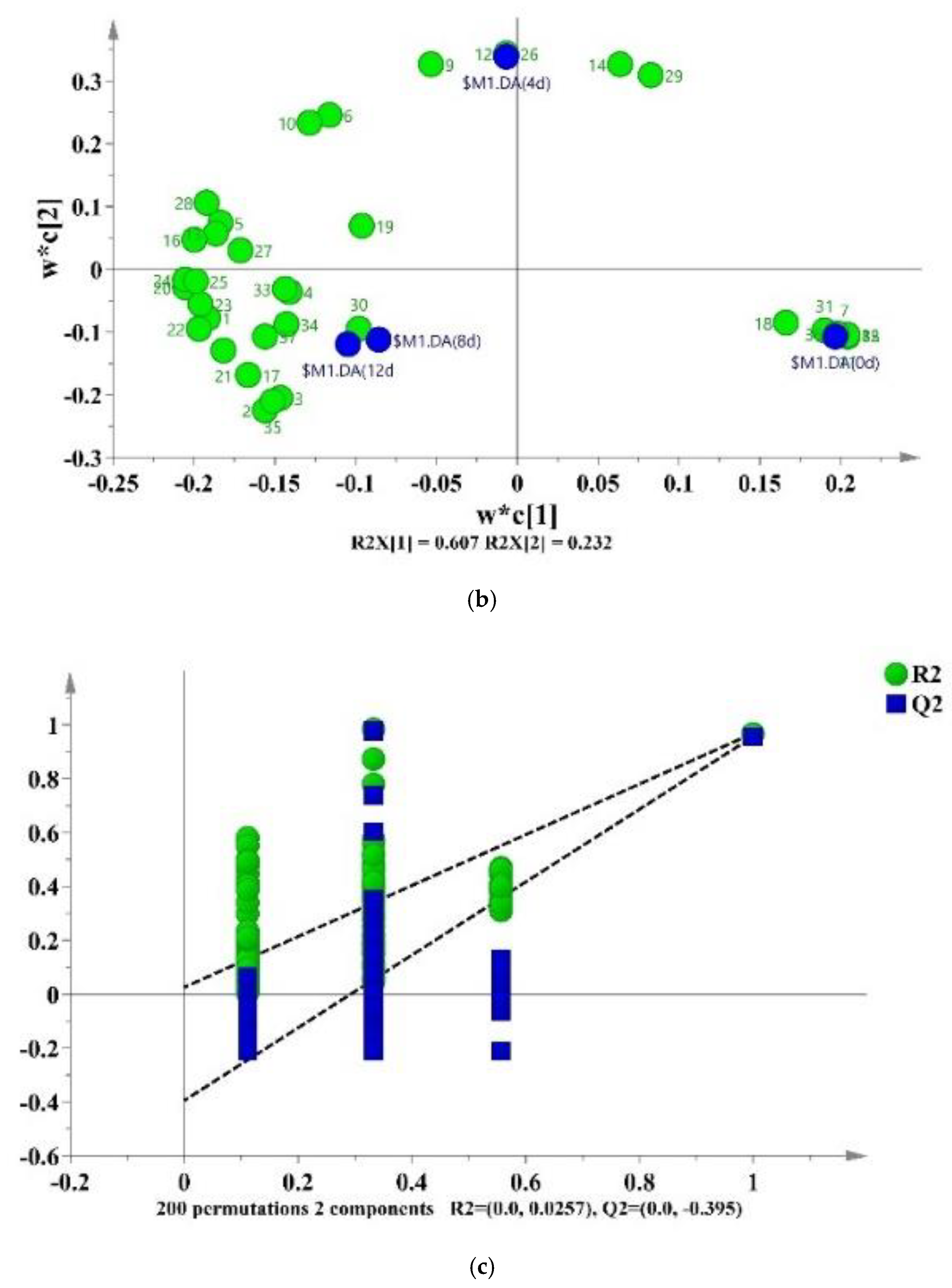
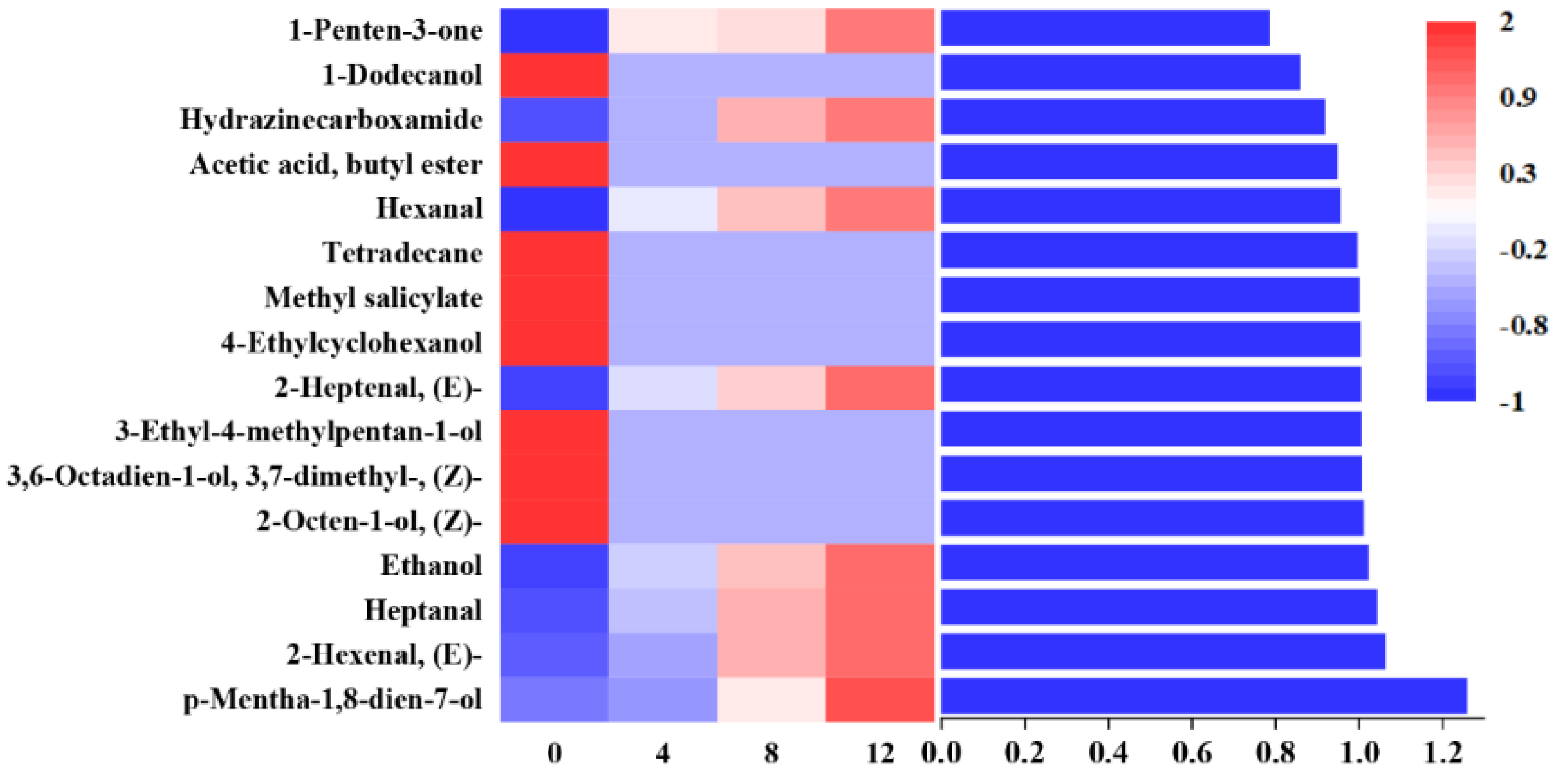
A partial least-squares discriminant analysis (PLS-DA) was used to clarify the effects of storage time on the changes of VOCs in potato samples and to screen for potential spoilage markers. The PLS-DA score plot, loading plot and permutation test at 200 times are displayed in Figure 6. The PLS-DA is a supervised model which can filter system noise and extract variable information. The model fit parameters of R2X, R2Y and Q2 were 0.916, 0.962 and 0.919, respectively, which indicated a good fit and acceptable predictability of the PLS-DA model. Using the PLS-DA model, potato samples in different storage periods were clearly distinguished (Figure 6a). In order to screen for the potential spoilage markers, VOCs related to time evolution were extracted for further analysis (Figure 7). The spoilage markers were defined as VOCs with a variable importance in projection (VIP) ≥ 1. Based on the criterion, p-mentha-1,8-dien-7-ol, (E)-2-hexenal, heptanal, ethanol, (Z)-2-octen-1-ol, (Z)-3,7-dimethyl-3,6-octadien-1-ol, 3-ethyl-4-methylpentan-1-ol, (E)-2-heptenal, 4-ethylcyclohexanol and methyl salicylate were selected. Among these compounds, ethanol was the most abundant, with a steady increasing content starting from 1517.1 μg/kg on day 4 and reaching 3843.27 μg/kg on day 12. Moreover, ethanol was related to microbial metabolism and accompanied by the formation of an off-odor, which could lead to consumer rejection [55]. Therefore, ethanol could serve as a better spoilage marker for vacuum-packaged potatoes. Some studies have also treated ethanol as a spoilage marker of fresh-cut produce due to its high concentration [55,56]. Figure 6b shows the corresponding loading plot of potato samples with the VOCs as variables. All VOCs had a relatively important contribution to the discrimination of potatoes in different storage periods. Furthermore, the permutation test demonstrated that the PLS-DA model was reliable, because the slope of both the R2 (0.969) and Q2 (0.957) regression curves were almost near 1 (Figure 6c).
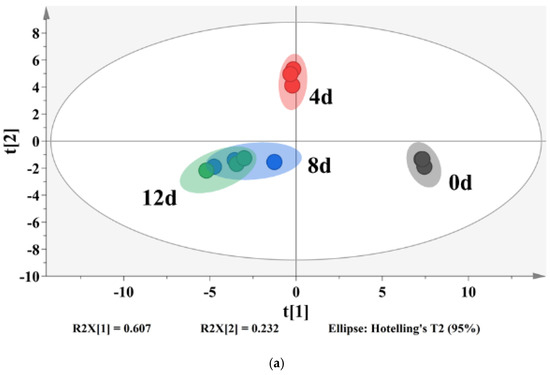
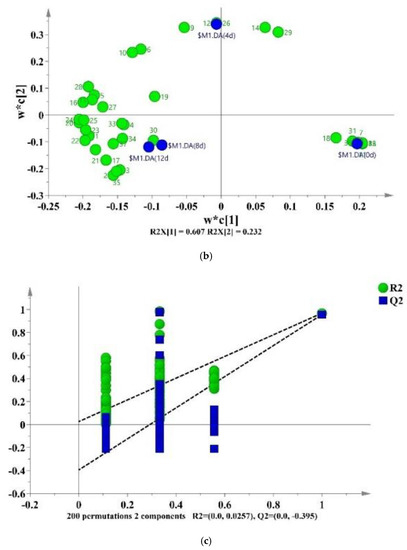
Figure 6.
PLS-DA score plot (a), loading plot (b) and a permutation test at 200 times (c) of vacuum-packaged peeled potato samples during storage.
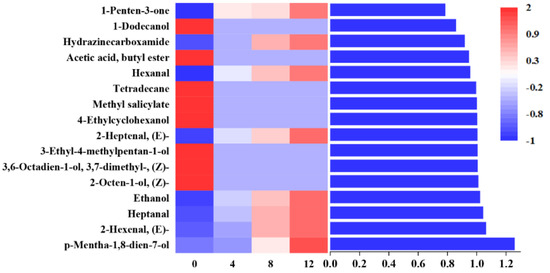
Figure 7.
Profile of VOCs with regular changes and values of the VIP of metabolites.
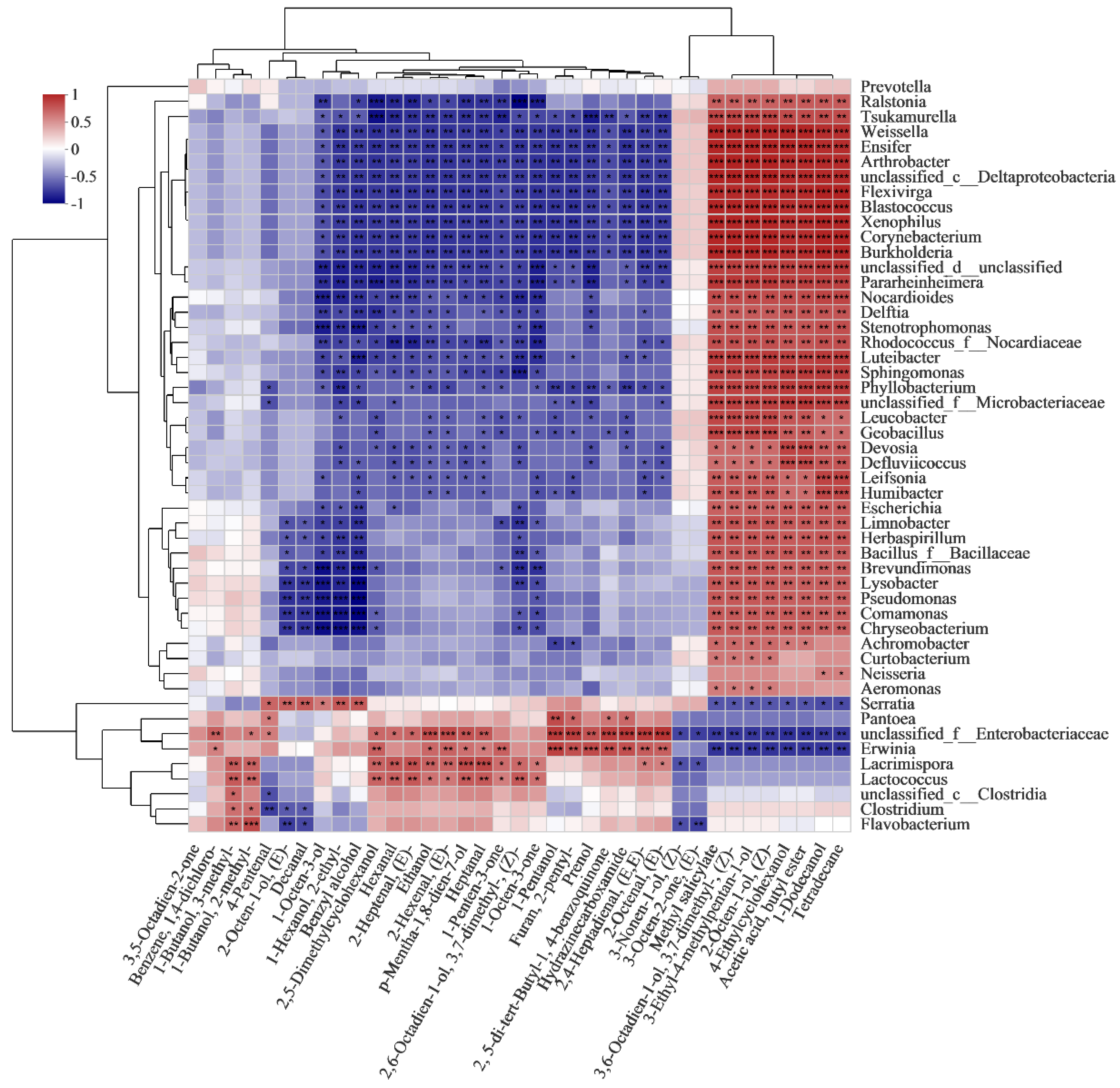
3.7. Correlation Analysis between Bacterial Genera and VOCs
Among the bacterial genus, the VOCs detected were significantly correlated with 9 bacterial genera. As shown in Figure 8, Enterobacteriaceae unclassified was positively correlated with the synthesis of most VOCs, including 17 VOCs (6 alcohols, 7 aldehydes, 1 ketone, 1 furan and 3 hydrocarbons). Erwinia was positively correlated with 14 VOCs (5 alcohols, 4 aldehydes, 1 ketone, 1 furan and 3 hydrocarbons). Lacrimispora was positively correlated with 14 VOCs (6 alcohols, 6 aldehydes and 2 ketones). Lactococcus was positively correlated with 12 VOCs (6 alcohols, 4 aldehydes and 2 ketones). The results suggest that the metabolism of Enterobacteriaceae, Erwinia, Lacrimispora and Lactococcus could have a great influence on the changes of VOCs. Rao et al. [57] found that Enterobacteriaceae unclassified and Lactococcus were dominant in traditional pickled radishes and contributed to the VOCs. Erwinia caused red onions stored at 8 °C to produce VOCs of alcohols and aldehydes [58]. With the extension of storage time, the accumulation of alcohols in potatoes, such as the formation of branched chain and linear alcohols, may be related to amino acid metabolism and fatty acyl esters under the action of bacteria, respectively [58]. Besides this, Serratia, Pantoea, Clostridium, Flavobacterium and Clostridia were positively correlated with 6, 5, 2, 2 and 1 VOCs, respectively. 1-octen-3-ol, 2-ethyl-1-hexanol, benzyl alcohol, (E)-2-octen-1-ol and 4-pentenal and decanal were positively correlated with Serratia. Similarly, 1-octen-3-ol and the isomers of (E)-2-octen-1-ol (2-octen-1-ol) were also found in beef inoculated with Serratia [59]. 1-pentanol, 4-pentenal, 2-pentyl-furan, hydrazinecarboxamide and 2,5-di-tert-butyl-1,4-benzoquinone were positively correlated with Pantoea, which was also reported to be positively correlated with the synthesis of alcohols [60]. The results indicate that Serratia and Pantoea also impacted the VOCs. Furthermore, spoilage markers were also closely related to the role of bacteria. Ethanol, p-mentha-1,8-dien-7-ol, (E)-2-octen-1-ol, (E)-2-hexenal, heptanal and (E)-2-heptenal were positively correlated with Enterobacteriaceae, Erwinia, Lacrimispora and Lactococcus. Among these, ethanol has been reported to be produced through biosynthesis and bioconversion during the growth of Lactococcus and other microorganisms in fresh vegetables [57]. Ioannidis et al. [55] also found that the biosynthesis of ethanol was accompanied by the growth of Lactococcus in fresh-cut lettuce during the storage. furthermore, Enterobacteriaceae has the ability to ferment cheese to produce ethanol [61]. In addition, 3-methyl-1-butanol and 2-methyl-1-butanol, reported as off-odor components, were positively correlated with Lacrimispora, Lactococcus, Clostridia, Clostridium and Flavobacterium. Several studies have identified 3-methyl-1-butanol and 2-methyl-1-butanol as being associated with Lactococcus in cheese. and they could be derived from the proteolytic activity of the bacteria and leucine catabolism [62,63]. The VOCs detected in this study are complex, and several VOCs (ethanol, hexanal, 3-methyl-1-butanol, 2-methyl-1-butanol and 1-octen-3-ol) may produce an unpleasant flavor. In this research, the combination analysis of the bacterial community dynamics and qualities was applied to fresh-cut products, and the potential impact of the bacterial community on the qualities, especially VOCs, of vacuum-packaged peeled potatoes was predicted. It is necessary to further study the VOCs released by specific microorganisms in potatoes to support the possibility of a comprehensive use of some metabolites as a spoilage marker.
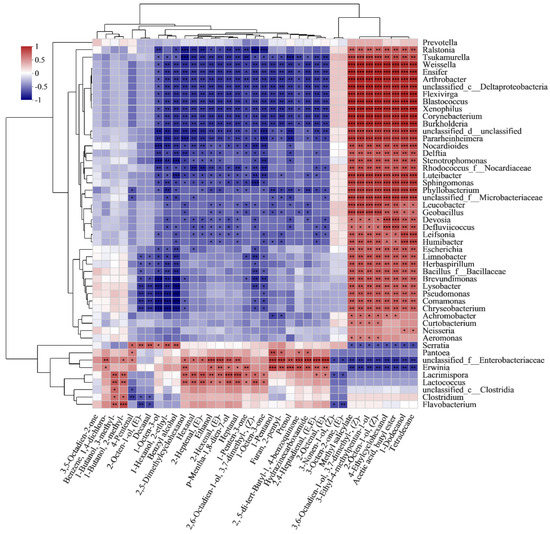
Figure 8.
Heatmaps of Spearman correlations between dominant bacterial genera and VOCs during storage.
4. Conclusions
This study revealed the shifts of bacterial diversity and quality attributes of vacuum-packaged peeled potatoes during storage, as well as their potential correlation. Initially, Ralstonia, Pseudomonas, Pantoea and Comamonas were dominant, while Clostridia, Clostridium, Lacrimispora, Lactococcus and Leuconostoc became more abundant and dominated the bacterial community with the extension of storage time. The visual quality and hardness deteriorated and the contents of lactic acid and acetic acid increased significantly on the 12th day. The flavor quality of potatoes deteriorated significantly after 8 days, as evidenced by results of the e-nose. A total of 37 VOCs were detected, among which alcohols, aldehydes and hydrocarbons accounted for a large proportion. The correlation analysis showed that the accumulation of VOCs was significantly positively correlated to Enterobacteriaceae, Erwinia, Lacrimispora, Lactococcus, Serratia, Pantoea, Clostridium, Flavobacterium and Clostridia. According to a VIP ≥ 1, 10 spoilage markers were screened. Ethanol, which was the most abundant spoilage marker, was significantly related to Enterobacteriaceae, Erwinia, Lacrimispora and Lactococcus. Further research should focus on the effects of specific or mixed bacteria on the quality of fresh-cut fruits and vegetables.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11081147/s1. Table S1: Distribution of the identified OTUs of the bacterial genus during storage of vacuum-packaged peeled potatoes.
Author Contributions
Conceptualization, Z.L. and W.Z.; methodology, Z.L.; validation, Z.L., W.Z. and Y.M.; formal analysis, H.L.; investigation, Z.L.; data curation, Z.L.; writing—original draft preparation, Z.L. and W.Z.; writing—review and editing, D.W. and H.L; visualization, Z.L.; supervision, W.Z. and X.Z.; project administration, Y.M.; funding acquisition, W.Z., Y.M. and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32101971), the Special Innovation Ability Construction Fund of the Beijing Academy of Agricultural and Forestry Sciences (KJCX20220417, KJCX20200208), the Collaborative Innovation Center of the Beijing Academy of Agricultural and Forestry Sciences (KJCX201915), the China Agriculture Research System of MOF and MARA (CARS-23) and the Efficient Ecological Agriculture Innovation Project of Taishan Industry Leading Talent Programming, Shandong Province (LJNY201705).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We express our thanks to the National Natural Science Foundation of China, the Beijing Academy of Agricultural and Forestry Sciences and the Ministry of Agriculture and Rural Affairs for the financial support.
Conflicts of Interest
The authors declare no conflict of interest. Institute of Agri-food Processing and Nutrition, Beijing Academy of Agriculture and Forestry Sciences and Longda Food Group Co. LTD are partners. Longda Food Group Co. LTD where Hao Liang come from also has no potential conflicts. The roles of Hao Liang in this study are formal analysis and writing—review and editing.
References
- Montouto-Grana, M.; Cabanas-Arias, S.; Porto-Fojo, S.; Lourdes Vazquez-Oderiz, M.; Angeles Romero-Rodriguez, M. Sensory characteristics and consumer acceptance and purchase intention toward fresh-cut potatoes. J. Food Sci. 2012, 77, S40–S46. [Google Scholar] [CrossRef] [PubMed]
- Tudela, J.A.; Cantos, E.; Espin, J.C.; Tomas-Barberan, F.A.; Gil, M.I. Induction of antioxidant flavonol biosynthesis in fresh-cut potatoes. Effect of domestic cooking. J. Agric. Food Chem. 2002, 50, 5925–5931. [Google Scholar] [CrossRef] [PubMed]
- Bobo-Garcia, G.; Arroqui, C.; Merino, G.; Virseda, P. Antibrowning compounds for minimally processed potatoes: A review. Food Rev. Int. 2020, 36, 529–546. [Google Scholar] [CrossRef]
- Tsikrika, K.; Tzima, K.; Rai, D.K. Recent advances in anti-browning methods in minimally processed potatoes-A review. J. Food Process. Preserv. 2022, 46, e16298. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Denoya, G.I.; Vaudagna, S.R.; Polenta, G. Effect of high pressure processing and vacuum packaging on the preservation of fresh-cut peaches. LWT 2015, 62, 801–806. [Google Scholar] [CrossRef]
- Min, T.; Liu, E.-C.; Xie, J.; Yi, Y.; Wang, L.-M.; Ai, Y.-W.; Wang, H.-X. Effects of vacuum packaging on enzymatic browning and ethylene response factor (ERF) gene expression of fresh-cut lotus root. HortScience 2019, 54, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Min, T.; Liu, E.-C.; Xie, J.; Yi, Y.; Wang, L.-M.; Ai, Y.-W.; Wang, H.-X. Effects of vacuum packaging on NAC gene expression in fresh-cut lotus root. J. Am. Soc. Hort. Sci. 2020, 145, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Dite Hunjek, D.; Pranjic, T.; Repajic, M.; Levaj, B. Fresh-cut potato quality and sensory: Effect of cultivar, age, processing, and cooking during storage. J. Food Sci. 2020, 85, 2296–2309. [Google Scholar] [CrossRef]
- Ge, L.; Lai, H.; Huang, Y.; Wang, Y.; Li, Y.; Zhu, S.; Shi, Q.; Li, H.; Zhu, Y.; Zhao, N. Comparative evaluation of package types in alleviating textural softening and package-swelling of Paocai during storage: Insight into microbial invasion, cell wall pectinolysis and alteration in sugar and organic acid profiles. Food Chem. 2021, 365, 130489. [Google Scholar] [CrossRef]
- Tudela, J.A.; Hernandez, J.A.; Gil, M.I.; Espin, J.C. L-Galactono-γ-lactone dehydrogenase activity and vitamin C content in fresh-cut potatoes stored under controlled atmospheres. J. Agric. Food Chem. 2003, 51, 4296–4302. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Hong, S.I.; Kim, D. Microbiological and visual quality of fresh-cut cabbage as affected by packaging treatments. Food Sci. Biotechnol. 2011, 20, 229–235. [Google Scholar] [CrossRef]
- Zudaire, L.; Vinas, I.; Abadias, M.; Lafarga, T.; Bobo, G.; Simo, J.; Aguilo-Aguayo, I. Effects of long-term controlled atmosphere storage, minimal processing, and packaging on quality attributes of calcots (Allium cepa L.). Food Sci. Technol. Int. 2020, 26, 403–412. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F.; Ruiz-Cruz, S.; Acedo-Felix, E.; Diaz-Cinco, M.E. Effect of temperature and modified atmosphere packaging on overall quality of fresh-cut bell peppers. Lebensm. Wiss. Und-Technol. Food Sci. Technol. 2004, 37, 817–826. [Google Scholar] [CrossRef]
- Pothakos, V.; Stellato, G.; Ercolini, D.; Devlieghere, F.; Griffiths, M.W.J.A.; Microbiology, E. Processing environment and ingredients are both sources of Leuconostoc gelidum, which emerges as a major spoiler in ready-to-eat meals. Appl. Environ. Microbiol. 2015, 81, 3529–3541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vihavainen, E.J.; Murros, A.E.; Bjrkroth, K.J. Leuconostoc spoilage of vacuum-packaged vegetable sausages. J. Food Prot. 2008, 71, 2312–2315. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.M.; Coulon, E.C.; Morais, A.M. Effects of vacuum packaging on the physical quality of minimally processed potatoes. Food Serv. Technol. 2003, 3, 81–88. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura de Sena Aquino, A.C.; Azevedo, M.S.; Baggio Ribeiro, D.H.; Oliveira Costa, A.C.; Amante, E.R. Validation of HPLC and CE methods for determination of organic acids in sour cassava starch wastewater. Food Chem. 2015, 172, 725–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paillart, M.J.M.; van der Vossen, J.M.B.M.; Levin, E.; Lommen, E.; Otma, E.C.; Snels, J.C.M.A.; Woltering, E.J. Bacterial population dynamics and sensorial quality loss in modified atmosphere packed fresh-cut iceberg lettuce. Postharvest Biol. Technol. 2017, 124, 91–99. [Google Scholar] [CrossRef]
- Solomon, H.M.; Rhodehamel, E.J.; Kautter, D.A. Growth and toxin production by clostridium botulinum in sliced raw potatoes under vacuum with and without sulfite. J. Food Prot. 1994, 57, 878–881. [Google Scholar] [CrossRef]
- Avci, A.; Kamiloglu, A.; Donmez, S. Efficient production of acetone butanol ethanol from sole fresh and rotten potatoes by various Clostridium strains. Biomass Convers. Biorefin. 2021, 189, 1–9. [Google Scholar]
- Lauridsen, L.; Knochel, S. Microbiological stability and diversity in raw pre-peeled potatoes packed in different atmospheres. Eur. Food Res. Technol. 2003, 217, 421–426. [Google Scholar] [CrossRef]
- Paillart, M.; Vossen, J.M.B.M.v.d.; Levin, E.; Otma, E.; Lommen, E.; Snels, J.; Woltering, E.J. Organic acids produced by lactic acid bacteria (Leuconostoc sp.) contribute to sensorial quality loss in modified-atmosphere-packed fresh-cut iceberg lettuce. Acta Hortic. 2016, 1141, 289–296. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Aguilo-Aguayo, I.; Martin-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments on quality and antioxidant properties of fresh-cut mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2010, 56, 216–222. [Google Scholar] [CrossRef]
- Waimaleongora-Ek, P.; Corredor, A.J.H.; No, H.K.; Prinyawiwatkul, W.; King, J.M.; Janes, M.E.; Sathivel, S. Selected quality characteristics of fresh-cut sweet potatoes coated with chitosan during 17-day refrigerated storage. J. Food Sci. 2008, 73, S418–S423. [Google Scholar] [CrossRef]
- Zhai, Y.; Perez-Diaz, I.M.; Diaz, J.T. Viability of commercial cucumber fermentation without nitrogen or air purging. Trends Food Sci. Technol. 2018, 81, 185–192. [Google Scholar] [CrossRef]
- Pothimon, R.; Podjanee, U.; Krusong, W.; Thompson, A.K.; Massa, S. Inhibition of Pantoea agglomerans contamination of fresh-cut jackfruit by exposure to weak organic acid vapors. LWT 2021, 139, 110586. [Google Scholar] [CrossRef]
- Corbo, M.R.; del Nobile, M.A.; Sinigaglia, M. A novel approach for calculating shelf life of minimally processed vegetables. Int. J. Food Microbiol. 2006, 106, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.A.; Morris, C.E. Bacterial population dynamics and decay on leaves of different ages of ready-to-use broad-leaved endive. Int. J. Food Sci. Tech. 2010, 30, 221–236. [Google Scholar] [CrossRef]
- Ragaert, P.; Devlieghere, F.; Debevere, J. Role of microbiological and physiological spoilage mechanisms during storage of minimally processed vegetables. Postharvest Biol. Technol. 2007, 44, 185–194. [Google Scholar] [CrossRef]
- Robbs, P.G.; Bartz, J.A.; McFie, G.; Hodge, N.C. Causes of decay of fresh-cut celery. J. Food Sci. 1996, 61, 444–448. [Google Scholar] [CrossRef]
- Xiong, T.; Li, J.; Liang, F.; Wang, Y.; Guan, Q.; Xie, M. Effects of salt concentration on Chinese sauerkraut fermentation. LWT 2016, 69, 169–174. [Google Scholar] [CrossRef]
- Huang, S.J.; Lin, S.Y.; Wang, T.T.; Hsu, F.C. Combining acetic acid and ethanol as an anti-browning treatment for lettuce butt discoloration through repression of the activity and expression of phenylalanine ammonia lyase. Postharvest Biol. Technol. 2020, 164, 111151. [Google Scholar] [CrossRef]
- Shabuer, G.; Ishida, K.; Pidot, S.J.; Roth, M.; Dahse, H.-M.; Hertweck, C. Plant pathogenic anaerobic bacteria use aromatic polyketides to access aerobic territory. Science 2015, 350, 670–674. [Google Scholar] [CrossRef]
- Supapvanich, S.; Tucker, G.A. Physicochemical changes in fresh-cut Honeydew melon fruit during storage. Afr. J. Agric. Res. 2011, 6, 2737–2742. [Google Scholar]
- Zhang, B.Y.; Samapundo, S.; Pothakos, V.; Surengil, G.; Devlieghere, F. Effect of high oxygen and high carbon dioxide atmosphere packaging on the microbial spoilage and shelf-life of fresh-cut honeydew melon. Int. J. Food Microbiol. 2013, 166, 378–390. [Google Scholar] [CrossRef] [PubMed]
- El-Hendawy, H.H.; Osman, M.E.; Ramadan, H.A. Pectic enzymes produced in vitro and in vivo by Erwinia spp. isolated from carrot and pepper in Egypt. J. Phytopathol. Phytopathol. 2002, 150, 431–438. [Google Scholar] [CrossRef]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Guo, Z. Evaluation of the freshness of fresh-cut green bell pepper (Capsicum annuum var. grossum) using electronic nose. LWT 2018, 87, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Jacxsens, L.; Devlieghere, F.; Ragaert, P.; Vanneste, E.; Debevere, J. Relation between microbiological quality, metabolite production and sensory quality of equilibrium modified atmosphere packaged fresh-cut produce. Int. J. Food Microbiol. 2003, 83, 263–280. [Google Scholar] [CrossRef]
- Chen, H.Z.; Zhang, M.; Guo, Z. Discrimination of fresh-cut broccoli freshness by volatiles using electronic nose and gas chromatography-mass spectrometry. Postharvest Biol. Technol. 2019, 148, 168–175. [Google Scholar] [CrossRef]
- Dresow, J.F.; Boehm, H. The influence of volatile compounds of the flavour of raw, boiled and baked potatoes: Impact of agricultural measures on the volatile components. Landbauforsch. Volkenrode 2009, 59, 309–337. [Google Scholar]
- Beaulieu, J.C. Within-season volatile and quality differences in stored fresh-cut cantaloupe cultivars. J. Agric. Food Chem. 2005, 53, 8679–8687. [Google Scholar] [CrossRef]
- Lamikanra, O.; Richard, O.A. Effect of storage on some volatile aroma compounds in fresh-cut cantaloupe melon. J. Agric. Food Chem. 2002, 50, 4043–4047. [Google Scholar] [CrossRef]
- Costello, B.P.J.D.L.; Evans, P.; Ewen, R.J.; Gunson, H.E.; Spencer-Phillips, P.T.N. Gas chromatography-mass spectrometry analyses of volatile organic compounds from potato tubers inoculated with Phytophthora infestans or Fusarium coeruleum. Plant Pathol. 2010, 50, 489–496. [Google Scholar] [CrossRef]
- Petersen, M.A.; Poll, L.; Larsen, L.M. Comparison of volatiles in raw and boiled potatoes using a mild extraction technique combined with GC odour profiling and GC-MS. Food Chem. 1998, 61, 461–466. [Google Scholar] [CrossRef]
- Waterer, D.R.; Pritchard, M.K. Monitoring of volatiles: A technique for detection of soft rot (Erwinia carotovora) in potato tubers. Can. J. Plant Pathol. 1984, 6, 165–171. [Google Scholar] [CrossRef]
- Schütz, S.; Weißbecker, B.; Koch, U.T.; Hummel, H.E. Detection of volatiles released by diseased potato tubers using a biosensor on the basis of intact insect antennae. Biosens. Bioelectron. 1999, 14, 221–228. [Google Scholar] [CrossRef]
- Vollbrecht, D.; Radler, F. Die bildung höherer alkohole bei aminosäuremangelmutanten von saccharomyces cerevisiae III. höhere alkohole als nebenprodukte der aminosäuresynthese. Zent. Für Bakteriol. Parasitenkd. Infekt. Und Hygiene. Zweite Nat. Abt. Allg. Landwirtsch. Und Tech. Mikrobiol. 1975, 130, 238–244. [Google Scholar] [CrossRef]
- Ragaert, P.; Devlieghere, F.; Devuyst, E.; Dewulf, J.; van Langenhove, H.; Debevere, J. Volatile metabolite production of spoilage micro-organisms on a mixed-lettuce agar during storage at 7 degrees C in air and low oxygen atmosphere. Int. J. Food Microbiol. 2006, 112, 162–170. [Google Scholar] [CrossRef]
- Ioannidis, A.G.; Kerckhof, F.M.; Drif, Y.R.; Vanderroost, M.; Boon, N.; Ragaert, P.; de Meulenaer, B.; Devlieghere, F. Characterization of spoilage markers in modified atmosphere packaged iceberg lettuce. Int. J. Food Microbiol. 2018, 279, 1–13. [Google Scholar] [CrossRef]
- Lopez-Galvez, F.; Ragaert, P.; Hague, M.A.; Eriksson, M.; van Labeke, M.C.; Devlieghere, F. High oxygen atmospheres can induce russet spotting development in minimally processed iceberg lettuce. Postharvest Biol. Technol. 2015, 100, 168–175. [Google Scholar] [CrossRef]
- Rao, Y.; Tao, Y.; Chen, X.; She, X.; Qian, Y.; Li, Y.; Du, Y.; Xiang, W.; Li, H.; Liu, L. The characteristics and correlation of the microbial communities and flavors in traditionally pickled radishes. LWT 2020, 118, 108804. [Google Scholar] [CrossRef]
- Tiwari, S.; Goswami, U.; Kate, A.; Modhera, B.; Tripathi, M.K.; Mohapatra, D. Biological relevance of VOCs emanating from red onions infected with Erwinia (Pectobacterium) carotovora under different storage conditions. Postharvest Biol. Technol. 2022, 184, 111761. [Google Scholar] [CrossRef]
- Ercolini, D.; Russo, F.; Nasi, A.; Ferranti, P.; Villani, F. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl. Environ. Microbiol. 2009, 75, 1990–2001. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhang, F.; Zhang, C.; Yang, L.; Fan, G.; Xu, Y.; Sun, B.; Li, X. Dynamic microbial succession of Shanxi aged vinegar and its correlation with flavor metabolites during different stages of acetic acid fermentation. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Morales, P.; Feliu, I.; Fernandez-Garcia, E.; Nunez, M. Volatile compounds produced in cheese by Enterobacteriaceae strains of dairy origin. J. Food Prot. 2004, 67, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Garde, S.; Carbonell, M.; Fernández-García, E.; Medina, M.; Nuñez, M. Volatile compounds in Hispánico cheese manufactured using a mesophilic starter, a thermophilic starter, and bacteriocin-producing Lactococcus lactis subsp. lactis INIA 415. J. Agric. Food Chem. 2002, 50, 6752–6757. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, J.; Arce, C.; Jordano, R.; Arce, L.; Medina, L.M. Target identification of volatile metabolites to allow the differentiation of lactic acid bacteria by gas chromatography-ion mobility spectrometry. Food Chem. 2017, 220, 362–370. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).